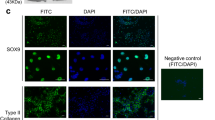
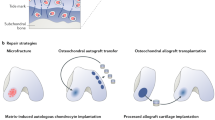
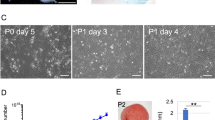
Abstract
Articular cartilage was expected to be one of the first successfully engineered tissues, but today, cartilage repair products are few and they exhibit considerable limitations. For example, of the cell-based products that are available globally, only one is marketed for non-knee indications, none are indicated for severe osteoarthritis or rheumatoid arthritis, and only one is approved for marketing in the USA. However, advances in cartilage tissue engineering might now finally lead to the development of new cartilage repair products. To understand the potential in this field, it helps to consider the current landscape of tissue-engineered products for articular cartilage repair and particularly cell-based therapies. Advances relating to cell sources, bioactive stimuli and scaffold or scaffold-free approaches should now contribute to progress in therapeutic development. Engineering for an inflammatory environment is required because of the need for implants to withstand immune challenge within joints affected by osteoarthritis or rheumatoid arthritis. Bringing additional cartilage repair products to the market will require an understanding of the translational vector for their commercialization. Advances thus far can facilitate the future translation of engineered cartilage products to benefit the millions of patients who suffer from cartilage injuries and arthritides.
Key points
-
Of the cell-based articular cartilage products available globally, only one is marketed for non-knee indications, none are marketed for severe osteoarthritis and none are marketed for rheumatoid arthritis.
-
Cartilage tissue engineering advances include making scaffold-based and scaffold-free cartilage with robust mechanical properties that might enable implants to survive in the loaded-joint environment.
-
Tissue engineering cartilage implants to survive the inflammatory environment through the use of immunomodulatory biomaterials and synthetic biology is a current research highlight.
-
Most large-animal studies for cartilage tissue engineering in the knee use porcine, caprine and ovine models, whereas porcine models are used for temporomandibular joint studies.
-
Several tissue-engineered cartilage products in clinical development use allogeneic cell sources; the use of cell banks that employ allogeneic cells can improve reproducibility and consistency.
-
Owing to the arduous nature of translating biologics, academic researchers should familiarize themselves with the translational vector (including regulatory, manufacturing, funding and intellectual property aspects) to facilitate clinical development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).
Huey, D. J., Hu, J. C. & Athanasiou, K. A. Unlike bone, cartilage regeneration remains elusive. Science 338, 917–921 (2012).
Matthews, L. S., Sonstegard, D. A. & Henke, J. A. Load bearing characteristics of the patello-femoral joint. Acta Orthop. Scand. 48, 511–516 (1977).
Kellathur, S. N. & Lou, H.-X. Cell and tissue therapy regulation: worldwide status and harmonization. Biologicals 40, 222–224 (2012).
Nordberg, R. C., Otarola, G. A., Wang, D., Hu, J. C. & Athanasiou, K. A. Navigating regulatory pathways for translation of biologic cartilage repair products. Sci. Transl. Med. 14, eabp8163 (2022).
US Food and Drug Administration. Expedited programs for regenerative medicine therapies for serious conditions. fda.gov https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-regenerative-medicine-therapies-serious-conditions (2019).
Muthu, S. et al. Failure of cartilage regeneration: emerging hypotheses and related therapeutic strategies. Nat. Rev. Rheumatol. 19, 403–416 (2023).
Sun, H. B. Mechanical loading, cartilage degradation, and arthritis. Ann. N. Y. Acad. Sci. 1211, 37–50 (2010).
Torzilli, P. A., Grigiene, R., Borrelli, J. & Helfet, D. L. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J. Biomech. Eng. 121, 433–441 (1999).
Long, H. et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease study 2019. Arthritis Rheumatol. 74, 1172–1183 (2022).
Almutairi, K., Nossent, J., Preen, D., Keen, H. & Inderjeeth, C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol. Int. 41, 863–877 (2021).
Andriacchi, T. P. & Favre, J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr. Rheumatol. Rep. 16, 463 (2014).
Bullock, J. et al. Rheumatoid arthritis: a brief overview of the treatment. Med. Princ. Pract. 27, 501–507 (2018).
Ruiz, D. J. et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J. Bone Jt Surg. Am. 95, 1473–1480 (2013).
Birnbaum, H. et al. Societal cost of rheumatoid arthritis patients in the US. Curr. Med. Res. Opin. 26, 77–90 (2010).
Torio, C. & Moore, B. National inpatient hospital costs: the most expensive conditions by payer, 2013. in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. PMID: 27359025 (2016).
Takahashi, T. et al. Commercialization of regenerative-medicine therapies. Nat. Rev. Bioeng. 1, 906–929 (2023).
Lee, D. H., Kim, S. J., Kim, S. A. & Ju, G. Past, present, and future of cartilage restoration: from localized defect to arthritis. Knee Surg. Relat. Res. 34, 1 (2022).
Kwon, H. et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 15, 550–570 (2019).
Murray, I. R. et al. Regulatory and ethical aspects of orthobiologic therapies. Orthop. J. Sports Med. 10, 23259671221101624 (2022).
European Medicines Agency. Spherox. em.europa.eu https://www.ema.europa.eu/en/medicines/human/EPAR/spherox (2023).
National Institute for Health and Care Excellence. Autologous chondrocyte implantation using chondrosphere for treating symptomatic articular cartilage defects of the knee. nice.org.uk https://www.nice.org.uk/guidance/ta508/resources/autologous-chondrocyte-implantation-using-chondrosphere-for-treating-symptomatic-articular-cartilage-defects-of-the-knee-pdf-82606726260421 (2018).
Jiang, S. et al. Clinical application status of articular cartilage regeneration techniques: tissue-engineered cartilage brings new hope. Stem Cell Int. 2020, 5690252 (2020).
National Institute for Health and Care Research. NOVOCART 3D for articular cartilage defects of the knee. io.nihr.ac.uk https://www.io.nihr.ac.uk/wp-content/uploads/2022/01/13181-Autologous-Chondrocyte-Implant-for-Articular-Cartilage-Defects-V1.0-SEP2019-NON-CONF.pdf (2019).
US Food and Drug Administration. Approved cellular and gene therapy products. fda.gov https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (2024).
Pharmaceuticals and Medical Devices Agency. Review reports: regenerative medical products. pmda.go.jp https://www.pmda.go.jp/english/review-services/reviews/approved-information/0004.html (2023).
Ministry of Food and Drug Safety. 2022 Drug approval report. mfds.go.kr https://www.mfds.go.kr/eng/brd/m_19/down.do?brd_id=eng0004&seq=70438&data_tp=A&file_seq=1 (2023).
World Health Organization. Ageing and health. who.int https://who.int/news-room/fact-sheets/detail/ageing-and-health (2022).
Bielajew, B. J. et al. Knee orthopedics as a template for the temporomandibular joint. Cell Rep. Med. 2, 100241 (2021).
Manchikanti, L. et al. Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet. Disord. 5, 15 (2004).
Lawrence, R. C. et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 58, 26–35 (2008).
Evenbratt, H. et al. Insights into the present and future of cartilage regeneration and joint repair. Cell Regen. 11, 3 (2022).
LaPrade, R. F. & Botker, J. C. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy 20, e69–e73 (2004).
Li, Y., Wei, X., Zhou, J. & Wei, L. The age-related changes in cartilage and osteoarthritis. Biomed. Res. Int. 2013, 916530 (2013).
Darling, E. M. & Athanasiou, K. A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23, 425–432 (2005).
US Department of Health & Human Services. Points to consider in the characterization of cell lines used to produce biologicals. fda.gov https://www.fda.gov/media/76255/download (1993).
Park, Y.-B., Ha, C.-W., Lee, C.-H., Yoon, Y. C. & Park, Y.-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cell Transl. Med. 6, 613–621 (2017).
Gille, J., Behrens, P., Schulz, A. P., Oheim, R. & Kienast, B. Matrix-associated autologous chondrocyte implantation. Cartilage 7, 309–315 (2016).
Hoburg, A. et al. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroids for patellofemoral or tibiofemoral defects: a 5-year follow-up of a phase 2, dose-confirmation trial. Orthop. J. Sports Med. 10, 232596712110533 (2022).
Thorp, H. et al. Trends in articular cartilage tissue engineering: 3D mesenchymal stem cell sheets as candidates for engineered hyaline-like cartilage. Cells 10, 643 (2021).
Vonk, L. A., De Windt, T. S., Slaper-Cortenbach, I. C. M. & Saris, D. B. F. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: a concise review. Stem Cell Res. Ther. 6, 94 (2015).
Lin, Z. et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 26, 1230–1237 (2008).
Cavalli, E. et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci. Rep. 9, 4275 (2019).
Tsvetkova, A. V. et al. Chondrogeneic potential of MSC from different sources in spheroid culture. Bull. Exp. Biol. Med. 170, 528–536 (2021).
Mohamed-Ahmed, S. et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res. Ther. 9, 168 (2018).
Pievani, A. et al. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy 16, 893–905 (2014).
Hennig, T. et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFβ receptor and BMP profile and is overcome by BMP-6. J. Cell. Physiol. 211, 682–691 (2007).
Mueller, M. B. & Tuan, R. S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 58, 1377–1388 (2008).
Le, H. et al. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 11, 2041731420943839 (2020).
Chen, S., Fu, P., Cong, R., Wu, H. & Pei, M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2, 76–95 (2015).
Rikkers, M., Korpershoek, J. V., Levato, R., Malda, J. & Vonk, L. A. The clinical potential of articular cartilage-derived progenitor cells: a systematic review. NPJ Regen. Med. 7, 2 (2022).
Levato, R. et al. The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 61, 41–53 (2017).
Carluccio, S. et al. Progenitor cells activated by platelet lysate in human articular cartilage as a tool for future cartilage engineering and reparative strategies. Cells 9, 1052 (2020).
Xue, K. et al. Cartilage progenitor cells combined with PHBV in cartilage tissue engineering. J. Transl. Med. 17, 104 (2019).
Neumann, A. J. et al. Human articular cartilage progenitor cells are responsive to mechanical stimulation and adenoviral-mediated overexpression of bone-morphogenetic protein 2. PLoS ONE 10, e0136229 (2015).
Adkar, S. S. et al. Step-wise chondrogenesis of human induced pluripotent stem cells and purification via a reporter allele generated by CRISPR-Cas9 genome editing. Stem Cell 37, 65–76 (2019).
Rodríguez Ruiz, A. et al. Cartilage from human-induced pluripotent stem cells: comparison with neo-cartilage from chondrocytes and bone marrow mesenchymal stromal cells. Cell Tissue Res. 386, 309–320 (2021).
Huang, B. J., Hu, J. C. & Athanasiou, K. A. Effects of passage number and post-expansion aggregate culture on tissue engineered, self-assembled neocartilage. Acta Biomater. 43, 150–159 (2016).
Kwon, H., Brown W. E, O’Leary, S. A., Hu, J. C. & Athanasiou, K. A. Rejuvenation of extensively passaged human chondrocytes to engineer functional articular cartilage. Biofabrication 13, 035002 (2021).
Qin, S. et al. Research progress of functional motifs based on growth factors in cartilage tissue engineering: a review. Front. Bioeng. Biotechnol. 11, 1127949 (2023).
Jelodari, S. et al. New insights into cartilage tissue engineering: improvement of tissue-scaffold integration to enhance cartilage regeneration. Biomed. Res. Int. 2022, 7638245 (2022).
Daou, F., Cochis, A., Leigheb, M. & Rimondini, L. Current advances in the regeneration of degenerated articular cartilage: a literature review on tissue engineering and its recent clinical translation. Materials 15, 31 (2021).
Takematsu, E. et al. Optimizing delivery of therapeutic growth factors for bone and cartilage regeneration. Gels 9, 377 (2023).
Patel, J. M., Saleh, K. S., Burdick, J. A. & Mauck, R. L. Bioactive factors for cartilage repair and regeneration: improving delivery, retention, and activity. Acta Biomater. 93, 222–238 (2019).
Ranmuthu, C. K. I., Ranmuthu, C. D. S., Wijewardena, C. K., Seah, M. K. T. & Khan, W. S. Evaluating the effect of hypoxia on human adult mesenchymal stromal cell chondrogenesis in vitro: a systematic review. Int. J. Mol. Sci. 23, 15210 (2022).
Kwon, H., Paschos, N. K., Hu, J. C. & Athanasiou, K. Articular cartilage tissue engineering: the role of signaling molecules. Cell Mol. Life Sci. 73, 1173–1194 (2016).
Otarola, G. A., Hu, J. C. & Athanasiou, K. A. Ion modulatory treatments toward functional self-assembled neocartilage. Acta Biomater. 153, 85–96 (2022).
Rojas, A. et al. Dickkopf-1 reduces hypertrophic changes in human chondrocytes derived from bone marrow stem cells. Gene 687, 228–237 (2019).
Cruz, M. A. et al. Micronutrient optimization for tissue engineered articular cartilage production of type II collagen. Front. Bioeng. Biotechnol. 11, 1179332 (2023).
Lee, J. K. et al. Tension stimulation drives tissue formation in scaffold-free systems. Nat. Mater. 16, 864–873 (2017).
Ouyang, X., Xie, Y. & Wang, G. Mechanical stimulation promotes the proliferation and the cartilage phenotype of mesenchymal stem cells and chondrocytes co-cultured in vitro. Biomed. Pharmacother. 117, 109146 (2019).
Du, G. et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca2+ response in chondrocytes. Exp. Biol. Med. 245, 180–189 (2020).
Salinas, E. Y. et al. Shear stress induced by fluid flow produces improvements in tissue-engineered cartilage. Biofabrication 12, 045010 (2020).
Farhang, N. et al. Synergistic CRISPRa-regulated chondrogenic extracellular matrix deposition without exogenous growth factors. Tissue Eng. Part. A 26, 1169–1179 (2020).
Seidl, C. I., Fulga, T. A. & Murphy, C. L. CRISPR-Cas9 targeting of MMP13 in human chondrocytes leads to significantly reduced levels of the metalloproteinase and enhanced type II collagen accumulation. Osteoarthr. Cartil. 27, 140–147 (2019).
Nguyen, N. T. K. et al. CRISPR activation of long non-coding RNA DANCR promotes bone regeneration. Biomaterials 275, 120965 (2021).
Li, C. et al. “Genetic scissors” CRISPR/Cas9 genome editing cutting-edge biocarrier technology for bone and cartilage repair. Bioact. Mater. 22, 254–273 (2023).
Uzieliene, I., Kalvaityte, U., Bernotiene, E. & Mobasheri, A. Non-viral gene therapy for osteoarthritis. Front. Bioeng. Biotechnol. 8, 618399 (2021).
Cucchiarini, M. & Madry, H. Advances in gene therapy for cartilage repair. Ann. Jt. 3, 97 (2018).
Han, X. et al. Advances of hydrogel-based bioprinting for cartilage tissue engineering. Front. Bioeng. Biotechnol. 9, 746564 (2021).
Stone, R. N., Reeck, J. C. & Oxford, J. T. Advances in cartilage tissue engineering using bioinks with decellularized cartilage and three-dimensional printing. Int. J. Mol. Sci. 24, 5526 (2023).
Huang, Y., Li, X., Poudel, A. J., Zhang, W. & Xiao, L. Hydrogel-based bioinks for 3D bioprinting articular cartilage: a comprehensive review with focus on mechanical reinforcement. Appl. Mater. Today 29, 101668 (2022).
Yilmaz, E. N. & Zeugolis, D. I. Electrospun polymers in cartilage engineering—state of play. Front. Bioeng. Biotechnol. 8, 77 (2020).
Niemczyk-Soczynska, B., Zaszczyńska, A., Zabielski, K. & Sajkiewicz, P. Hydrogel, electrospun and composite materials for bone/cartilage and neural tissue engineering. Materials 14, 6899 (2021).
Wei, W. et al. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 6, 998–1011 (2021).
Zhu, S. et al. Advanced injectable hydrogels for cartilage tissue engineering. Front. Bioeng. Biotechnol. 10, 954501 (2022).
Liu, M. et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 5, 17014 (2017).
Hafezi, M., Nouri Khorasani, S., Zare, M., Esmaeely Neisiany, R. & Davoodi, P. Advanced hydrogels for cartilage tissue engineering: recent progress and future directions. Polymers 13, 4199 (2021).
Zhou, L. et al. Functionalized hydrogels for articular cartilage tissue engineering. Engineering 13, 71–90 (2022).
Qiu, F. et al. Recent progress in hydrogel-based synthetic cartilage: focus on lubrication and load-bearing capacities. Gels 9, 144 (2023).
Girão, A. F., Semitela, Â., Ramalho, G., Completo, A. & Marques, P. A. A. P. Mimicking nature: fabrication of 3D anisotropic electrospun polycaprolactone scaffolds for cartilage tissue engineering applications. Compos. B Eng. 154, 99–107 (2018).
Garrigues, N. W., Little, D., Sanchez-Adams, J., Ruch, D. S. & Guilak, F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. A 102, 3998–4008 (2014).
Galarraga, J. H., Kwon, M. Y. & Burdick, J. A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 9, 19987 (2019).
Beketov, E. E. et al. Bioprinting of cartilage with bioink based on high-concentration collagen and chondrocytes. Int. J. Mol. Sci. 22, 11351 (2021).
Larson, B. L. et al. Chondrogenic, hypertrophic, and osteochondral differentiation of human mesenchymal stem cells on three-dimensionally woven scaffolds. J. Tissue Eng. Regen. Med. 13, 1453–1465 (2019).
Huynh, N. P. T. et al. Genetic engineering of mesenchymal stem cells for differential matrix deposition on 3D woven scaffolds. Tissue Eng. Part A 24, 1531–1544 (2018).
US Food and Drug Administration. Use of International Standard ISO 10993-1, ‘Biological evaluation of medical devices - part 1: Evaluation and testing within a risk management process’. fda.gov https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (2020).
Yang, F. et al. A synthetic hydrogel composite with the mechanical behavior and durability of cartilage. Adv. Funct. Mater. 30, 2003451 (2020).
Huang, B. et al. Hydrogel composite scaffolds achieve recruitment and chondrogenesis in cartilage tissue engineering applications. J. Nanobiotechnol. 20, 25 (2022).
Chen, J. et al. Tough hydrophobic association hydrogels with self-healing and reforming capabilities achieved by polymeric core-shell nanoparticles. Mater. Sci. Eng. C 99, 460–467 (2019).
Jiang, Y., Guo, S., Jiao, J. & Li, L. A biphasic hydrogel with self-healing properties and a continuous layer structure for potential application in osteochondral defect repair. Polymers 15, 2744 (2023).
Li, P. et al. Building a poly(amino acid)/chitosan-based self-healing hydrogel via host–guest interaction for cartilage regeneration. ACS Biomater. Sci. Eng. 9, 4855–4866 (2023).
Huerta-López, C. & Alegre-Cebollada, J. Protein hydrogels: the Swiss army knife for enhanced mechanical and bioactive properties of biomaterials. Nanomaterials 11, 1656 (2021).
Fu, L. et al. Cartilage-like protein hydrogels engineered via entanglement. Nature 618, 740–747 (2023).
Athanasiou, K. A., Eswaramoorthy, R., Hadidi, P. & Hu, J. C. Self-organization and the self-assembling process in tissue engineering. Annu. Rev. Biomed. Eng. 15, 115–136 (2013).
Zhang, L. et al. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol. Lett. 32, 1339–1346 (2010).
Ullah, M., Hamouda, H., Stich, S., Sittinger, M. & Ringe, J. A reliable protocol for the isolation of viable, chondrogenically differentiated human mesenchymal stem cells from high-density pellet cultures. Biores. Open Access 1, 297–305 (2012).
Zhang, Z., McCaffery, J. M., Spencer, R. G. & Francomano, C. A. Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J. Anat. 205, 229–237 (2004).
Griffith, L. A., Arnold, K. M., Sengers, B. G., Tare, R. S. & Houghton, F. D. A scaffold-free approach to cartilage tissue generation using human embryonic stem cells. Sci. Rep. 11, 18921 (2021).
Grigull, N. P. et al. Chondrogenic potential of pellet culture compared to high-density culture on a bacterial cellulose hydrogel. Int. J. Mol. Sci. 21, 2785 (2020).
Zheng, K. et al. Co-culture pellet of human Wharton’s jelly mesenchymal stem cells and rat costal chondrocytes as a candidate for articular cartilage regeneration: in vitro and in vivo study. Stem Cell Res. Ther. 13, 386 (2022).
Thorp, H., Kim, K., Kondo, M., Grainger, D. W. & Okano, T. Fabrication of hyaline-like cartilage constructs using mesenchymal stem cell sheets. Sci. Rep. 10, 20869 (2020).
Takao, T. et al. A novel chondrocyte sheet fabrication using human-induced pluripotent stem cell-derived expandable limb-bud mesenchymal cells. Stem Cell Res. Ther. 14, 34 (2023).
Maehara, M. et al. Characterization of polydactyly-derived chondrocyte sheets versus adult chondrocyte sheets for articular cartilage repair. Inflamm. Regen. 37, 22 (2017).
Kondo, M. et al. Safety and efficacy of human juvenile chondrocyte-derived cell sheets for osteochondral defect treatment. NPJ Regen. Med. 6, 65 (2021).
Huang, B. J., Brown, W. E., Keown, T., Hu, J. C. & Athanasiou, K. A. Overcoming challenges in engineering large, scaffold-free neocartilage with functional properties. Tissue Eng. Part. A 24, 1652–1662 (2018).
Brown, W. E., Huang, B. J., Hu, J. C. & Athanasiou, K. A. Engineering large, anatomically shaped osteochondral constructs with robust interfacial shear properties. NPJ Regen. Med. 6, 42 (2021).
Vapniarsky, N. et al. Adult dermal stem cells for scaffold-free cartilage tissue engineering: exploration of strategies. Tissue Eng. Part C Methods 26, 598–607 (2020).
Vapniarsky, N. et al. Tissue engineering of canine cartilage from surgically debrided osteochondritis dissecans fragments. Ann. Biomed. Eng. 50, 56–77 (2022).
Bielajew, B. J. et al. Proteomic, mechanical, and biochemical development of tissue-engineered neocartilage. Biomater. Res. 26, 34 (2022).
Griffin, T. M. & Scanzello, C. R. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin. Exp. Rheumatol. 37, 57–63 (2019).
Chen, Y. et al. Macrophages in osteoarthritis: pathophysiology and therapeutics. Am. J. Transl. Res. 12, 261–268 (2020).
Kesharwani, D., Paliwal, R., Satapathy, T. & Das Paul, S. Rheumatiod arthritis: an updated overview of latest therapy and drug delivery. J. Pharmacopuncture 22, 210–224 (2019).
Klimak, M. et al. Immunoengineering the next generation of arthritis therapies. Acta Biomater. 133, 74–86 (2021).
Jüni, P. et al. Intra‐articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 10, CD005328 (2015).
Ansboro, S., Roelofs, A. J. & De Bari, C. Mesenchymal stem cells for the management of rheumatoid arthritis: immune modulation, repair or both? Curr. Opin. Rheumatol. 29, 201–207 (2017).
Mautner, K. et al. Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial. Nat. Med. 29, 3120–3126 (2023).
McBride, D. A., Jones, R. M., Bottini, N. & Shah, N. J. The therapeutic potential of immunoengineering for systemic autoimmunity. Nat. Rev. Rheumatol. 20, 203–215 (2024).
Zhou, F. et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 63, 64–75 (2017).
Tian, G. et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: in vitro and in vivo preclinical study. Acta Biomater. 127, 131–145 (2021).
Ziadlou, R. et al. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. C 120, 111701 (2021).
McWhorter, F. Y., Wang, T., Nguyen, P., Chung, T. & Liu, W. F. Modulation of macrophage phenotype by cell shape. Proc. Natl Acad. Sci. USA 110, 17253–17258 (2013).
Wang, T., Luu, T. U., Chen, A., Khine, M. & Liu, W. F. Topographical modulation of macrophage phenotype by shrink-film multi-scale wrinkles. Biomater. Sci. 4, 948–952 (2016).
Luu, T. U., Gott, S. C., Woo, B. W. K., Rao, M. P. & Liu, W. F. Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl. Mater. Interfaces 7, 28665–28672 (2015).
Badylak, S. F., Valentin, J. E., Ravindra, A. K., McCabe, G. P. & Stewart-Akers, A. M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. Part A 14, 1835–1842 (2008).
Hsieh, J. Y. et al. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 47, 14–24 (2017).
Cha, B.-H. et al. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Adv. Healthc. Mater. 6, 1700289 (2017).
Donahue, R. P. et al. Stiffness- and bioactive factor-mediated protection of self-assembled cartilage against macrophage challenge in a novel co-culture system. Cartilage 13, 19476035221081464 (2022).
Glass, K. A. et al. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 35, 5921–5931 (2014).
Moutos, F. T. et al. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl Acad. Sci. USA 113, E4513–E4522 (2016).
Nims, R. J. et al. A synthetic mechanogenetic gene circuit for autonomous drug delivery in engineered tissues. Sci. Adv. 7, 9858–9885 (2021).
US Food and Drug Administration. Guidance for Industry: preparation of IDEs and INDs for products intended to repair or replace knee cartilage. fda.gov https://www.fda.gov/regulatory-information/search-fda-guidance-documents/preparation-ides-and-inds-products-intended-repair-or-replace-knee-cartilage (2011).
Hurtig, M. B. et al. Preclinical studies for cartilage repair: recommendations from the international cartilage repair society. Cartilage 2, 137–152 (2011).
US Food and Drug Administration. Guidance for industry product development under the animal rule. fda.gov https://www.fda.gov/regulatory-information/search-fda-guidance-documents/product-development-under-animal-rule (2015).
Wai, K. M., Leng, T. & Goldberg, J. National Institutes of Health. Putting stem cell-based therapies in context. nih.gov https://www.nih.gov/about-nih/what-we-do/science-health-public-trust/perspectives/putting-stem-cell-based-therapies-context (2022).
US Department of Health and Human Services. Guidance for industry, investigators, and reviewers exploratory IND studies. fda.gov https://www.fda.gov/media/72325/download (2006).
US Food and Drug Administration. CFR - Code of Federal Regulations Title 21. fda.gov https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm (2023).
European Medicines Agency. ICH harmonised tripartite guideline: derivation and characterisation of cell substrates used for production of biotechnological/biological products Q5D. ema.europa.eu https://www.ema.europa.eu/en/ich-q5d-derivation-characterisation-cell-substrates-used-production-biotechnological-biological-products-scientific-guideline (1997).
No authors listed. FDA Report: Guidance for industry: guidance for human somatic cell therapy and gene therapy. Hum. Gene Ther. 9, 1513–1524 (1998).
Federal Register. Medical devices; quality system regulation amendments. federalregister.gov https://www.federalregister.gov/documents/2022/02/23/2022-03227/medical-devices-quality-system-regulation-amendments (2022).
CIRM. CLIN1: Late stage preclinical projects. cirm.ca.gov https://www.cirm.ca.gov/our-funding/research-rfas/late-stage-preclinical-projects/ (2022).
Orbach, M.; CTECH. Biomed company CartiHeal acquired by Smith & Nephew for up to $330 million. calcalistech.com https://www.calcalistech.com/ctechnews/article/b19u54oet (2023).
Kim, J., Park, J., Song, S.-Y. & Kim, E. Advanced therapy medicinal products for autologous chondrocytes and comparison of regulatory systems in target countries. Regen. Ther. 20, 126–137 (2022).
Moon-hee, C. Korea’s drug ministry cancels Kolon Life Sciences’ Invossa license. Business Korea https://www.businesskorea.co.kr/news/articleView.html?idxno=32318 (2019).
MEDIPOST. MEDIPOST clears product approval renewal of CARTISTEM by the Ministry of Food and Drug Safety (MFDS). medi-post.co.kr https://en.medi-post.co.kr/stem-cell-therapeutic/2019/03/36443/ (2019).
J-TEC. What was gained through the re-examination of JACC. jpte.co.jp https://www.jpte.co.jp/en/columns/details/367 (2022).
UMIN. Multicenter parallel group comparative study of autologous cultured cartilage ACC-01 with intra-articular injection treatment using sodium hyaluronate preparation for knee osteoarthritis. https://rctportal-niph-go-jp.translate.goog/s/detail/um?trial_id=UMIN000034801&_x_tr_sl=ja&_x_tr_tl=en&_x_tr_hl=en&_x_tr_pto=sc (2018).
PR Newswire. FDA approves CartiHeal’s implant for the treatment of cartilage and osteochondral defects. prnewswire.com https://www.prnewswire.com/in/news-releases/fda-approves-cartiheal-s-implant-for-the-treatment-of-cartilage-and-osteochondral-defects-833871872.html#:~:Text=KFAR%20SABA%2C%20Israel%2C%20March%2030,its%20Agili%2DC%E2%84%A2%20implant (2022).
Ocugen. Ocugen provides business update with fourth quarter and full year 2022 financial results. ocugen.com https://ir.ocugen.com/news-releases/news-release-details/ocugen-provides-business-update-fourth-quarter-and-full-year-0 (2023).
Arzi, B. et al. Cartilage immunoprivilege depends on donor source and lesion location. Acta Biomater. 23, 72–81 (2015).
Vapniarsky, N. et al. Tissue engineering toward temporomandibular joint disc regeneration. Sci. Transl. Med. 10, eaaq1802 (2018).
Chen, D. et al. Tissue engineered autologous cartilage-bone grafts for temporomandibular joint regeneration. Sci. Transl. Med. 12, eabb6683 (2020).
O’Leary, S. A. et al. Facet joints of the spine: structure–function relationships, problems and treatments, and the potential for regeneration. Annu. Rev. Biomed. Eng. 20, 145–170 (2018).
Nordberg, R. C. et al. Biochemical and biomechanical characterization of the cervical, thoracic, and lumbar facet joint cartilage in the Yucatan minipig. J. Biomech. 142, 111238 (2022).
Murab, S. et al. Tissue engineering strategies for treating avascular necrosis of the femoral head. Bioengineering 8, 200 (2021).
Athanasiou, K. A., Rosenwasser, M. P., Buckwalter, J. A., Malinin, T. I. & Mow, V. C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 9, 330–340 (1991).
Kohn, M. D., Sassoon, A. A. & Fernando, N. D. Classifications in brief: Kellgren–Lawrence classification of osteoarthritis. Clin. Orthop. Relat. Res. 474, 1886–1893 (2016).
Kellgren, J. H. & Lawrence, J. S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16, 494–502 (1957).
Bothe, F. et al. Treatment of focal cartilage defects in minipigs with zonal chondrocyte/mesenchymal progenitor cell constructs. Int. J. Mol. Sci. 20, 653 (2019).
Steele, J. A. M. et al. In vitro and in vivo investigation of a zonal microstructured scaffold for osteochondral defect repair. Biomaterials 286, 121548 (2022).
Kutaish, H. et al. Articular cartilage repair after implantation of hyaline cartilage beads engineered from adult dedifferentiated chondrocytes: cartibeads preclinical efficacy study in a large animal model. Am. J. Sports Med. 51, 237–249 (2023).
Ruvinov, E., Tavor Re’em, T., Witte, F. & Cohen, S. Articular cartilage regeneration using acellular bioactive affinity-binding alginate hydrogel: a 6-month study in a mini-pig model of osteochondral defects. J. Orthop. Transl. 16, 40–52 (2019).
Brown, B. N. et al. Inductive remodeling of extracellular matrix scaffolds in the temporomandibular joint of pigs. Tissue Eng. Part A 28, 447–457 (2022).
Zhang, Y. et al. Human umbilical cord Wharton’s jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthr. Cartil. 26, 954–965 (2018).
Wei, X. et al. Mesenchymal stem cell-loaded porous tantalum integrated with biomimetic 3D collagen-based scaffold to repair large osteochondral defects in goats. Stem Cell Res. Ther. 10, 72 (2019).
Rothrauff, B. B. et al. Point-of-care procedure for enhancement of meniscal healing in a goat model utilizing infrapatellar fat pad-derived stromal vascular fraction cells seeded in photocrosslinkable hydrogel. Am. J. Sports Med. 47, 3396–3405 (2019).
Critchley, S. et al. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 113, 130–143 (2020).
Jia, S. et al. Multilayered scaffold with a compact interfacial layer enhances osteochondral defect repair. ACS Appl. Mater. Interfaces 10, 20296–20305 (2018).
Cunniffe, G. M. et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 188, 63–73 (2019).
Browe, D. C. et al. Promoting endogenous articular cartilage regeneration using extracellular matrix scaffolds. Mater. Today Bio 16, 100343 (2022).
Vukasovic, A. et al. Bioreactor-manufactured cartilage grafts repair acute and chronic osteochondral defects in large animal studies. Cell Prolif. 52, e12653 (2019).
Keller, L. et al. Preclinical safety study of a combined therapeutic bone wound dressing for osteoarticular regeneration. Nat. Commun. 10, 2156 (2019).
Patel, J. M., Ghodbane, S. A., Brzezinski, A., Gatt, C. J. J. & Dunn, M. G. Tissue-engineered total meniscus replacement with a fiber-reinforced scaffold in a 2-year ovine model. Am. J. Sports Med. 46, 1844–1856 (2018).
Yang, Z. et al. Microenvironmentally optimized 3D-printed TGFβ-functionalized scaffolds facilitate endogenous cartilage regeneration in sheep. Acta Biomater. 150, 181–198 (2022).
Mancini, I. A. D. et al. A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: in vivo performance in a long-term equine model. Biofabrication 12, 35028 (2020).
Abe, K. et al. Engraftment of allogeneic iPS cell-derived cartilage organoid in a primate model of articular cartilage defect. Nat. Commun. 14, 804 (2023).
Matsushita, T. et al. A phase I/IIa clinical trial of third-generation autologous chondrocyte implantation (IK-01) for focal cartilage injury of the knee. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 28, 6–12 (2022).
Copp, G., Robb, K. P. & Viswanathan, S. Culture-expanded mesenchymal stromal cell therapy: does it work in knee osteoarthritis? A pathway to clinical success. Cell Mol. Immunol. 20, 626–650 (2023).
Han, J.-Y. Medipost completes first dose of Cartistem in phase 3 clinical trials in Japan. Korea Economic Daily https://www.kedglobal.com/bio-pharma/newsView/ked202301170002 (2023).
Buntz, B. Why medipost is upbeat about its stem cell-based treatment of osteoarthritis. Drug Discovery & Development https://www.drugdiscoverytrends.com/why-medipost-is-upbeat-about-stem-cell-based-treatment-of-osteoarthritis/ (2022).
Medipost. MEDIPOST speeds up its global commercialization of CARTISTEM by requesting official product approval in Malaysia. medi-post.co.kr https://en.medi-post.co.kr/stem-cell-therapeutic/2021/03/36889/ (2021).
Choi, N.-Y. et al. Gel-type autologous chondrocyte (Chondron) implantation for treatment of articular cartilage defects of the knee. BMC Musculoskelet. Disord. 11, 103 (2010).
Kedage, V. V., Sanghavi, S. Y., Badnre, A. & Desai, N. S. Autologous chondrocyte implantation (ACI): an innovative technique for articular cartilage defects. J. Clin. Orthop. Trauma. 1, 33–36 (2010).
J-TEC. Re-examination of autologous cultured cartilage “JACC”. jpte.co.jp https://www.jpte.co.jp/sys/upload/save/63378210962c394272708b.pdf (2022).
Sternberg, C. Vericel shares accelerated launch timeline for MACI arthroscopic program. Orthopedic Design & Technology https://www.odtmag.com/contents/view_breaking-news/2023-01-11/vericel-shares-accelerated-launch-timeline-for-maci-arthroscopic-program/ (2023).
Orthocell. OrthoACI. Autologous chondrocyte implantation. orthocell.com https://orthocell.com/wp-content/uploads/2023/02/OrthoACI-Consumer-Medicines-Information-IFU-0000116.pdf (2023).
European Medicines Agency. Spherox: EPAR product information. ema.europa.eu https://www.ema.europa.eu/en/documents/product-information/spherox-epar-product-information_en.pdf (2017).
Niemeyer, P. et al. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects. Orthop. J. Sports Med. 7, 2325967119854442 (2019).
Hoburg, A. et al. Matrix-associated autologous chondrocyte implantation with spheroid technology is superior to arthroscopic microfracture at 36 months regarding activities of daily living and sporting activities after treatment. Cartilage 13, 437S–448S (2021).
European Medicines Agency. Spherox: summary of the risk management plan. ema.europa.eu https://www.ema.europa.eu/en/documents/rmp-summary/spherox-epar-risk-management-plan-summary_en.pdf (2017).
US Food and Drug Administration. PMA P210034: summary of safety and effectiveness data. fda.gov https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210034B.pdf (2022).
CartiHeal. CartiHeal receives FDA “Breakthrough Device Designation” for the novel Agili-C implant. CartiHeal.com https://www.cartiheal.com/bioventus-makes-15-million-equity-investment-in-cartiheal-with-an-agreed-option-structure-to-acquire-company-upon-milestone-achievements-https-ca-finance-yahoo-com-news-bioventus-makes-15-million-2/ (2020).
US Food and Drug Administration. Premarket approval (PMA). fda.gov https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150017 (2016).
Wang, L. et al. Key considerations on the development of biodegradable biomaterials for clinical translation of medical devices: With cartilage repair products as an example. Bioact. Mater. 9, 332–342 (2022).
Geistlich. Chondro-Gide® articular cartilage cover: FDA approval as a breakthrough device. GeistlichPharma.com https://www.geistlich-pharma.com/about-us/news/news-detail/chondro-gider-articular-cartilage-cover-fda-approval-as-a-breakthrough-device (2021).
Geistlich. AMIC® chondro-gide®. GeistlichPharma.com https://www.geistlich-pharma.com/orthopedic/cartilage-regeneration/amic-chondro-gide (2024).
Anika. Anika completes enrollment in Hyalofast® U.S. pivotal phase III study achieving key milestone. anika.com https://ir.anika.com/2023-05-30-Anika-Completes-Enrollment-in-Hyalofast-R-U-S-Pivotal-Phase-III-Study-Achieving-Key-Milestone (2023).
MiMedx. MIMEDX reports top-line data from two late-stage musculoskeletal trials with proprietary amniotic tissue technology. MiMedx.gcs-web.com https://mimedx.gcs-web.com/news-releases/news-release-details/mimedx-reports-top-line-data-two-late-stage-musculoskeletal (2021).
Clavé, A. et al. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J. Orthop. Res. 34, 658–665 (2016).
Chugai Pharmaceutical Co. Ltd. Chugai and TWOCELLS announce the decision of the termination of license agreement on investigational regenerative cellular medicine for knee chondrogenesis (gMSC®1). chugal-pharm.co.jp https://www.chugai-pharm.co.jp/english/news/detail/20230413150000_979.html (2023).
Shahryari, A. et al. in Comprehensive Pharmacology (ed. Kenakin, T.) 2.16. 326–368 (Elsevier, 2022).
Evans, C. H., Ghivizzani, S. C. & Robbins, P. D. Orthopaedic gene therapy twenty-five years on. JBJS Rev. 9, e20.00220 (2021).
Cherian, J. J. et al. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr. Cartil. 23, 2109–2118 (2015).
Kim, K.-I. et al. Clinical efficacy and safety of the intra-articular injection of autologous adipose-derived mesenchymal stem cells for knee osteoarthritis: a phase III, randomized, double-blind, placebo-controlled trial. Am. J. Sports Med. 51, 2243–2253 (2023).
Han-soo, L.; Korea Biomedical Review. Regulators give final rejection for nature cell’s jointstem. Korea Biomed. Rev. https://www.koreabiomed.com/news/articleView.html?idxno=21443 (2023).
Gomoll, A. H. et al. Safety and efficacy of an amniotic suspension allograft injection over 12 months in a single-blinded, randomized controlled trial for symptomatic osteoarthritis of the knee. Arthroscopy 37, 2246–2257 (2021).
Wolf, M. T. et al. Two-year follow-up and remodeling kinetics of ChonDux hydrogel for full-thickness cartilage defect repair in the knee. Cartilage 11, 447–457 (2020).
Bajuri, M. Y., Sabri, S., Mazli, N., Sarifulnizam, F. A. & Mohd Apandi, H. Osteochondral injury of the talus treated with cell-free hyaluronic acid-based scaffold (Hyalofast®) - a reliable solution. Cureus 13, e17928 (2021).
Anderson, D. E., Gridley, A. & Crawford, D. C. Next generation cartilage repair and the pre-arthroplasty patient. Oper. Tech. Sports Med. 30, 150956 (2022).
Ocugen. Press release. Ocugen announces phase 3 confirmatory clinical trial agreement for Neocart®. occugen.com https://ir.ocugen.com/news-releases/news-release-details/ocugen-announces-phase-3-confirmatory-clinical-trial-agreement (2022).
Acknowledgements
This work was supported by the US National Institutes of Health (NIH) R01 AR067821 (K.A.A.), NIH R01 AR078389 (K.A.A.), NIH TL1 TR001415 (R.C.N.) and NIH F32 DE032896 (B.J.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
R.C.N., B.J.B., T.T. and S.D. researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. R.C.N., B.J.B., J.C.H. and K.A.A reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
K.A.A. and J.C.H. have an equity interest in Cartilage, Inc. Their relationship with Cartilage, Inc. has been reviewed and approved by the University of California, Irvine in accordance with its conflict-of-interest policies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Adetola Adesida, who co-reviewed with Hilda Ma; Elizabeth Vinod; and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ARS Arthro Bioteknology: https://arsarthro.com.tr/en/products/cares/for-the-patients/
Australian Government Department of Health and Aged Care Therapeutic Goods Administration: https://www.tga.gov.au/products/australian-register-therapeutic-goods-artg
clinicaltrials.gov: https://clinicaltrials.gov
International Council for Harmonisation: https://www.ich.org/page/quality-guidelines
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nordberg, R.C., Bielajew, B.J., Takahashi, T. et al. Recent advancements in cartilage tissue engineering innovation and translation. Nat Rev Rheumatol 20, 323–346 (2024). https://doi.org/10.1038/s41584-024-01118-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01118-4