Abstract
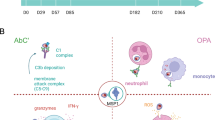
Plasmodium vivax causes approximately 100 million clinical malaria cases yearly1,2. The basis of protective immunity is poorly understood and thought to be mediated by antibodies3,4. Cytotoxic CD8+ T cells protect against other intracellular parasites by detecting parasite peptides presented by human leukocyte antigen class I on host cells. Cytotoxic CD8+ T cells kill parasite-infected mammalian cells and intracellular parasites by releasing their cytotoxic granules5,6. Perforin delivers the antimicrobial peptide granulysin and death-inducing granzymes into the host cell, and granulysin then delivers granzymes into the parasite. Cytotoxic CD8+ T cells were thought to have no role against Plasmodium spp. blood stages because red blood cells generally do not express human leukocyte antigen class I7. However, P. vivax infects reticulocytes that retain the protein translation machinery. Here we show that P. vivax–infected reticulocytes express human leukocyte antigen class I. Infected patient circulating CD8+ T cells highly express cytotoxic proteins and recognize and form immunological synapses with P. vivax–infected reticulocytes in a human leukocyte antigen–dependent manner, releasing their cytotoxic granules to kill both host cell and intracellular parasite, preventing reinvasion. P. vivax–infected reticulocytes and parasite killing is perforin independent, but depends on granulysin, which generally efficiently forms pores only in microbial membranes8. We find that P. vivax depletes cholesterol from the P. vivax–infected reticulocyte cell membrane, rendering it granulysin-susceptible. This unexpected T cell defense might be mobilized to improve P. vivax vaccine efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anstey, N. M., Douglas, N. M., Poespoprodjo, J. R. & Price, R. N. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv. Parasitol. 80, 151–201 (2012).
Miller, L. H., Ackerman, H. C., Su, X. Z. & Wellems, T. E. Malaria biology and disease pathogenesis: insights for new treatments. Nat. Med. 19, 156–167 (2013).
Zimmerman, P. A., Ferreira, M. U., Howes, R. E. & Mercereau-Puijalon, O. Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv. Parasitol. 81, 27–76 (2013).
Mueller, I., Shakri, A. R. & Chitnis, C. E. Development of vaccines for Plasmodium vivax malaria. Vaccine 33, 7489–7495 (2015).
Dotiwala, F. et al. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat. Med. 22, 210–216 (2016).
Walch, M. et al. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 157, 1309–1323 (2014).
Silvestre, D., Kourilsky, F. M., Nicolai, M. G. & Levy, J. P. Presence of HLA antigens on human reticulocytes as demonstrated by electron microscopy. Nature 228, 67–68 (1970).
Barman, H. et al. Cholesterol in negatively charged lipid bilayers modulates the effect of the antimicrobial protein granulysin. J. Membr. Biol. 212, 29–39 (2006).
Bassat, Q. & Alonso, P. L. Defying malaria: fathoming severe Plasmodium vivax disease. Nat. Med. 17, 48–49 (2011).
Murray, C. J. et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379, 413–431 (2012).
Lacerda, M. V. et al. Understanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literature. Malar. J. 11, 12 (2012).
Chakravarty, S., Baldeviano, G. C., Overstreet, M. G. & Zavala, F. Effector CD8 + T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect. Immun. 76, 3628–3631 (2008).
Spencer, A. J. et al. The threshold of protection from liver-stage malaria relies on a fine balance between the number of infected hepatocytes and effector CD8(+) T cells present in the liver. J. Immunol. 198, 2006–2016 (2017).
Schofield, L. et al. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330, 664–666 (1987).
Seguin, M. C. et al. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J. Exp. Med. 180, 353–358 (1994).
Miller, L. H., Mason, S. J., Dvorak, J. A., McGinniss, M. H. & Rothman, I. K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science 189, 561–563 (1975).
King, C. L. et al. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. Proc. Natl. Acad. Sci. USA 105, 8363–8368 (2008).
Safeukui, I., et al. Malaria induces anemia through CD8+ T cell-dependent parasite clearance and erythrocyte removal in the spleen. MBio 6, e02493-14 (2015).
Costa, P. A. et al. Induction of inhibitory receptors on T cells during Plasmodium vivax malaria impairs cytokine production. J. Infect. Dis. 212, 1999–2010 (2015).
Hojo-Souza, N. S. et al. Phenotypic profiling of CD8(+) T cells during Plasmodium vivax blood-stage infection. BMC Infect. Dis. 15, 35 (2015).
Burel, J. G., Apte, S. H., McCarthy, J. S. & Doolan, D. L. Plasmodium vivax but not Plasmodium falciparum blood-stage infection in humans is associated with the expansion of a CD8+ T cell population with cytotoxic potential. PLoS Negl. Trop. Dis. 10, e0005031 (2016).
Falanga, Y. T., et al. High pathogen burden in childhood promotes the development of unconventional innate-like CD8+ T cells. JCI Insight 2, e93814 (2017).
Wilson, M. C. et al. Comparison of the proteome of adult and cord erythroid cells, and changes in the proteome following reticulocyte maturation. Mol. Cell. Proteom. 15, 1938–1946 (2016).
Goh, S. H. et al. The human reticulocyte transcriptome. Physiol. Genom. 30, 172–178 (2007).
Malleret, B. et al. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci. Rep. 1, 118 (2011).
Hoffmann, J. J. & Pennings, J. M. Pseudo-reticulocytosis as a result of malaria parasites. Clin. Lab. Haematol. 21, 257–260 (1999).
Raghavan, M., Del Cid, N., Rizvi, S. M. & Peters, L. R. MHC class I assembly: out and about. Trends Immunol. 29, 436–443 (2008).
Antonelli, L. R. et al. The CD14+ CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog. 10, e1004393 (2014).
Rocha, B. C. et al. Type I interferon transcriptional signature in neutrophils and low-density granulocytes are associated with tissue damage in malaria. Cell Rep. 13, 2829–2841 (2015).
Heemskerk, M. H. et al. Dual HLA class I and class II restricted recognition of alloreactive T lymphocytes mediated by a single T cell receptor complex. Proc. Natl. Acad. Sci. USA 98, 6806–6811 (2001).
Martinvalet, D., Dykxhoorn, D. M., Ferrini, R. & Lieberman, J. Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell 133, 681–692 (2008).
Lauer, S. et al. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19, 3556–3564 (2000).
Holz, G. G. Jr. Lipids and the malarial parasite. Bull. World Health Organ. 55, 237–248 (1977).
Russell, B. et al. A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood 118, e74–e81 (2011).
Ramsey, J. M. et al. Plasmodium falciparum and P. vivax gametocyte-specific exoantigens stimulate proliferation of TCR gammadelta + lymphocytes. J. Parasitol. 88, 59–68 (2002).
Artavanis-Tsakonas, K. & Riley, E. M. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 169, 2956–2963 (2002).
Artavanis-Tsakonas, K. et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J. Immunol. 171, 5396–5405 (2003).
Costa, G. et al. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell-invasive merozoites. Blood 118, 6952–6962 (2011).
Troye-Blomberg, M. et al. Human gamma delta T cells that inhibit the in vitro growth of the asexual blood stages of the Plasmodium falciparum parasite express cytolytic and proinflammatory molecules. Scand. J. Immunol. 50, 642–650 (1999).
Böyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97, 77–89 (1968).
Betts, M. R. et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281, 65–78 (2003).
Cho, J. S. et al. Unambiguous determination of Plasmodium vivax reticulocyte invasion by flow cytometry. Int. J. Parasitol. 46, 31–39 (2016).
Russell, B. et al. Field-based flow cytometry for ex vivo characterization of Plasmodium vivax and P. falciparum antimalarial sensitivity. Antimicrob. Agents Chemother. 57, 5170–5174 (2013).
Wirjanata, G. et al. Quantification of Plasmodium ex vivo drug susceptibility by flow cytometry. Malar. J. 14, 417 (2015).
Rug, M. et al. Export of virulence proteins by malaria-infected erythrocytes involves remodeling of host actin cytoskeleton. Blood 124, 3459–3468 (2014).
Cyrklaff, M. et al. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science 334, 1283–1286 (2011).
Thiery, J., Walch, M., Jensen, D. K., Martinvalet, D. & Lieberman, J. Isolation of cytotoxic T cell and NK granules and purification of their effector proteins. Curr. Protoc. Cell Biol. Chapter 3, Unit 3.37 (2010).
Acknowledgements
We thank Dr. Kasturi Haldar and Kenneth Rock for scientific discussions and suggestions during the development of this work. We are grateful to the Program for Technological Development in Tools for Health–PDTIS-FIOCRUZ for use of its facilities, and to the clinic, laboratory and administrative staff, as well as field workers and subjects from Porto Velho who participated in the study. This study was funded by the National Institutes of Health (1R01NS098747 to R.T.G.; the Amazonian-ICEMR U19 AI089681 to C.J., L.R.A., and R.T.G.; 1R01AI116577 and R21AI131632-01 to R.T.G. and J.L.); National Institute of Science and Technology for Vaccines/Conselho Nacional de Desenvolvimento Científico e Tecnológico (465293/2014-0 to C.J., L.R.A., D.B.P. and R.T.G.) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (RED-00012-14 and APQ-00653-16 to C.J. and R.T.G.); and Fundação de Amparo à Pesquisa do Estado de São Paulo (2016/23618-8 to R.T.G.). C.J., L.R.A., A.T.-C., and R.T.G. are recipients of CNPq fellowships; P.A.C.C. and C.R.R.B. are fellows from FAPEMIG; and C.J. is a fellow from Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior.
Author information
Authors and Affiliations
Contributions
C.J., J.L., and R.T.G. conceived the study. C.J. and R.T.G. supervised this study. D.B.P. recruited the patients and healthy donors; C.J., L.R.A., A.T.-C., J.L., and R.T.G. designed and analyzed experiments. C.J., C.R.R.B., L.R.A., P.A.C.C., A.T.-C., G.C., and R.P.B. performed experiments. S.S.S. and F.D. contributed reagents. C.J., C.R.R.B., P.A.C.C., and L.R.A. prepared figures and helped with manuscript preparation. C.J., J.L., and R.T.G. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1 and 2
Rights and permissions
About this article
Cite this article
Junqueira, C., Barbosa, C.R.R., Costa, P.A.C. et al. Cytotoxic CD8+ T cells recognize and kill Plasmodium vivax–infected reticulocytes. Nat Med 24, 1330–1336 (2018). https://doi.org/10.1038/s41591-018-0117-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0117-4
This article is cited by
-
Pathogenetic mechanisms and treatment targets in cerebral malaria
Nature Reviews Neurology (2023)
-
Cytolytic circumsporozoite-specific memory CD4+ T cell clones are expanded during Plasmodium falciparum infection
Nature Communications (2023)
-
Immunological characteristics of CD103+CD8+ Tc cells in the liver of C57BL/6 mouse infected with plasmodium NSM
Parasitology Research (2023)
-
Escaping the enemy’s bullets: an update on how malaria parasites evade host immune response
Parasitology Research (2023)
-
Identification of hub genes for adult patients with sepsis via RNA sequencing
Scientific Reports (2022)