Abstract
Lung-resident macrophages, which include alveolar macrophages and interstitial macrophages (IMs), exhibit a high degree of diversity, generally attributed to different activation states, and often complicated by the influx of monocytes into the pool of tissue-resident macrophages. To gain a deeper insight into the functional diversity of IMs, here we perform comprehensive transcriptional profiling of resident IMs and reveal ten distinct chemokine-expressing IM subsets at steady state and during inflammation. Similar IM subsets that exhibited coordinated chemokine signatures and differentially expressed genes were observed across various tissues and species, indicating conserved specialized functional roles. Other macrophage types shared specific IM chemokine profiles, while also presenting their own unique chemokine signatures. Depletion of CD206hi IMs in Pf4creR26EYFP+DTR and Pf4creR26EYFPCx3cr1DTR mice led to diminished inflammatory cell recruitment, reduced tertiary lymphoid structure formation and fewer germinal center B cells in models of allergen- and infection-driven inflammation. These observations highlight the specialized roles of IMs, defined by their coordinated chemokine production, in regulating immune cell influx and organizing tertiary lymphoid tissue architecture.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The sequencing raw data and processed data used in this article are available in the Gene Expression Omnibus under accession code GSE193782 (hBAL)9 and Super Series GSE225668 (mLu and all others). The processed data are also available for online visualization at https://ams-supercluster.cells.ucsc.edu, https://cells.ucsc.edu/?ds=ln-mono-dc and https://cells.ucsc.edu/?ds=lung-interstitial-macrophage. In addition to our in-house datasets, we incorporated several publicly available datasets for auxiliary analyses. For confirmation of IM subsets, we consulted dataset GSE179276 (ref. 33), as illustrated in Extended Data Fig. 6. Furthermore, the specificity of Pf4 and Cx3cr1 expression in IMs was corroborated by referencing datasets GSE147668 (ref. 42), Inflammatory Memory Neutophils in Asthma (ref. 43), GSE149563 (ref. 44) and E.MTAB.10026 (ref. 45), as presented in Extended Data Fig. 9, whose visualizations with complete cell types labels are also available on a different dataset depository at https://app.lungmap.net/app/shinycell-mm-timecourse, https://cells.ucsc.edu/?ds=mouse-lung-immune, https://cells.ucsc.edu/?ds=mouse-asthma+all-cells and https://cells.ucsc.edu/?ds=covid19-pbmc. Source data are provided with this paper.
Code availability
Code is available at https://github.com/XinLi-0419/MacrophageChemokineHeterogeneity.
References
Guilliams, M. et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 185, 379–396 (2022).
Aegerter, H., Lambrecht, B. N. & Jakubzick, C. V. Biology of lung macrophages in health and disease. Immunity 55, 1564–1580 (2022).
Bain, C. C. & MacDonald, A. S. The impact of the lung environment on macrophage development, activation and function: diversity in the face of adversity. Mucosal Immunol. 15, 223–234 (2022).
Li, X., Rawat, K. & Jakubzick, C. V. Targeting resident macrophages in cancer. Nat. Immunol. 22, 1078–1079 (2021).
Janssen, W. J. et al. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.201011-1891oc (2011).
Gibbings, S. L. et al. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood 126, 1357–1366 (2015).
Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214, 2387–2404 (2017).
Guilliams, M. et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 210, 1977–1992 (2013).
Li, X. et al. ScRNA-seq expression of IFI27 and APOC2 identifies four alveolar macrophage superclusters in healthy BALF. Life Sci. Alliance 5, e202201458 (2022).
Dick, S. A. et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 7, eabf7777 (2022).
Gibbings, S. L. et al. Three unique interstitial macrophages in the murine lung at steady state. Am. J. Respir. Cell Mol. Biol. 57, 66–76 (2017).
Moore, P. K. et al. Single-cell RNA sequencing reveals unique monocyte-derived interstitial macrophage subsets during lipopolysaccharide-induced acute lung inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. https://doi.org/10.1152/ajplung.00223.2022 (2023).
Vanneste, D. et al. MafB-restricted local monocyte proliferation precedes lung interstitial macrophage differentiation. Nat. Immunol. 24, 827–840 (2023).
Hume, P. S. et al. Localization of macrophages in the human lung via design-based stereology. Am. J. Respir. Crit. Care Med. 201, 1209–1217 (2020).
Cho, C. H. et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ. Res. 100, e47–e57 (2007).
Han, Y., Ma, F. Y., Tesch, G. H., Manthey, C. L. & Nikolic-Paterson, D. J. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab. Invest. 91, 978–991 (2011).
Pridans, C. et al. Pleiotropic impacts of macrophage and microglial deficiency on development in rats with targeted mutation of the Csf1r locus. J. Immunol. 201, 2683–2699 (2018).
Ural, B. B. et al. Identification of a nerve-associated, lung-resident interstitial macrophage subset with distinct localization and immunoregulatory properties. Sci. Immunol. 5, eaax8756 (2020).
Keerthivasan, S. et al. Homeostatic functions of monocytes and interstitial lung macrophages are regulated via collagen domain-binding receptor LAIR1. Immunity 54, 1511–1526 (2021).
Schyns, J. et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat. Commun. 10, 3964 (2019).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019).
Zhou, X. & Moore, B. B. Location or origin? What is critical for macrophage propagation of lung fibrosis? Eur. Respir. J. 51, 1800103 (2018).
Nakano, H. et al. Complement receptor C5aR1/CD88 and dipeptidyl peptidase-4/CD26 define distinct hematopoietic lineages of dendritic cells. J. Immunol. 194, 3808–3819 (2015).
Rawat, K. et al. CCL5-producing migratory dendritic cells guide CCR5+ monocytes into the draining lymph nodes. J. Exp. Med. 220, e20222129 (2023).
Bosteels, C. et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 52, 1039–1056 (2020).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Tang, X. Z., Kreuk, L. S. M., Cho, C., Metzger, R. J. & Allen, C. D. C. Bronchus-associated macrophages efficiently capture and present soluble inhaled antigens and are capable of local Th2 cell activation. eLife 11, e63296 (2022).
Zheng, S. C., Stein-O’Brien, G., Boukas, L., Goff, L. A. & Hansen, K. D. Pumping the brakes on RNA velocity by understanding and interpreting RNA velocity estimates. Genome Biol. 24, 246 (2023).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Forero, A. et al. Differential activation of the transcription factor IRF1 underlies the distinct immune responses elicited by type I and type III interferons. Immunity 51, 451–464 (2019).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Pitarresi, J. R. et al. Stromal ETS2 regulates chemokine production and immune cell recruitment during acinar-to-ductal metaplasia. Neoplasia 18, 541–552 (2016).
Revelo, X. S. et al. Cardiac resident macrophages prevent fibrosis and stimulate angiogenesis. Circ. Res. 129, 1086–1101 (2021).
Heng, T. S. & Painter, M. W. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Neyt, K., Perros, F., GeurtsvanKessel, C. H., Hammad, H. & Lambrecht, B. N. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 33, 297–305 (2012).
Bracke, K. R. et al. Role of CXCL13 in cigarette smoke-induced lymphoid follicle formation and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 188, 343–355 (2013).
Rangel-Moreno, J., Moyron-Quiroz, J. E., Hartson, L., Kusser, K. & Randall, T. D. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc. Natl Acad. Sci. USA 104, 10577–10582 (2007).
Ansel, K. M., Harris, R. B. & Cyster, J. G. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16, 67–76 (2002).
Koscso, B. et al. Gut-resident CX3CR1(hi) macrophages induce tertiary lymphoid structures and IgA response in situ. Sci. Immunol. 5, eaax0062. (2020).
Tiedt, R., Schomber, T., Hao-Shen, H. & Skoda, R. C. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 109, 1503–1506 (2007).
Han, J. et al. Human serous cavity macrophages and dendritic cells possess counterparts in the mouse with a distinct distribution between species. Nat. Immunol. 25, 155–165 (2024).
Domingo-Gonzalez, R. et al. Diverse homeostatic and immunomodulatory roles of immune cells in the developing mouse lung at single cell resolution. eLife 9, e56890 (2020).
Li, Z. et al. Single-cell transcriptomics of mouse lung reveal inflammatory memory neutrophils in allergic asthma. Allergy 77, 1911–1915 (2022).
Zepp, J. A. et al. Genomic, epigenomic, and biophysical cues controlling the emergence of the lung alveolus. Science 371, eabc3172 (2021).
Stephenson, E. et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat. Med. 27, 904–916 (2021).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Lee, S. H. et al. Dermis resident macrophages orchestrate localized ILC2 eosinophil circuitries to promote non-healing cutaneous leishmaniasis. Nat. Commun. 14, 7852 (2023).
Kim, J. S. et al. A binary Cre transgenic approach dissects microglia and CNS border-associated macrophages. Immunity 54, 176–190 (2021).
Gibbings, S. L. & Jakubzick, C. V. Isolation and characterization of mononuclear phagocytes in the mouse lung and lymph nodes. Methods Mol. Biol. 1809, 33–44 (2018).
Jakubzick, C., Helft, J., Kaplan, T. J. & Randolph, G. J. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J. Immunol. Methods 337, 121–131 (2008).
Bosnjak, B. et al. MCK2-mediated MCMV infection of macrophages and virus dissemination to the salivary gland depends on MHC class I molecules. Cell Rep. 42, 112597 (2023).
Brinkman, E. K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
R Development Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2014).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Marini, F. & Binder, H. pcaExplorer: an R/Bioconductor package for interacting with RNA-seq principal components. BMC Bioinform. 20, 331 (2019).
Rantakari, P. et al. Fetal liver endothelium regulates the seeding of tissue-resident macrophages. Nature 538, 392–396 (2016).
Soetaert, K. plot3D: plotting multi-dimensional data (2021).
Wickham, H. ggplot2: Elegant Graphics For Data Analysis (Springer, 2016).
Crow, M., Paul, A., Ballouz, S., Huang, Z. J. & Gillis, J. Characterizing the replicability of cell types defined by single cell RNA-sequencing data using MetaNeighbor. Nat. Commun. 9, 884 (2018).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Zhang, H. SCP: Single Cell Pipeline (2023).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Huynh-Thu, V. A., Irrthum, A., Wehenkel, L. & Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 5, e12776 (2010).
Van de Sande, B. et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 15, 2247–2276 (2020).
Acknowledgements
We are grateful to W. Wells, S. H. Boyle, N. R. Lemelin, J. N. Ray, S. N. Schutz, A. J. Wood and P. P. Seery at the Department of Pathology at Dartmouth Hitchcock Medical Center for kindly providing us with human breast reduction skin samples, W. T. King at the Department of Microbiology and Immunology at Dartmouth Hitchcock Medical Center for technical support, K. Rawat and A. Tewari at the Department of Microbiology and Immunology at Dartmouth Hitchcock Medical Center for the mTME dataset and A. Balasubramanian at the Department of Microbiology and Immunology at Dartmouth Hitchcock Medical Center for the assistance with the CRISPR-mediated knockout experiments. This work was funded by National Institutes of Health (NIH) grants R01 HL115334 (C.V.J.); NIH grants R01 HL135001 (C.V.J.); NIH grants R35 HL155458 (C.V.J.); NIH grants T32AI007363 (A.B.M.); RS-TS UGent grant BOF.MET.2021.0007.01 (contract no. 01M01521) (N.G.); National Cancer Institute Cancer Center Support Grant 5P30CA023108 (F.W.K.); NIH S10 1S10OD030242 (F.W.K.); NIH NIGMS P20GM130454 (F.W.K.); and NIH S10 S10OD025235 (F.W.K.). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
X.L. and C.V.J. developed and organized study infrastructure; X.L., A.B.M., S.M., F.W.K., N.G. and C.V.J. developed the methodology. X.L., A.B.M., S.M., F.W.K. and S.L.G. collected samples; X.L., C.V.J. and A.B.M. conducted experiments and analyzed data; C.V.J., A.B.M. and F.W.K. provided supervision and obtained regulatory compliance and funding; R.S., X.L. and C.V.J. wrote the manuscript with editing input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Andreas Schlitzer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ioana Staicu, in collaboration with the Nature Immunology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
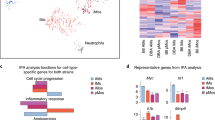
Extended Data Fig. 1 Distinct transcriptional profiles and gene ontology enrichment observed in IM subsets.
(a) Dot plot depicts the expression of top 20 DEGs in CD206hi IMs and CD206lo IMs under naïve state. (b) Dot plot depicts the expression of top 20 DEGs in CD206hi IMs and CD206lo IMs under LPS treatment. (c) Heat map shows the expression of top 10 DEGs in CD206hi IMs and CD206lo IMs, ncIM and recMac under naïve state. (d) Heat map shows the expression of top 10 DEGs in CD206hi IMs and CD206lo IMs, ncIM and recMac under LPS treatment. (e) Heat map shows the expression of top 10 overall DEGs in CD206hi IMs and CD206lo IMs, ncIM and recMac. (f) Heat map shows the top 20 biological process-related gene ontology (GO) terms for CD206hi IMs and CD206lo IMs, ncIM and recMac. Statistical analysis was conducted using Fisher’s exact test.
Extended Data Fig. 2 Absence of unique growth factor or substantial M1/M2 gene signature expression in IM populations.
(a) Dot plot depicts the expression of growth factor genes (GO: 0008083) in each major cell type. (b) Feature plots depict the expression of selected growth factor genes, complemented by violin plots illustrating the expression of those genes in individual experimental groups. (c) Feature plots depict the expression of Nos2 and Arg1, the markers for M1/M2 macrophages. (d) Heat map shows the expression of M1/M2 signature genes (PMID: 31178859) in CD206hi IMs and CD206lo IMs, ncIM and recMac79.
Extended Data Fig. 3 Unbiased clustering analysis with higher resolution shows chemokine gene enrichment in IM subtypes.
(a) UMAP plot illustrates the 58 clusters generated through an unbiased high-resolution clustering analysis, including 38 IM clusters. (b) Heat map shows the expression of top 10 DEGs in each IM cluster; chemokine genes are distinctly emphasized. (c) UMAP plots illustrates IM chemokine expression. (d) UMAP plots illustrates ten IM subsets (IMck0 through IMck9) delineated by their respective chemokine expression profiles, within the original and re-clustered UMAP plot. (e) Feature plots further detail the expression of pan chemokine genes among IM subsets.
Extended Data Fig. 4 Identification of regulons governing differential chemokine expression among IM subsets.
(a) With the motif enriched 500 bp upstream to 100 bp downstream of the targeted gene TSS, left panel: heat map shows the specificity of top 10 regulons enriched in each IM subset, as identified by SCENIC analysis, with the ones that putatively regulate the indicated chemokine genes are accentuated; right panel Heat map shows the expression of the transcription factors from the top 10 enriched in each IM subset, with the ones that putatively regulate the indicated chemokine genes are accentuated. (b) With the motif enriched 10k bp around the targeted gene TSS, right panel: heat map shows the specificity of top 10 regulons enriched in each IM subset, as identified by SCENIC analysis, with the ones that putatively regulate the indicated chemokine genes are accentuated; right panel Heat map shows the expression of the transcription factors from the top 10 enriched in each IM subset, with the ones that putatively regulate the indicated chemokine genes are accentuated. (c) Workflow illustrates the verification of putative transcription factors through CRISPR-mediated knockout in the RAW 264.7 mouse macrophage cell line. Following nucleofection of ribonucleoprotein with various gRNAs, the cells were treated with either PBS or LPS for 24 hours. RT-qPCR assessed chemokine gene expression.
Extended Data Fig. 5 IMck subsets are conserved across mouse and human tissues.
a-g, Dot plots depict the expression of complete chemokine gene set (GO: 0008009) in IM datasets across diverse tissues and species: (a) Mouse lung (mLu); (b) Mouse tumor microenvironment (mTME); (c) Mouse peritoneal lavage (mPL); (d) Mouse skin (mSk); (e) Mouse bronchioalveolar lavage (mBAL); (f) Human skin (hSk); (g) Human bronchioalveolar lavage (hBAL). Highlighted regions accentuate the chemokine-expression patterns of IMs observed in mLu.
Extended Data Fig. 6 Chemokine-expressing IM subsets are conserved in mouse heart (GSE179276).
(a-d) mouse heart (mHr) IMs (a) Dot plot depicts the expression of complete chemokine gene set (GO: 0008009) across the identified IM subsets (IMck0 through IMck9). (b) UMAP plots illustrates ten IM subsets delineated by their respective chemokine expression profiles. (c) Feature plots further detail the expression of pan chemokine genes among IM subsets. (d) Feature plots further detail the expression of remaining individual chemokine genes among IM subsets. (e) Heat map shows the expression of the top 5 DEGs, excluding chemokine genes, originally identified in the mLu dataset across corresponding IM subsets in additional datasets from different tissues and species (mTME, mHr, mSk, hSk). Expression data are normalized within each dataset and merged for the combined visualization. IM subsets not represented in certain datasets are indicated in gray. (f) Bar graph illustrates the distribution of IMck DEGs across five distinct datasets (mLu, mTME, mHr, mSk, hSk). The y-axis categorizes the IM subsets, while the x-axis quantifies the number of DEGs. The DEGs are color-coded based on their occurrence across the datasets, with five shades of gray indicating the level of overlap. The darkest shade represents genes shared in all five datasets, indicating the highest conservation, while the lightest shade represents genes unique to a single dataset, indicating no overlap. (g) Donut charts illustrates the distribution of IMck DEGs five distinct datasets (mLu, mTME, mHr, mSk, hSk) in the format of percentage values, complementing the bar graph.
Extended Data Fig. 7 Tissue-specific macrophages also comprise chemokine-expressing subsets.
a-f, Dot plots depict the expression of complete chemokine gene set (GO: 0008009) in tissue-specific macrophage datasets across diverse tissues and species: (a) Large peritoneal macrophages (LPMs) in mPL; (b) Small peritoneal macrophages (SPMs) in mPL; (c) Langerhans cells (LCs) in mSk; (d) Alveolar macrophages (AMs) in mBAL; (e) Langerhans cells (LCs) in hSk; (f) Alveolar macrophages (AMs) in hBAL. Regions highlighted in black accentuate the chemokine-expression patterns observed in IMs and regions highlighted in red accentuate the unique chemokine-expression patterns observed in tissue-specific macrophages. (g) Schematic representation of the different tissue-specific macrophages investigated for chemokine expression in this figure.
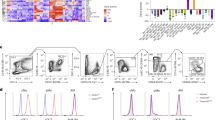
Extended Data Fig. 8 IMs contribute to iBALT formation in Pf4creR26DTR mice.
(a) Flow plots illustrates the verification of Pf4 specificity through Cre recombinase-induced EYFP activation in Pf4crecreR26EYFP mice. Live cells were gated based on DAPI− cells, and it was observed that EYFP expression was exclusive to interstitial macrophages without non-specific targeting effects. (b) Flow plots illustrates the verification of the integrity of dendritic cells (DCs) and other myeloid cells in Pf4creR26EYFP+DTR mice following a single DT administration. DCs were further subdivided into DC1 and DC2 subsets based on the expression of CD11b and CD11c. Cells were gated on DAPI−CD88- myeloid cells. c-e, CD206hi IMs contribute to iBALT formation and B cells maturation in the type 2 inflammation model in Pf4creR26DTR mice. (c) Representative H&E-stained sections of lungs in R26DTR mice and Pf4creR26DTR mice treated with HDM (left, scale bars = 1000 µm). Lung histopathology scores in R26DTR mice and Pf4creR26DTR mice treated with HDM. Two independent experiments with n = 9-10 per group. (d) H&E-stained sections of lungs in R26DTR mice and Pf4creR26DTR mice treated with HDM (4X camera lens magnification, scale bars = 1000 µm or 20X camera lens magnification, scale bars = 200 µm). Two independent experiments with n = 4-5 per group. (e) Flow plots of lung GL7+CD95+ B cells, and the percentage of GL7+ B cells in lung extravascular CD45+ cell population, from R26DTR mice and Pf4creR26DTR mice treated with HDM. Two independent experiments with n = 10 per group. f-h, CD206hi IMs contribute to iBALT formation and B cells maturation in the bacterial infection model in Pf4creR26DTR mice. (f) Representative H&E-stained sections of lungs in R26DTR mice and Pf4creR26DTR mice treated with M. pneu (left, scale bars = 1000 µm). Lung histopathology scores in R26DTR mice and Pf4creR26DTR mice treated with M. pneu. Two independent experiments with n = 9 per group. (g) H&E-stained sections of lungs in R26DTR mice and Pf4creR26DTR mice treated with M. pneu (4X camera lens magnification, scale bars = 1000 µm or 20X camera lens magnification, scale bars = 200 µm). Two independent experiments with n = 4 per group. (h) Flow plots of lung GL7+CD95+ B cells, and the percentage of GL7+ B cells in lung extravascular CD45+ cell population, from R26DTR mice and Pf4creR26DTR mice treated with M. pneu. Two independent experiments with n = 8 per group. Data are shown as median with interquartile range. P values were calculated using two-sided Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Exact P values are listed in Source Data.
Extended Data Fig. 9 Enhanced specificity in targeting CD206hi IMs using the Pf4cre mouse model in combination with Cx3cr1DTR mice.
(a) Breeding scheme to produce Pf4creR26EYFPCx3cr1DTR offspring. (b) A time course analysis presents the depletion kinetics of CD206hi IMs in Pf4creR26EYFPCx3cr1DTR mice following a single DT administration. Three independent experiments. (c) Bioinformatical verification of the specificity in Pf4creCx3cr1DTR mice by analyzing public datasets: GSE147668 (Domingo-Gonzalez - Lung), CRA004586 (Li - Lung), GSE149563 (Zapp - Lung), and E.MTAB.10026 (Stephenson - PBMC), highlighting the specific expression of both Pf4 and Cx3cr1 by IMs. Visualizations with complete cell types labels are available on different dataset repositories (listed in Data Availability section) and original publications.
Extended Data Fig. 10 Analogous to cytokine-producing helper T cells, macrophages’ specific chemokine expression may also be under tight regulation.
(a) The illustration summarizes the established T cell subsets, emphasizing the distinct differentiation trajectories, cytokines, and chemokine receptor expression profiles for each subset. (b) The illustration summarizes the proposed universal classification of macrophage subsets, emphasizing the distinct differentiation trajectories and associated chemokine expression profiles for each subset.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Supplementary Data 1
Source data for Supplementary Fig. 2.
Supplementary Data 2
Source data for Supplementary Fig. 3.
Supplementary Data 3
Oligonucleotide sequences for gRNA and qPCR.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data Fig. 7
Statistical source data for Fig. 7.
Source Data Extended Data Fig. 1
Statistical source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 3
Statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 8
Statistical source data for Extended Data Fig. 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Mara, A.B., Musial, S.C. et al. Coordinated chemokine expression defines macrophage subsets across tissues. Nat Immunol (2024). https://doi.org/10.1038/s41590-024-01826-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41590-024-01826-9