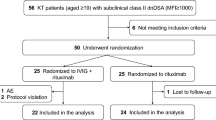
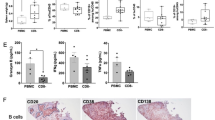
Abstract
Antibody-mediated rejection (AMR), including chronic AMR (cAMR), causes ~50% of kidney allograft losses each year. Despite attempts to develop well-tolerated and effective therapeutics for the management of AMR, to date, none has obtained FDA approval, thereby highlighting an urgent unmet medical need. Discoveries over the past decade from basic, translational and clinical studies of transplant recipients have provided a foundation for developing novel therapeutic approaches to preventing and treating AMR and cAMR. These interventions are aimed at reducing donor-specific antibody levels, decreasing graft injury and fibrosis, and preserving kidney function. Innovative approaches emerging from basic science findings include targeting interactions between alloreactive T cells and B cells, and depleting alloreactive memory B cells, as well as donor-specific antibody-producing plasmablasts and plasma cells. Therapies aimed at reducing the cytotoxic antibody effector functions mediated by natural killer cells and the complement system, and their associated pro-inflammatory cytokines, are also undergoing evaluation. The complexity of the pathogenesis of AMR and cAMR suggest that multiple approaches will probably be required to treat these disease processes effectively. Definitive answers await results from large, double-blind, multicentre, randomized controlled clinical trials.
Key points
-
Understanding the expression pattern of surface antigens — including CD19, CD20 and CD38 — on B cell subsets can guide strategies for using monoclonal antibodies to deplete relevant effector B cell subsets.
-
Enhanced understanding of the pleiotropic effects of IL-6–IL-6 receptor signalling in antibody-mediated rejection has led to early trials targeting IL-6R (tocilizumab) or IL-6 (clazakizumab) as potential effective therapies.
-
Plasma cell depletion strategies used in the treatment of multiple myeloma, including proteosome inhibition and an anti-CD38 monoclonal antibody (daratumumab)-mediated depletion of plasma cells, are being tested in kidney transplant recipients with chronic antibody-mediated rejection.
-
New approaches to targeting antibody effector functions with C1 esterase inhibitor (blocks classical and mannose-binding lectin complement pathways), eculizumab (inhibits terminal complement and membrane-attack complex (MAC) formation), imlifidase (cleaves antibody Fc region) and neonatal Fc receptor monoclonal antibodies (prevent antibody recycling and shorten antibody half-lives) show promise for preventing or treating antibody-mediated rejection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bohmig, G. A., Halloran, P. F. & Feucht, H. E. On a long and winding road: alloantibodies in organ transplantation. Transplantation 107, 1027–1041 (2023).
Patel, R. & Terasaki, P. I. Significance of the positive crossmatch test in kidney transplantation. N. Engl. J. Med. 280, 735–739 (1969).
Mickey, M. R., Singal, D. P. & Terasaki, P. I. Serotyping for homotransplantation. XXV. Evidence for three HL-A subloci. Transpl. Proc. 1, 347–351 (1969).
Bray, R. A., Nickerson, P. W., Kerman, R. H. & Gebel, H. M. Evolution of HLA antibody detection: technology emulating biology. Immunol. Res. 29, 41–54 (2004).
Schinstock, C. A. et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 expert consensus from the transplantion society working group. Transplantation 104, 911–922 (2020).
Loupy, A., Hill, G. S. & Jordan, S. C. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat. Rev. Nephrol. 8, 348–357 (2012).
Nandiwada, S. L. Overview of human B-cell development and antibody deficiencies. J. Immunol. Methods 519, 113485 (2023).
Legler, D. F. et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187, 655–660 (1998).
Hobeika, E., Nielsen, P. J. & Medgyesi, D. Signaling mechanisms regulating B-lymphocyte activation and tolerance. J. Mol. Med. 93, 143–158 (2015).
Bruton, O. C. Agammaglobulinemia. Pediatrics 9, 722–728 (1952).
Rawlings, D. J. et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science 261, 358–361 (1993).
Smith, F. L. & Baumgarth, N. B-1 cell responses to infections. Curr. Opin. Immunol. 57, 23–31 (2019).
Somoza, C. & Lanier, L. L. T-cell costimulation via CD28-CD80/CD86 and CD40-CD40 ligand interactions. Res. Immunol. 146, 171–176 (1995).
Feng, Y., Seija, N., Di Noia, J. M. & Martin, A. AID in antibody diversification: there and back again. Trends Immunol. 41, 586–600 (2020).
Stavnezer, J. Antibody class switching. Adv. Immunol. 61, 79–146 (1996).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 40, 413–442 (2022).
Choi, Y. S., Eto, D., Yang, J. A., Lao, C. & Crotty, S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190, 3049–3053 (2013).
Raybuck, A. L. et al. B cell-intrinsic mTORC1 promotes germinal center-defining transcription factor gene expression, somatic hypermutation, and memory B cell generation in humoral immunity. J. Immunol. 200, 2627–2639 (2018).
Weisel, F. & Shlomchik, M. Memory B cells of mice and humans. Annu. Rev. Immunol. 35, 255–284 (2017).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171, 1684–1690 (2003).
Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 (2007).
Basler, M., Li, J. & Groettrup, M. On the role of the immunoproteasome in transplant rejection. Immunogenetics 71, 263–271 (2019).
Anolik, J. H., Looney, R. J., Lund, F. E., Randall, T. D. & Sanz, I. Insights into the heterogeneity of human B cells: diverse functions, roles in autoimmunity, and use as therapeutic targets. Immunol. Res. 45, 144–158 (2009).
Crotty, S. et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171, 4969–4973 (2003).
Chen, M. et al. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat. Med. 20, 503–510 (2014).
Fribourg, M. et al. Allospecific memory B cell responses are dependent on autophagy. Am. J. Transpl. 18, 102–112 (2018).
Goenka, R., Scholz, J. L., Sindhava, V. J. & Cancro, M. P. New roles for the BLyS/BAFF family in antigen-experienced B cell niches. Cytokine Growth Factor. Rev. 25, 107–113 (2014).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Chavele, K. M., Merry, E. & Ehrenstein, M. R. Cutting edge: circulating plasmablasts induce the differentiation of human T follicular helper cells via IL-6 production. J. Immunol. 194, 2482–2485 (2015).
Papillion, A. et al. Inhibition of IL-2 responsiveness by IL-6 is required for the generation of GC-TFH cells. Sci. Immunol. 4, eaaw7636 (2019).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug. Discov. 17, 395–412 (2018).
Jordan, S. C. et al. Interleukin-6: an important mediator of allograft injury. Transplantation 104, 2497–2506 (2020).
Jordan, S. C., Ammerman, N., Huang, E. & Vo, A. Importance of IL-6 inhibition in prevention and treatment of antibody-mediated rejection in kidney allografts. Am. J. Transpl. 22, 28–37 (2022).
Liu, X., Jones, G. W., Choy, E. H. & Jones, S. A. The biology behind interleukin-6 targeted interventions. Curr. Opin. Rheumatol. 28, 152–160 (2016).
Xie, C. B. et al. Complement-activated interferon-γ-primed human endothelium transpresents interleukin-15 to CD8+ T cells. J. Clin. Invest. 130, 3437–3452 (2020).
Ekdahl, K. N. et al. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol. Rev. 274, 245–269 (2016).
Cravedi, P. & Heeger, P. S. Complement as a multifaceted modulator of kidney transplant injury. J. Clin. Invest. 124, 2348–2354 (2014).
Carroll, M. C. & Isenman, D. E. Regulation of humoral immunity by complement. Immunity 37, 199–207 (2012).
Brooks, D. & Ravetch, J. V. Fc receptor signaling. Adv. Exp. Med. Biol. 365, 185–195 (1994).
Barb, A. W. Fc γ receptor compositional heterogeneity: considerations for immunotherapy development. J. Biol. Chem. 296, 100057 (2021).
Anania, J. C., Chenoweth, A. M., Wines, B. D. & Hogarth, P. M. The human FcγRII (CD32) family of leukocyte FcR in health and disease. Front. Immunol. 10, 464 (2019).
Jordan, S. C., Vo, A. A., Peng, A., Toyoda, M. & Tyan, D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am. J. Transpl. 6, 459–466 (2006).
Smith, K. G. & Clatworthy, M. R. FcγRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat. Rev. Immunol. 10, 328–343 (2010).
Baldwin, W. M. III, Valujskikh, A. & Fairchild, R. L. The neonatal Fc receptor: key to homeostasic control of IgG and IgG-related biopharmaceuticals. Am. J. Transpl. 19, 1881–1887 (2019).
Dragun, D. et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N. Engl. J. Med. 352, 558–569 (2005).
Reinsmoen, N. L. et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation 90, 1473–1477 (2010).
Dinavahi, R. et al. Antibodies reactive to non-HLA antigens in transplant glomerulopathy. J. Am. Soc. Nephrol. 22, 1168–1178 (2011).
Jackson, A. M. et al. Endothelial cell antibodies associated with novel targets and increased rejection. J. Am. Soc. Nephrol. 26, 1161–1171 (2015).
Li, L. et al. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc. Natl Acad. Sci. USA 106, 4148–4153 (2009).
Reindl-Schwaighofer, R. et al. Contribution of non-HLA incompatibility between donor and recipient to kidney allograft survival: genome-wide analysis in a prospective cohort. Lancet 393, 910–917 (2019).
Jordan, S. C. et al. Spontaneous anti-tubular-basement-membrane antibody production by lymphocytes isolated from a rejected allograft. Transplantation 41, 173–176 (1986).
Zorn, E. & See, S. B. Is there a role for natural antibodies in rejection following transplantation? Transplantation 103, 1612–1619 (2019).
Asano, Y. et al. Innate-like self-reactive B cells infiltrate human renal allografts during transplant rejection. Nat. Commun. 12, 4372 (2021).
Cumpelik, A. et al. Cutting edge: neutrophil complement receptor signaling is required for BAFF-dependent humoral responses in mice. J. Immunol. 210, 19–23 (2023).
Coca, A. & Sanz, I. Updates on B-cell immunotherapies for systemic lupus erythematosus and Sjogren’s syndrome. Curr. Opin. Rheumatol. 24, 451–456 (2012).
Kim, I., Wu, G., Chai, N. N., Klein, A. S. & Jordan, S. Ibrutinib suppresses alloantibody responses in a mouse model of allosensitization. Transpl. Immunol. 45, 59–64 (2017).
Cumpelik, A. et al. Dynamic regulation of B cell complement signaling is integral to germinal center responses. Nat. Immunol. 22, 757–768 (2021).
Leibler, C. et al. Control of humoral response in renal transplantation by belatacept depends on a direct effect on B cells and impaired T follicular helper-B cell crosstalk. J. Am. Soc. Nephrol. 29, 1049–1062 (2018).
Chen, J. et al. Cutting edge: CTLA-4Ig inhibits memory B cell responses and promotes allograft survival in sensitized recipients. J. Immunol. 195, 4069–4073 (2015).
Kim, I., Wu, G., Chai, N. N., Klein, A. S. & Jordan, S. C. Immunological characterization of de novo and recall alloantibody suppression by CTLA4Ig in a mouse model of allosensitization. Transpl. Immunol. 38, 84–92 (2016).
Leibler, C. et al. Belatacept in renal transplant recipient with mild immunologic risk factor: a pilot prospective study (BELACOR). Am. J. Transpl. 19, 894–906 (2019).
Vincenti, F. et al. Belatacept and long-term outcomes in kidney transplantation. N. Engl. J. Med. 374, 333–343 (2016).
Cerwenka, A. & Lanier, L. L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 16, 112–123 (2016).
Negrin, R. S. Immune regulation in hematopoietic cell transplantation. Bone Marrow Transpl. 54, 765–768 (2019).
Callemeyn, J. et al. Missing self-induced microvascular rejection of kidney allografts: a population-based study. J. Am. Soc. Nephrol. 32, 2070–2082 (2021).
Hidalgo, L. G. et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am. J. Transpl. 10, 1812–1822 (2010).
Koenig, A. et al. Missing self triggers NK cell-mediated chronic vascular rejection of solid organ transplants. Nat. Commun. 10, 5350 (2019).
Koenig, A. et al. Missing self-induced activation of NK cells combines with non-complement-fixing donor-specific antibodies to accelerate kidney transplant loss in chronic antibody-mediated rejection. J. Am. Soc. Nephrol. 32, 479–494 (2021).
van Bergen, J. et al. KIR-ligand mismatches are associated with reduced long-term graft survival in HLA-compatible kidney transplantation. Am. J. Transpl. 11, 1959–1964 (2011).
Yazdani, S. et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int. 95, 188–198 (2019).
Yokoyama, W. M. & Plougastel, B. F. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 3, 304–316 (2003).
Horowitz, A. et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 5, 208ra145 (2013).
Kohei, N. et al. Natural killer cells play a critical role in mediating inflammation and graft failure during antibody-mediated rejection of kidney allografts. Kidney Int. 89, 1293–1306 (2016).
Yagisawa, T. et al. In the absence of natural killer cell activation donor-specific antibody mediates chronic, but not acute, kidney allograft rejection. Kidney Int. 95, 350–362 (2019).
Halloran, P. F. et al. The molecular phenotype of kidney transplants. Am. J. Transpl. 10, 2215–2222 (2010).
Hidalgo, L. G. et al. Interpreting NK cell transcripts versus T cell transcripts in renal transplant biopsies. Am. J. Transpl. 12, 1180–1191 (2012).
Fogal, B. et al. Neutralizing IL-6 reduces human arterial allograft rejection by allowing emergence of CD161+ CD4+ regulatory T cells. J. Immunol. 187, 6268–6280 (2011).
Liu, L. et al. Endothelial cell-derived interleukin-18 released during ischemia reperfusion injury selectively expands T peripheral helper cells to promote alloantibody production. Circulation 141, 464–478 (2020).
Xie, C. B., Zhou, J., Mackay, S. & Pober, J. S. Complement-activated human endothelial cells stimulate increased polyfunctionality in alloreactive T cells. Am. J. Transpl. 21, 1902–1909 (2021).
Basler, M. & Groettrup, M. On the role of the immunoproteasome in protein homeostasis. Cells 10, 3216 (2021).
Hainz, N. et al. The proteasome inhibitor bortezomib prevents lupus nephritis in the NZB/W F1 mouse model by preservation of glomerular and tubulointerstitial architecture. Nephron Exp. Nephrol. 120, e47–58, (2012).
Ejaz, N. S. et al. Review of bortezomib treatment of antibody-mediated rejection in renal transplantation. Antioxid. Redox Signal. 21, 2401–2418 (2014).
Ezekian, B. et al. Pretransplant desensitization with costimulation blockade and proteasome inhibitor reduces DSA and delays antibody-mediated rejection in highly sensitized nonhuman primate kidney transplant recipients. J. Am. Soc. Nephrol. 30, 2399–2411 (2019).
Li, J. et al. Immunoproteasome inhibition induces plasma cell apoptosis and preserves kidney allografts by activating the unfolded protein response and suppressing plasma cell survival factors. Kidney Int. 95, 611–623 (2019).
Kwun, J. et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J. Am. Soc. Nephrol. 28, 1991–1996 (2017).
Burghuber, C. K. et al. Dual targeting: combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am. J. Transpl. 19, 724–736 (2019).
Siedlecki, A., Irish, W. & Brennan, D. C. Delayed graft function in the kidney transplant. Am. J. Transpl. 11, 2279–2296 (2011).
Mannon, R. B. Delayed graft function: the AKI of kidney transplantation. Nephron 140, 94–98 (2018).
Budhiraja, P. et al. Duration of delayed graft function and its impact on graft outcomes in deceased donor kidney transplantation. BMC Nephrol. 23, 154 (2022).
Gill, J., Dong, J., Rose, C. & Gill, J. S. The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function. Kidney Int. 89, 1331–1336 (2016).
Lim, W. H., Johnson, D. W., Teixeira-Pinto, A. & Wong, G. Association between duration of delayed graft function, acute rejection, and allograft outcome after deceased donor kidney transplantation. Transplantation 103, 412–419 (2019).
Cippa, P. E. et al. Transcriptional trajectories of human kidney injury progression. JCI Insight 3, e123151 (2018).
Chun, N., Horwitz, J. & Heeger, P. S. Role of complement activation in allograft inflammation. Curr. Transpl. Rep. 6, 52–59 (2019).
Loupy, A. et al. The Banff 2019 kidney meeting report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transpl. 20, 2318–2331 (2020).
Allen, N. H. et al. Plasma exchange in acute renal allograft rejection. A controlled trial. Transplantation 35, 425–428 (1983).
Bonomini, V., Vangelista, A., Frasca, G. M., Di Felice, A. & Liviano D’Arcangelo, G. Effects of plasmapheresis in renal transplant rejection. A controlled study. Trans. Am. Soc. Artif. Intern. Organs 31, 698–703 (1985).
Kirubakaran, M. G., Disney, A. P., Norman, J., Pugsley, D. J. & Mathew, T. H. A controlled trial of plasmapheresis in the treatment of renal allograft rejection. Transplantation 32, 164–165, (1981).
Bohmig, G. A. et al. Immunoadsorption in severe C4d-positive acute kidney allograft rejection: a randomized controlled trial. Am. J. Transpl. 7, 117–121 (2007).
Bartel, G. et al. Peritransplant immunoadsorption for positive crossmatch deceased donor kidney transplantation. Am. J. Transpl. 10, 2033–2042 (2010).
Perez, E. E. et al. Update on the use of immunoglobulin in human disease: a review of evidence. J. Allergy Clin. Immunol. 139, S1–S46 (2017).
Jordan, S. C. et al. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation 66, 800–805 (1998).
Lefaucheur, C. et al. Complement-activating anti-HLA antibodies in kidney transplantation: allograft gene expression profiling and response to treatment. J. Am. Soc. Nephrol. 29, 620–635 (2018).
Luke, P. P. et al. IVIG rescue therapy in renal transplantation. Transpl. Proc. 33, 1093–1094 (2001).
Vo, A. A. et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N. Engl. J. Med. 359, 242–251 (2008).
Sautenet, B. et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation 100, 391–399 (2016).
Bailly, E. et al. An extension of the RITUX-ERAH study, multicenter randomized clinical trial comparing rituximab to placebo in acute antibody-mediated rejection after renal transplantation. Transpl. Int. 33, 786–795 (2020).
Lara, S., Heilig, J., Virtanen, A. & Kleinau, S. Exploring complement-dependent cytotoxicity by rituximab isotypes in 2D and 3D-cultured B-cell lymphoma. BMC Cancer 22, 678 (2022).
Kamburova, E. G. et al. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am. J. Transpl. 13, 1503–1511 (2013).
Looney, C. M. et al. Obinutuzumab effectively depletes key B-cell subsets in blood and tissue in end-stage renal disease patients. Transpl. Direct 9, e1436 (2023).
Arnold, J. et al. Efficacy and safety of obinutuzumab in systemic lupus erythematosus patients with secondary non-response to rituximab. Rheumatology 61, 4905–4909 (2022).
Banham, G. D. et al. Belimumab in kidney transplantation: an experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet 391, 2619–2630 (2018).
Agarwal, D. et al. BLyS neutralization results in selective anti-HLA alloantibody depletion without successful desensitization. Transpl. Immunol. 69, 101465 (2021).
Eskandary, F. et al. A randomized trial of bortezomib in late antibody-mediated kidney transplant rejection. J. Am. Soc. Nephrol. 29, 591–605 (2018).
Ensor, C. et al. Proteasome inhibitor carfilzomib-based therapy for antibody-mediated rejection of the pulmonary allograft: use and short-term findings. Am. J. Transplant. 17, 1380–1388 (2017).
Moreno Gonzales, M. A. et al. 32 doses of bortezomib for desensitization is not well tolerated and is associated with only modest reductions in anti-HLA antibody. Transplantation 101, 1222–1227 (2017).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT05017545 (2023).
Deaglio, S., Mehta, K. & Malavasi, F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk. Res. 25, 1–12 (2001).
Krejcik, J. et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128, 384–394 (2016).
Hill, E., Morrison, C. & Kazandjian, D. Daratumumab: a review of current indications and future directions. Semin. Oncol. 49, 48–59 (2022).
Doberer, K. et al. CD38 antibody daratumumab for the treatment of chronic active antibody-mediated kidney allograft rejection. Transplantation 105, 451–457 (2021).
Scalzo, R. E. et al. Daratumumab use prior to kidney transplant and T cell-mediated rejection: a case report. Am. J. Kidney Dis. 81, 616–620 (2023).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04827979 (2023).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04294459 (2023).
Mayer, K. A. et al. Safety, tolerability, and efficacy of monoclonal CD38 antibody felzartamab in late antibody-mediated renal allograft rejection: study protocol for a phase 2 trial. Trials 23, 270 (2022).
Vo, A. et al. Daratumumab (anti-cd38) for desensitization of treatment-resistant highly-sensitized ESRD patients. Am. J. Transpl. 23, S376 (2023).
van de Donk, N. & Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 402, 142–158 (2023).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT05092347 (2023).
Mitra, A. et al. From bench to bedside: the history and progress of CAR T cell therapy. Front. Immunol. 14, 1188049 (2023).
Du, Z., Zhu, S., Zhang, X., Gong, Z. & Wang, S. Non-conventional allogeneic anti-BCMA chimeric antigen receptor-based immune cell therapies for multiple myeloma treatment. Cancers 15, 567 (2023).
Hill, J. A. et al. Anti-HLA antibodies in recipients of CD19 versus BCMA-targeted CAR T-cell therapy. Am. J. Transpl. 23, 416–422 (2023).
Choi, J. et al. Assessment of tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am. J. Transpl. 17, 2381–2389 (2017).
Irish, W. et al. Change in estimated GFR and risk of allograft failure in patients diagnosed with late active antibody-mediated rejection following kidney transplantation. Transplantation 105, 648–659 (2021).
Cabezas, L. et al. Tocilizumab and active antibody-mediated rejection in kidney transplantation: a literature review. Front. Immunol. 13, 839380 (2022).
Pottebaum, A. A. et al. Efficacy and safety of tocilizumab in the treatment of acute active antibody-mediated rejection in kidney transplant recipients. Transpl. Direct 6, e543 (2020).
Jouve, T. et al. Immune responses following tocilizumab therapy to desensitize HLA-sensitized kidney transplant candidates. Am. J. Transpl. 22, 71–84 (2022).
Jordan, S. C. et al. Evaluation of clazakizumab (Anti-Interleukin-6) in patients with treatment-resistant chronic active antibody-mediated rejection of kidney allografts. Kidney Int. Rep. 7, 720–731 (2022).
Doberer, K. et al. A randomized clinical trial of anti-IL-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection. J. Am. Soc. Nephrol. 32, 708–722 (2021).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03744910 (2023).
Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. A guide to complement biology, pathology and therapeutic opportunity. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-023-00926-1 (2023).
Cornell, L. D., Schinstock, C. A., Gandhi, M. J., Kremers, W. K. & Stegall, M. D. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am. J. Transpl. 15, 1293–1302 (2015).
Wiebe, C. et al. Evaluation of C1q status and titer of de novo donor-specific antibodies as predictors of allograft survival. Am. J. Transpl. 17, 703–711 (2017).
Roth, A. et al. Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies. Blood Adv. 2, 2176–2185 (2018).
Sheridan, D. et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS One 13, e0195909 (2018).
Huang, E. et al. Three-year outcomes of a randomized, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 15, 109–116 (2020).
Jordan, S. C. et al. A phase I/II, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Am. J. Transpl. 18, 2955–2964 (2018).
Eerhart, M. J. et al. Complement blockade in recipients prevents delayed graft function and delays antibody-mediated rejection in a nonhuman primate model of kidney transplantation. Transplantation 106, 60–71 (2022).
Chun, N. et al. Complement dependence of murine costimulatory blockade-resistanT cellular cardiac allograft rejection. Am. J. Transpl. 17, 2810–2819 (2017).
Vo, A. A. et al. A phase I/II placebo-controlled trial of C1-inhibitor for prevention of antibody-mediated rejection in HLA sensitized patients. Transplantation 99, 299–308 (2015).
Montgomery, R. A. et al. Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: results of a randomized double-blind placebo-controlled pilot study. Am. J. Transpl. 16, 3468–3478 (2016).
Wahrmann, M. et al. Effect of the anti-C1s humanized antibody TNT009 and its parental mouse variant TNT003 on HLA antibody-induced complement activation-a preclinical in vitro study. Am. J. Transpl. 17, 2300–2311 (2017).
Eskandary, F. et al. Anti-C1s monoclonal antibody BIVV009 in late antibody-mediated kidney allograft rejection-results from a first-in-patient phase 1 trial. Am. J. Transpl. 18, 916–926 (2018).
Huang, E., Maldonado, A. Q., Kjellman, C. & Jordan, S. C. Imlifidase for the treatment of anti-HLA antibody-mediated processes in kidney transplantation. Am. J. Transpl. 22, 691–697 (2022).
Jordan, S. C. et al. Imlifidase desensitization in crossmatch-positive, highly sensitized kidney transplant recipients: results of an international phase 2 trial (Highdes). Transplantation 105, 1808–1817 (2021).
Jordan, S. C., Lorant, T. & Choi, J. IgG endopeptidase in highly sensitized patients undergoing transplantation. N. Engl. J. Med. 377, 1693–1694 (2017).
Al-Salama, Z. T. Imlifidase: first approval. Drugs 80, 1859–1864 (2020).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT05369975 (2023).
Lorant, T. et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti-HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am. J. Transpl. 18, 2752–2762 (2018).
Howard, J. F. Jr et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 92, e2661–e2673 (2019).
Wiebe, C. et al. HLA-DR/DQ molecular mismatch: a prognostic biomarker for primary alloimmunity. Am. J. Transpl. 19, 1708–1719 (2019).
Davis, S. et al. Tacrolimus intrapatient variability, time in therapeutic range, and risk of de novo donor-specific antibodies. Transplantation 104, 881–887 (2020).
Davis, S. et al. Adequate tacrolimus exposure modulates the impact of HLA class II molecular mismatch: a validation study in an American cohort. Am. J. Transpl. 21, 322–328 (2021).
Wiebe, C. et al. Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J. Am. Soc. Nephrol. 28, 3353–3362 (2017).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT05917522 (2023).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03644667 (2023).
Acknowledgements
The authors acknowledge and thank all the kidney allograft recipients and their families who have participated in clinical trials aimed at eliminating antibody rejection and improving outcomes. We also want to acknowledge the team members of the Kidney Transplant Program, Transplant Immunotherapy Program, Transplant Immunology Laboratory, and HLA Laboratory at Cedars-Sinai Medical Center for their commitment to improving lives through transplantation. P.S.H. is supported by R01AI141434 and M.C.H. is supported by T32 AI078892.
Author information
Authors and Affiliations
Contributions
All authors researched data and wrote the article. S.J. and P.S.H. made substantial contributions to discussions of the content, and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.J. has grants and consulting fees from Hansa Biopharma, CSL-Behring, Regeneron, Sana Biotechnology and Argenx. P.S.H. and M.C.H. declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks G. Böhmig, T. Jouve and J. Kwun for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antibody recycling
-
A cellular process in which antibodies bound to FcRn are endocytosed and then released back into the circulation without undergoing degradation.
- Idiotypic
-
An idiotype is the distinctive amino acid sequence within the variable region that makes any immunoglobulin (or T cell receptor) unique. Anti-idiotypic antibodies are specifically reactive to these idiotypes.
- Somatic hypermutation
-
A process within B cells in which point mutations accumulate in the V regions of antibody light and heavy chains to enhance antibody affinity for a given antigen.
- Transitional B cells
-
Immature B cells that have recently left the bone marrow.
- Type 1 anti-CD20
-
Examples include rituximab and ofatumumab. These antibodies cause redistribution of CD20 into lipid rafts within the plasma membrane, which can enhance their effector mechanisms, but are more susceptible to internalization and proteolytic degradation than type 2 anti-CD20 monoclonal antibodies.
- Type 2 anti-CD20
-
Obinutuzumab. Compared with the type 1 counterparts, this antibody shows enhanced binding to FcγRIII and enhanced antibody-dependent cytotoxicity function, as well as reduced internalization, which might enhance its efficacy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heeger, P.S., Haro, M.C. & Jordan, S. Translating B cell immunology to the treatment of antibody-mediated allograft rejection. Nat Rev Nephrol 20, 218–232 (2024). https://doi.org/10.1038/s41581-023-00791-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00791-0