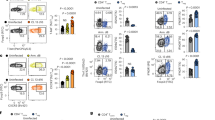
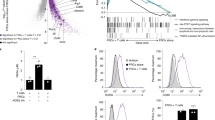
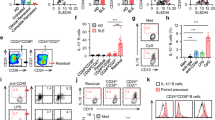
Abstract
Lymph-node (LN) stromal cell populations expand during the inflammation that accompanies T cell activation. Interleukin-17 (IL-17)-producing helper T cells (TH17 cells) promote inflammation through the induction of cytokines and chemokines in peripheral tissues. We demonstrate a critical requirement for IL-17 in the proliferation of LN and splenic stromal cells, particularly fibroblastic reticular cells (FRCs), during experimental autoimmune encephalomyelitis and colitis. Without signaling via the IL-17 receptor, activated FRCs underwent cell cycle arrest and apoptosis, accompanied by signs of nutrient stress in vivo. IL-17 signaling in FRCs was not required for the development of TH17 cells, but failed FRC proliferation impaired germinal center formation and antigen-specific antibody production. Induction of the transcriptional co-activator IκBζ via IL-17 signaling mediated increased glucose uptake and expression of the gene Cpt1a, encoding CPT1A, a rate-limiting enzyme of mitochondrial fatty acid oxidation. Hence, IL-17 produced by locally differentiating TH17 cells is an important driver of the activation of inflamed LN stromal cells, through metabolic reprogramming required to support proliferation and survival.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Patel, D. D. & Kuchroo, V. K. Th17 cell pathway in human immunity: lessons from genetics andtherapeutic interventions. Immunity 43, 1040–1051 (2015).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelialpermeability. Immunity 43, 727–738 (2015).
Maxwell, J. R. et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 43, 739–750 (2015).
Grogan, J. L. & Ouyang, W. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur. J. Immunol. 42, 2255–2262 (2012).
Pikor, N. B. et al. Integration of Th17- and lymphotoxin-derived signals initiates meningeal-resident stromal cell remodeling to propagate neuroinflammation. Immunity 43, 1160–1173 (2015).
Brown, F. D. & Turley, S. J. Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle. J. Immunol. 194, 1389–1394 (2015).
Rodda, L. B. et al. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity 48, 1014–1028 e1016 (2018).
Huang, H. Y. et al. Identification of a new subset of lymph node stromal cells involved in regulating plasma cell homeostasis. Proc. Natl Acad. Sci. USA 115, E6826–E6835 (2018).
Cremasco, V. et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat. Immunol. 15, 973–981 (2014).
Chai, Q. et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 38, 1013–1024 (2013).
Zeng, M. et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Invest. 121, 998–1008 (2011).
Estes, J. D. et al. Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ T-cell populations and improves immune reconstitution with antiretroviral therapy. J. Infect. Dis. 211, 744–754 (2015).
Kityo, C. et al. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J. Clin. Invest. 128, 2763–2773 (2018).
Khan, O. et al. Regulation of T cell priming by lymphoid stroma. PLoS ONE 6, e26138 (2011).
Lukacs-Kornek, V. et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat. Immunol. 12, 1096–1104 (2011).
Siegert, S. et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS ONE 6, e27618 (2011).
Gil-Cruz, C. et al. Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs. Nat. Immunol. 17, 1388–1396 (2016).
Fletcher, A. L. et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J. Exp. Med. 207, 689–697 (2010).
Dubrot, J. et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J. Exp. Med. 211, 1153–1166 (2014).
Cyster, J. G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
Astarita, J. L. et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat. Immunol. 16, 75–84 (2015).
Chyou, S. et al. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J. Immunol. 187, 5558–5567 (2011).
Yang, C. Y. et al. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc. Natl Acad. Sci. USA 111, E109–E118 (2014).
Katakai, T., Hara, T., Sugai, M., Gonda, H. & Shimizu, A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J. Exp. Med. 200, 783–795 (2004).
Teesalu, T., Hinkkanen, A. E. & Vaheri, A. Coordinated induction of extracellular proteolysis systems during experimental autoimmune encephalomyelitis in mice. Am. J. Pathol. 159, 2227–2237 (2001).
Han, M. H. et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 (2008).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Garg, A. V. et al. MCPIP1 endoribonuclease activity negatively regulates interleukin-17-mediated signaling and inflammation. Immunity 43, 475–487 (2015).
Khader, S. A., Gaffen, S. L. & Kolls, J. K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2, 403–411 (2009).
Chung, J. et al. Fibroblastic niches prime T cell alloimmunity through delta-like notch ligands. J. Clin. Invest. 127, 1574–1588 (2017).
Amatya, N., Garg, A. V. & Gaffen, S. L. IL-17 signaling: the yin and the yang. Trends Immunol. 38, 310–322 (2017).
Coller, H. A., Sang, L. & Roberts, J. M. A new description of cellular quiescence. PLoS Biol. 4, e83 (2006).
Okoshi, R. et al. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J. Biol. Chem. 283, 3979–3987 (2008).
Loberg, R. D., Vesely, E. & Brosius, F. C. III. Enhanced glycogen synthase kinase-3 beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. J. Biol. Chem. 277, 41667–41673 (2002).
Yamamoto, M. et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 430, 218–222 (2004).
Ha, H. L. et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl Acad. Sci. USA 111, E3422–E3431 (2014).
Wu, L. et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 212, 1571–1587 (2015).
Wang, C. et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat. Commun. 8, 15508 (2017).
Datta, S. K. et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl Acad. Sci. USA 107, 10638–10643 (2010).
Hsu, H. C. et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 9, 166–175 (2008).
Mitsdoerffer, M. et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl Acad. Sci. USA 107, 14292–14297 (2010).
Hirota, K. et al. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14, 372–379 (2013).
Ding, Y. et al. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J. Immunol. 191, 1614–1624 (2013).
Sonder, S. U. et al. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J. Biol. Chem. 286, 12881–12890 (2011).
Okuma, A. et al. Enhanced apoptosis by disruption of the STAT3-IkappaB-zeta signaling pathway in epithelial cells induces Sjogren’s syndrome-like autoimmune disease. Immunity 38, 450–460 (2013).
Nogai, H. et al. IkappaB-zeta controls the constitutive NF-kappaB target gene network and survival of ABC DLBCL. Blood 122, 2242–2250 (2013).
Mauro, C. et al. NF-κB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell. Biol. 13, 1272–1279 (2011).
Johnson, R. F., Witzel, I. I. & Perkins, N. D. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-kappaB. Cancer Res. 71, 5588–5597 (2011).
Sommermann, T. G., O’Neill, K., Plas, D. R. & Cahir-McFarland, E. IKKbeta and NF-kappaB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 71, 7291–7300 (2011).
Kumar, P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671 (2016).
Claudio, E. et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J. Immunol. 182, 1617–1630 (2009).
Awasthi, A. et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 182, 5904–5908 (2009).
Jin, Z., Liang, J., Wang, J. & Kolattukudy, P. E. MCP-induced protein 1 mediates the minocycline-induced neuroprotection against cerebral ischemia/reperfusion injury in vitro and in vivo. J. Neuroinflamm. 12, 39 (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Acknowledgements
Funding for this study was provided by the following grants: NIH Nos. AI110822 and AI128991 (to M.J.M.), No. T32-AI089443 (to I.R.), No. DK104680 (to P.S.B.), Nos. DE022550, DE023815 and AI107825 (to S.L.G.), and No. DP2AI136598 (to G.M.D); and R.K. Mellon Institute for Pediatric Research No. AACR SU2C-AACR-IRG-04-16 (to T.W.H). This research was supported in part by the University of Pittsburgh Center for Research Computing through the resources provided. We thank V. Kuchroo (Harvard University) for Il23r–/– mice, P. Kolattukudy (University of Central Florida) for Zc3h12a+/– mice, J. Kolls (Tulane University) for Il17rafl/fl mice (now available at JAX labs) and L. D’Cruz for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
S.M. and M.J.M. conceptualized and designed the study, performed analysis and wrote the manuscript. S.M., N.A., S.R., C.V.J., P.S.B., D.W. and A.M. performed experiments. N.R., I.R., N.A., A.C.P. and S.K. performed or assisted with analysis. F.D., A.B., U.S., T.W.H., G.M.D., S.L.G., P.S.B. and M.J.M. assisted with methodology, resources and analysis of experiments. T.W.H., A.P., P.S.B. and M.J.M. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7
Rights and permissions
About this article
Cite this article
Majumder, S., Amatya, N., Revu, S. et al. IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat Immunol 20, 534–545 (2019). https://doi.org/10.1038/s41590-019-0367-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0367-4
This article is cited by
-
Post-transcriptional checkpoints in autoimmunity
Nature Reviews Rheumatology (2023)
-
The IL-17 family in diseases: from bench to bedside
Signal Transduction and Targeted Therapy (2023)
-
Interleukin-17 as a key player in neuroimmunometabolism
Nature Metabolism (2023)
-
Comprehensive immunophenotypic analysis reveals the pathological involvement of Th17 cells in Graves' disease
Scientific Reports (2022)
-
Fibroblasts as immune regulators in infection, inflammation and cancer
Nature Reviews Immunology (2021)