Abstract
Sequential expression of claudins, a family of tight junction proteins, along the nephron mirrors the sequential expression of ion channels and transporters. Only by the interplay of transcellular and paracellular transport can the kidney efficiently maintain electrolyte and water homeostasis in an organism. Although channel and transporter defects have long been known to perturb homeostasis, the contribution of individual tight junction proteins has been less clear. Over the past two decades, the regulation and dysregulation of claudins have been intensively studied in the gastrointestinal tract. Claudin expression patterns have, for instance, been found to be affected in infection and inflammation, or in cancer. In the kidney, a deeper understanding of the causes as well as the effects of claudin expression alterations is only just emerging. Little is known about hormonal control of the paracellular pathway along the nephron, effects of cytokines on renal claudin expression or relevance of changes in paracellular permeability to the outcome in any of the major kidney diseases. By summarizing current findings on the role of specific claudins in maintaining electrolyte and water homeostasis, this Review aims to stimulate investigations on claudins as prognostic markers or as druggable targets in kidney disease.
Key points
-
Claudins are a family of transmembane proteins that assemble into tight junction strands and thus restrict the paracellular pathway.
-
Whereas some claudins exclusively form paracellular barriers, others confer ion selectivity with or without water permeability by forming paracellular channels.
-
Each nephron segment has its own specific claudin expression pattern that complements the specific set of transcellular channels and transporters.
-
Mutations in several claudins (claudin-2, claudin-10, claudin-16 and claudin-19) cause human hereditary diseases that affect kidney function and compromise calcium homeostasis. Common genetic variants in the claudin-2 and claudin-14 genes are risk factors for nephrolithiasis.
-
Factors (such as hormones or cytokines) and processes (transcriptional, post-translational) that regulate renal claudin expression, as well as the role of claudin dysregulation in kidney disease, are only just emerging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Farquhar, M. G. & Palade, G. E. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963).
Staehelin, L. A., Mukherjee, T. M. & Williams, A. W. Fine structure of frozen-etched tight junctions. Naturwissenschaften 56, 142 (1969).
Claude, P. & Goodenough, D. A. Fracture faces of zonulae occludentes from ‘tight’ and ‘leaky’ epithelia. J. Cell Biol. 58, 390–400 (1973).
Kühn, K. & Reale, E. Junctional complexes of the tubular cells in the human kidney as revealed with freeze-fracture. Cell Tissue Res. 160, 193–205 (1975).
Taugner, R., Boll, U., Zahn, P. & Forssmann, W. G. Cell junctions in the epithelium of Bowman’s capsule. Cell Tissue Res. 172, 431–446 (1976).
Claude, P. Morphological factors influencing transepithelial permeability: a model for the resistance of the zonula occludens. J. Membr. Biol. 39, 219–232 (1978).
Roesinger, B., Schiller, A. & Taugner, R. A freeze-fracture study of tight junctions in the pars convoluta and pars recta of the renal proximal tubule. Cell Tissue Res. 186, 121–133 (1978).
Schiller, A. & Taugner, R. Are there specialized junctions in the pars maculata of the distal tubule? Cell Tissue Res. 200, 337–344 (1979).
Schiller, A. & Taugner, R. Junctions between interstitial cells of the renal medulla: a freeze-fracture study. Cell Tissue Res. 203, 231–240 (1979).
Schiller, A., Taugner, R. & Kriz, W. The thin limbs of Henle’s loop in the rabbit. A freeze fracture study. Cell Tissue Res. 207, 249–265 (1980).
Schiller, A., Forssmann, W. G. & Taugner, R. The tight junctions of renal tubules in the cortex and outer medulla. A quantitative study of the kidneys of six species. Cell Tissue Res. 212, 395–413 (1980).
Schiller, A. & Taugner, R. Heterogeneity of tight junctions along the collecting duct in the renal medulla. A freeze-fracture study in rat and rabbit. Cell Tissue Res. 223, 603–614 (1982).
Kottra, G. & Fromter, E. Functional properties of the paracellular pathway in some leaky epithelia. J. Exp. Biol. 106, 217–229 (1983).
Muto, S. Physiological roles of claudins in kidney tubule paracellular transport. Am. J. Physiol. Ren. Physiol. 312, F9–F24 (2017).
Boulpaep, E. L. & Seely, J. F. Electrophysiology of proximal and distal tubules in the autoperfused dog kidney. Am. J. Physiol. 221, 1084–1096 (1971).
Greger, R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflug. Arch. 390, 30–37 (1981).
Schneeberger, E. E. & Lynch, R. D. Structure, function, and regulation of cellular tight junctions. Am. J. Physiol. 262, L647–L661 (1992).
Furuse, M., Fujita, K., Hiiragi, T., Fujimoto, K. & Tsukita, S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141, 1539–1550 (1998).
Simon, D. B. et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285, 103–106 (1999).
Günzel, D. & Yu, A. S. L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 93, 525–569 (2013).
Suzuki, H. et al. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 344, 304–307 (2014).
Li, J., Angelow, S., Linge, A., Zhuo, M. & Yu, A. S. L. Claudin-2 pore function requires an intramolecular disulfide bond between two conserved extracellular cysteines. Am. J. Physiol. Cell Physiol. 305, C190–C196 (2013).
Suzuki, H., Tani, K., Tamura, A., Tsukita, S. & Fujiyoshi, Y. Model for the architecture of claudin-based paracellular ion channels through tight junctions. J. Mol. Biol. 427, 291–297 (2015).
Heinemann, U. & Schuetz, A. Structural features of tight-junction proteins. Int. J. Mol. Sci. 20, 6020 (2019).
Müller, D. et al. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am. J. Hum. Genet. 73, 1293–1301 (2003).
Alzahrani, A. S. et al. A novel claudin-10 mutation with a unique mechanism in two unrelated families with HELIX syndrome. Kidney Int. 100, 415–429 (2021).
Ikari, A. et al. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J. Cell Sci. 119, 1781–1789 (2006).
D’Souza, T., Indig, F. E. & Morin, P. J. Phosphorylation of claudin-4 by PKCepsilon regulates tight junction barrier function in ovarian cancer cells. Exp. Cell Res. 313, 3364–3375 (2007).
Aono, S. & Hirai, Y. Phosphorylation of claudin-4 is required for tight junction formation in a human keratinocyte cell line. Exp. Cell Res. 314, 3326–3339 (2008).
Ikari, A. et al. Claudin-16 is directly phosphorylated by protein kinase A independently of a vasodilator-stimulated phosphoprotein-mediated pathway. J. Cell Physiol. 214, 221–229 (2008).
Van Itallie, C. M., Gambling, T. M., Carson, J. L. & Anderson, J. M. Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci. 118, 1427–1436 (2005).
Heiler, S., Mu, W., Zöller, M. & Thuma, F. The importance of claudin-7 palmitoylation on membrane subdomain localization and metastasis-promoting activities. Cell Commun. Signal. 13, 29 (2015).
Rajagopal, N., Irudayanathan, F. J. & Nangia, S. Palmitoylation of claudin-5 proteins influences their lipid domain affinity and tight junction assembly at the blood-brain barrier interface. J. Phys. Chem. B 123, 983–993 (2019).
Hempel, C. et al. Assembly of tight junction strands: claudin-10b and claudin-3 form homo-tetrameric building blocks that polymerise in a channel-independent manner. J. Mol. Biol. 432, 2405–2427 (2020).
Kiuchi-Saishin, Y. et al. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J. Am. Soc. Nephrol. 13, 875–886 (2002).
Reyes, J. L. et al. The renal segmental distribution of claudins changes with development. Kidney Int. 62, 476–487 (2002).
Chen, L., Chou, C.-L. & Knepper, M. A. A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J. Am. Soc. Nephrol. 32, 897–912 (2021).
Limbutara, K., Chou, C. L. & Knepper, M. A. Quantitative proteomics of all 14 renal tubule segments in rat. J. Am. Soc. Nephrol. 31, 1255–1266 (2020).
Ransick, A. et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev. Cell 51, 399–413.e7 (2019).
Hinze, C. et al. Single-cell transcriptomics reveals common epithelial response patterns in human acute kidney injury. Genome Med. 14, 103 (2022).
Wu, H. et al. Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J. Am. Soc. Nephrol. 29, 2069–2080 (2018).
Wu, H. et al. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23, 869–881.e8 (2018).
Wu, H., Kirita, Y., Donnelly, E. L. & Humphreys, B. D. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 30, 23–32 (2019).
Lee, J. W., Chou, C. L. & Knepper, M. A. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J. Am. Soc. Nephrol. 26, 2669–2677 (2015).
Tesch, F. et al. Super-resolved local recruitment of CLDN5 to filtration slits implicates a direct relationship with podocyte foot process effacement. J. Cell Mol. Med. 25, 7631–7641 (2021).
Dumas, S. J. et al. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J. Am. Soc. Nephrol. 31, 118–138 (2020).
Pei, L. et al. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J. Clin. Invest. 126, 2509–2518 (2016).
Beggs, M. R. et al. Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. Proc. Natl Acad. Sci. USA 118, e2111247118 (2021).
Breiderhoff, T. et al. Claudin-10a deficiency shifts proximal tubular Cl− permeability to cation selectivity via claudin-2 redistribution. J. Am. Soc. Nephrol. 33, 699–717 (2022).
Gonschior, H. et al. Nanoscale segregation of channel and barrier claudins enables paracellular ion flux. Nat. Commun. 13, 4985 (2022).
Sonntag, S. R. et al. Diuretic state affects ascending thin limb tight junctions. Am. J. Physiol. Ren. Physiol. 314, F190–F195 (2018).
Breiderhoff, T. et al. Deletion of claudin-10 rescues claudin-16–deficient mice from hypomagnesemia and hypercalciuria. Kidney Int. 93, 580–588 (2018).
Milatz, S. et al. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl Acad. Sci. USA 114, E219–E227 (2017).
Prot-Bertoye, C. et al. Differential localization patterns of claudin 10, 16, and 19 in human, mouse, and rat renal tubular epithelia. Am. J. Physiol. Ren. Physiol. 321, F207–F224 (2021).
Quintanova, C. et al. Unrecognized role of claudin‐10b in basolateral membrane infoldings of the thick ascending limb. Ann. N. Y. Acad. Sci. 1517, 266–278 (2022).
Li, W. Y., Huey, C. L. & Yu, A. S. L. Expression of claudin-7 and -8 along the mouse nephron. Am. J. Physiol. Ren. Physiol. 286, F1063–F1071 (2004).
Ziemens, A. et al. Claudin 19 is regulated by extracellular osmolality in rat kidney inner medullary collecting duct cells. Int. J. Mol. Sci. 20, 4401 (2019).
Otani, T. et al. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J. Cell Biol. 218, 3372–3396 (2019).
Furuse, M. et al. Reconstitution of functional tight junctions with individual claudin subtypes in epithelial cells. Cell Struct. Funct. 48, 1–17 (2023).
Inai, T., Kobayashi, J. & Shibata, Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol. 78, 849–855 (1999).
Milatz, S. et al. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta 1798, 2048–2057 (2010).
Shashikanth, N. et al. Tight junction channel regulation by interclaudin interference. Nat. Commun. 13, 3780 (2022).
Borovac, J. et al. Claudin-4 forms a paracellular barrier, revealing the interdependence of claudin expression in the loose epithelial cell culture model opossum kidney cells. Am. J. Physiol. Cell Physiol. 303, C1278–C1291 (2012).
Lanaspa, M. A., Andres-Hernando, A., Rivard, C. J., Dai, Y. & Berl, T. Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells. Proc. Natl Acad. Sci. USA 105, 15797–15802 (2008).
Lashhab, R. et al. The kidney anion exchanger 1 affects tight junction properties via claudin-4. Sci. Rep. 9, 3099 (2019).
Yu, A. S. L., Enck, A. H., Lencer, W. I. & Schneeberger, E. E. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J. Biol. Chem. 278, 17350–17359 (2003).
Angelow, S., Schneeberger, E. E. & Yu, A. S. L. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J. Membr. Biol. 215, 147–159 (2007).
Miyamoto, T. et al. Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J. Cell Biol. 169, 527–538 (2005).
Angelow, S., El-Husseini, R., Kanzawa, S. A. & Yu, A. S. L. Renal localization and function of the tight junction protein, claudin-19. Am. J. Physiol. Ren. Physiol. 293, F166–F177 (2007).
Hou, J. et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Invest. 118, 619–628 (2008).
Amasheh, S. et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115, 4969–4976 (2002).
Colegio, O. R., Van Itallie, C. M., McCrea, H. J., Rahner, C. & Anderson, J. M. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 283, C142–C147 (2002).
Van Itallie, C. M. et al. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Ren. Physiol. 291, F1288–F1299 (2006).
Günzel, D. et al. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J. Cell Sci. 122, 1507–1517 (2009).
Fujita, H. et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 19, 1912–1921 (2008).
Curry, J. N. et al. Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease. J. Clin. Invest. 130, 1948–1960 (2020).
Curry, J. N., Tokuda, S., McAnulty, P. & Yu, A. S. L. Combinatorial expression of claudins in the proximal renal tubule and its functional consequences. Am. J. Physiol. Ren. Physiol. 318, F1138–F1146 (2020).
Plain, A. et al. Claudin-12 knockout mice demonstrate reduced proximal tubule calcium permeability. Int. J. Mol. Sci. 21, 2074 (2020).
Ikari, A. et al. Tight junctional localization of claudin-16 is regulated by syntaxin 8 in renal tubular epithelial cells. J. Biol. Chem. 289, 13112–13123 (2014).
Hou, J. et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl Acad. Sci. USA 106, 15350–15355 (2009).
Gong, Y. et al. Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Mol. Biol. Cell 26, 4333–4346 (2015).
Krug, S. M. et al. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol. Life Sci. 69, 2765–2778 (2012).
Sewerin, S. et al. Defective claudin-10 causes a novel variation of HELIX syndrome through compromised tight junction strand assembly. Genes Dis. 9, 1301–1314 (2021).
Hou, J., Renigunta, A., Yang, J. & Waldegger, S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl Acad. Sci. USA 107, 18010–18015 (2010).
Rosenthal, R. et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 123, 1913–1921 (2010).
Muto, S. et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl Acad. Sci. USA 107, 8011–8016 (2010).
Schnermann, J., Huang, Y. & Mizel, D. Fluid reabsorption in proximal convoluted tubules of mice with gene deletions of claudin-2 and/or aquaporin1. Am. J. Physiol. Ren. Physiol. 305, F1352–F1364 (2013).
Wilmes, A., Aschauer, L., Limonciel, A., Pfaller, W. & Jennings, P. Evidence for a role of claudin 2 as a proximal tubular stress responsive paracellular water channel. Toxicol. Appl. Pharmacol. 279, 163–172 (2014).
Rosenthal, R. et al. Water channels and barriers formed by claudins. Ann. N. Y. Acad. Sci. 1397, 100–109 (2017).
Rosenthal, R. et al. Claudin-15 forms a water channel through the tight junction with distinct function compared to claudin-2. Acta Physiol. 228, e13334 (2020).
Ben-Yosef, T. et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 12, 2049–2061 (2003).
Sato, T. et al. Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein claudin14. Proc. Natl Acad. Sci. USA 114, E3344–E3353 (2017).
Gong, Y. et al. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 31, 1999–2012 (2012).
Raya-Sandino, A. et al. Claudin-23 strengthens the colonic epithelial barrier by regulating claudin-3 and -4 proteins in the tight junction plasma membrane. FASEB J. 36, R2002 (2022).
Sassi, A. et al. Interaction between epithelial sodium channel γ-subunit and claudin-8 modulates paracellular sodium permeability in renal collecting duct. J. Am. Soc. Nephrol. 31, 1009–1023 (2020).
Sassi, A. et al. Expression of claudin-8 is induced by aldosterone in renal collecting duct principal cells. Am. J. Physiol. Ren. Physiol. 321, F645–F655 (2021).
Gong, Y. et al. KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc. Natl Acad. Sci. USA 112, 4340–4345 (2015).
Tanaka, H. et al. Intestinal deletion of claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut 64, 1529–1538 (2015).
Alexandre, M. D., Lu, Q. & Chen, Y. H. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 118, 2683–2693 (2005).
Hou, J., Gomes, A. S., Paul, D. L. & Goodenough, D. A. Study of claudin function by RNA interference. J. Biol. Chem. 281, 36117–36123 (2006).
Tatum, R. et al. WNK4 phosphorylates ser206 of claudin-7 and promotes paracellular Cl− permeability. FEBS Lett. 581, 3887–3891 (2007).
Tatum, R. et al. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am. J. Physiol. Ren. Physiol. 298, F24–F34 (2010).
Saito, A. C. et al. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell 32, 722–738 (2021).
Yu, A. S. L. Paracellular transport as a strategy for energy conservation by multicellular organisms? Tissue Barriers 5, e1301852 (2017).
Yu, A. S. L. Paracellular transport and energy utilization in the renal tubule. Curr. Opin. Nephrol. Hypertens. 26, 398–404 (2017).
Rosenthal, R. et al. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 219, 521–536 (2017).
Berry, C. A. Water permeability and pathways in the proximal tubule. Am. J. Physiol. 245, F279–F294 (1983).
Barry, P. H. & Diamond, J. M. Effects of unstirred layers on membrane phenomena. Physiol. Rev. 64, 763–872 (1984).
Günzel, D. Is there a molecular basis for solvent drag in the renal proximal tubule? Pflug. Arch. 475, 277–281 (2023).
Grantham, J. J., Kurg, M. B. & Obloff, J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J. Clin. Invest. 49, 1815–1826 (1970).
Rau, W. S. & Frömter, E. Electrical properties of the medullary collecting ducts of the golden hamster kidney. I. The transepithelial potential difference. Pflug. Arch. 351, 99–111 (1974).
Koeppen, B. & Giebisch, G. Cellular electrophysiology of potassium transport in the mammalian cortical collecting tubule. Pflug. Arch. 405 (Suppl. 1), S143–S146 (1985).
Rector, F. C. Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am. J. Physiol. 244, F461–F471 (1983).
Ikari, A. et al. Activation of a polyvalent cation-sensing receptor decreases magnesium transport via claudin-16. Biochim. Biophys. Acta 1778, 283–290 (2008).
Gong, Y. & Hou, J. Claudin-14 underlies Ca++-sensing receptor-mediated Ca++ metabolism via NFAT-microRNA-based mechanisms. J. Am. Soc. Nephrol. 25, 745–760 (2014).
Gong, Y., Himmerkus, N., Plain, A., Bleich, M. & Hou, J. Epigenetic regulation of microRNAs controlling CLDN14 expression as a mechanism for renal calcium handling. J. Am. Soc. Nephrol. 26, 663–676 (2015).
Lee, J. J. et al. Activation of the calcium sensing receptor increases claudin-14 expression via a PLC -p38-Sp1 pathway. FASEB J. 35, e21982 (2021).
Dimke, H. et al. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am. J. Physiol. Ren. Physiol. 304, F761–F769 (2013).
Oh, I. H. et al. Thick ascending limb claudins are altered to increase calciuria and magnesiuria in metabolic acidosis. Am. J. Physiol. Ren. Physiol. 320, F418–F428 (2021).
Balkovetz, D. F., Chumley, P. & Amlal, H. Downregulation of claudin-2 expression in renal epithelial cells by metabolic acidosis. Am. J. Physiol. Ren. Physiol. 297, F604–F611 (2009).
Kim, G. H. Renal mechanisms for hypercalciuria induced by metabolic acidosis. Am. J. Nephrol. 53, 839–846 (2022).
Ferreira, P. G. et al. Renal claudin-14 expression is not required for regulating Mg2+ balance in mice. Am. J. Physiol. Ren. Physiol. 320, F897–F907 (2021).
De Baaij, J. H. F., Hoenderop, J. G. J. & Bindels, R. J. M. Regulation of magnesium balance: lessons learned from human genetic disease. Clin. Kidney J. 5 (Suppl. 1), i15–i24 (2012).
Moor, M. B. & Bonny, O. Ways of calcium reabsorption in the kidney. Am. J. Physiol. Ren. Physiol. 310, F1337–F1350 (2016).
Amasheh, S. et al. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem. Biophys. Res. Commun. 378, 45–50 (2009).
Molina-Jijón, E. et al. Aldosterone signaling regulates the over-expression of claudin-4 and -8 at the distal nephron from type 1 diabetic rats. PLoS ONE 12, e0177362 (2017).
Fan, J., Tatum, R., Hoggard, J. & Chen, Y. H. Claudin-7 modulates Cl− and Na+ homeostasis and WNK4 expression in renal collecting duct cells. Int. J. Mol. Sci. 20, 3798 (2019).
Fujii, N. et al. Hypotonic stress-induced down-regulation of claudin-1 and -2 mediated by dephosphorylation and clathrin-dependent endocytosis in renal tubular epithelial cells. J. Biol. Chem. 291, 24787–24799 (2016).
Ikari, A., Takiguchi, A., Atomi, K., Sato, T. & Sugatani, J. Decrease in claudin-2 expression enhances cell migration in renal epithelial Madin-Darby canine kidney cells. J. Cell Physiol. 226, 1471–1478 (2011).
Canuto, L. P. & Collares-Buzato, C. B. Increased osmolality enhances the tight junction-mediated barrier function in a cultured renal epithelial cell line. Cell Biol. Int. 43, 73–82 (2019).
Ikari, A. et al. Hyperosmolarity-induced down-regulation of claudin-2 mediated by decrease in PKCβ-dependent GATA-2 in MDCK cells. J. Cell Physiol. 230, 2776–2787 (2015).
Ikari, A. et al. Hyperosmolarity-induced up-regulation of claudin-4 mediated by NADPH oxidase-dependent H2O2 production and Sp1/c-Jun cooperation. Biochim. Biophys. Acta 1833, 2617–2627 (2013).
Hinze, C. et al. GRHL2 is required for collecting duct epithelial barrier function and renal osmoregulation. J. Am. Soc. Nephrol. 29, 857–868 (2018).
Ziemba, J. B. & Matlaga, B. R. Epidemiology and economics of nephrolithiasis. Investig. Clin. Urol. 58, 299–306 (2017).
Blaine, J., Chonchol, M. & Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 10, 1257–1272 (2015).
Zhang, Y. G. et al. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci. Rep. 5, 10652 (2015).
Kladnitsky, O., Rozenfeld, J., Azulay-Debby, H., Efrati, E. & Zelikovic, I. The claudin-16 channel gene is transcriptionally inhibited by 1,25-dihydroxyvitamin D. Exp. Physiol. 100, 79–94 (2015).
Loupy, A. et al. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J. Clin. Investig. 122, 3355–3367 (2012).
Bourdeau, J. E. & Burg, M. B. Effect of PTH on calcium transport across the cortical thick ascending limb of Henle’s loop. Am. J. Physiol. 239, 121–126 (1980).
Friedman, P. A. Basal and hormone-activated calcium absorption in mouse renal thick ascending limbs. Am. J. Physiol. 254, F62–F70 (1988).
Motoyama, H. I. & Friedman, P. A. Calcium-sensing receptor regulation of PTH-dependent calcium absorption by mouse cortical ascending limbs. Am. J. Physiol. Ren. Physiol. 283, F399–F406 (2002).
Nelson, J. W. et al. Local and downstream actions of proximal tubule angiotensin II signaling on Na+ transporters in the mouse nephron. Am. J. Physiol. Ren. Physiol. 321, F69–F81 (2021).
Poulsen, S. B., Limbutara, K., Fenton, R. A., Pisitkun, T. & Christensen, B. M. RNA sequencing of kidney distal tubule cells reveals multiple mediators of chronic aldosterone action. Physiol. Genom. 50, 343–354 (2018).
Le Moellic, C. et al. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am. J. Physiol. Cell Physiol. 289, C1513–C1521 (2005).
Salhadar, K. et al. Phosphoproteomic identification of vasopressin/cAMP/protein kinase A-dependent signaling in kidney. Mol. Pharmacol. 99, 358–369 (2021).
Mutig, K. et al. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am. J. Physiol. Ren. Physiol. 293, F1166–F1177 (2007).
Gunaratne, R. et al. Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc. Natl Acad. Sci. USA 107, 15653–15658 (2010).
Himmerkus, N. et al. AVP dynamically increases paracellular Na+ permeability and transcellular NaCl transport in the medullary thick ascending limb of Henle’s loop. Pflug. Arch. 469, 149–158 (2017).
Breiderhoff, T. et al. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc. Natl Acad. Sci. USA 109, 14241–14246 (2012).
Askari, M. et al. Identification of a missense variant in CLDN2 in obstructive azoospermia. J. Hum. Genet. 64, 1023–1032 (2019).
Evan, A. P., Coe, F. L., Lingeman, J., Bledsoe, S. & Worcester, E. M. Randall’s plaque in stone formers originates in ascending thin limbs. Am. J. Physiol. Ren. Physiol. 315, F1236–F1242 (2018).
Konrad, M. et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am. J. Hum. Genet. 79, 949–957 (2006).
Figueres, L. et al. Hypomagnesemia, hypocalcemia, and tubulointerstitial nephropathy caused by claudin-16 autoantibodies. J. Am. Soc. Nephrol. 33, 1402–1410 (2022).
Hou, J. et al. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J. Biol. Chem. 282, 17114–17122 (2007).
Blanchard, A. et al. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 59, 2206–2215 (2001).
Will, C. et al. Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am. J. Physiol. Ren. Physiol. 298, F1152–F1161 (2010).
Wilcox, E. R. et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104, 165–172 (2001).
Thorleifsson, G. et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat. Genet. 41, 926–930 (2009).
Guha, M. et al. Polymorphisms in CaSR and CLDN14 genes associated with increased risk of kidney stone disease in patients from the Eastern part of India. PLoS ONE 10, e0130790 (2015).
Ure, M. E. et al. A variant in a cis-regulatory element enhances claudin-14 expression and is associated with pediatric-onset hypercalciuria and kidney stones. Hum. Mutat. 38, 649–657 (2017).
Arcidiacono, T. et al. Claudin-14 gene polymorphisms and urine calcium excretion. Clin. J. Am. Soc. Nephrol. 13, 1542–1549 (2018).
Elshamaa, M. F. et al. Genetic polymorphisms in CLDN14 (rs219780) and ALP (rs1256328) genes are associated with risk of nephrolithiasis in Egyptian children. Turk. J. Urol. 47, 73–80 (2021).
Ullah, I. et al. Association study of CLDN14 variations in patients with kidney stones. Open Life Sci. 17, 81–92 (2022).
Wang, L. et al. A genetic polymorphism in the WDR72 gene is associated with calcium nephrolithiasis in the Chinese Han population. Front. Genet. 13, 897051 (2022).
Bongers, E. M. H. F. et al. A novel hypokalemic-alkalotic salt-losing tubulopathy in patients with CLDN10 mutations. J. Am. Soc. Nephrol. 28, 3118–3128 (2017).
Klar, J. et al. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet 13, e1006897 (2017).
Hadj-Rabia, S. et al. Multiplex epithelium dysfunction due to CLDN10 mutation: the HELIX syndrome. Genet. Med. 20, 190–201 (2018).
Meyers, N. et al. Hypokalemia associated with a claudin 10 mutation: a case report. Am. J. Kidney Dis. 73, 425–428 (2019).
Milatz, S. A novel claudinopathy based on claudin-10 mutations. Int. J. Mol. Sci. 20, 5396 (2019).
Faivre, A., Scholz, C. C. & De Seigneux, S. Hypoxia in chronic kidney disease: towards a paradigm shift? Nephrol. Dial. Transpl. 36, 1782–1790 (2021).
Furuse, M. et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 156, 1099–1111 (2002).
Hasegawa, K. et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing claudin-1 overexpression in podocytes. Nat. Med. 19, 1496–1504 (2013).
Wang, B. et al. VDR/Atg3 axis regulates slit diaphragm to tight junction transition via p62-mediated autophagy pathway in diabetic nephropathy. Diabetes 70, 2639–2651 (2021).
Gong, Y., Sunq, A., Roth, R. A. & Hou, J. Inducible expression of claudin-1 in glomerular podocytes generates aberrant tight junctions and proteinuria through slit diaphragm destabilization. J. Am. Soc. Nephrol. 28, 106–117 (2017).
Fujita, H., Hamazaki, Y., Noda, Y., Oshima, M. & Minato, N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS ONE 7, e52272 (2012).
Gong, Y. et al. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc. Natl Acad. Sci. USA 111, E3766–E3774 (2014).
Susa, K. et al. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum. Mol. Genet. 23, 5052–5060 (2014).
Gheith, O., Farouk, N., Nampoory, N., Halim, M. A. & Al-Otaibi, T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J. Nephropharmacol. 5, 49 (2016).
Mongelli-Sabino, B. M., Canuto, L. P. & Collares-Buzato, C. B. Acute and chronic exposure to high levels of glucose modulates tight junction-associated epithelial barrier function in a renal tubular cell line. Life Sci. 188, 149–157 (2017).
Molina-Jijón, E., Rodríguez-Muñoz, R., Namorado, M. D. C., Pedraza-Chaverri, J. & Reyes, J. L. Oxidative stress induces claudin-2 nitration in experimental type 1 diabetic nephropathy. Free. Radic. Biol. Med. 72, 162–175 (2014).
Rosas-Martínez, L. et al. Hyperglycemic levels in early stage of diabetic nephropathy affect differentially renal expression of claudins-2 and -5 by oxidative stress. Life Sci. 268, 119003 (2021).
Sun, H. et al. Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease. Nat. Commun. 13, 1600 (2022).
Amoozadeh, Y., Dan, Q., Xiao, J., Waheed, F. & Szászi, K. Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: role in barrier functions. Am. J. Physiol. Cell Physiol. 309, C38–C50 (2015).
Amoozadeh, Y. et al. Tumor necrosis factor-α increases claudin-1, 4, and 7 expression in tubular cells: role in permeability changes. J. Cell Physiol. 232, 2210–2220 (2017).
Meurer, M. & Höcherl, K. Deregulated renal magnesium transport during lipopolysaccharide-induced acute kidney injury in mice. Pflug. Arch. 471, 619–631 (2019).
Meurer, M. & Höcherl, K. Renal ischemia-reperfusion injury impairs renal calcium, magnesium, and phosphate handling in mice. Pflug. Arch. 471, 901–914 (2019).
Dan, Q. et al. Claudin-2 suppresses GEF-H1, RHOA, and MRTF, thereby impacting proliferation and profibrotic phenotype of tubular cells. J. Biol. Chem. 294, 15446–15465 (2019).
Tabariès, S. & Siegel, P. M. The role of claudins in cancer metastasis. Oncogene 36, 1176–1190 (2017).
Hwang, S. J., Yang, Q., Meigs, J. B., Pearce, E. N. & Fox, C. S. A genome-wide association for kidney function and endocrine-related traits in the NHLBI’s Framingham Heart Study. BMC Med. Genet. 8 (Suppl. 1), S10 (2007).
Pattaro, C. et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat. Commun. 7, 10023 (2016).
Wuttke, M. et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 51, 957–972 (2019).
Watanabe, K. et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 51, 1339–1348 (2019).
Lechpammer, M. et al. The diagnostic and prognostic utility of claudin expression in renal cell neoplasms. Mod. Pathol. 21, 1320–1329 (2008).
Prat, A. et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12, R68 (2010).
Zhang, C. et al. Identification of a claudin-low subtype in clear cell renal cell carcinoma with implications for the evaluation of clinical outcomes and treatment efficacy. Front. Immunol. 13, 1020729 (2022).
Mihajlovic, M. et al. Role of vitamin D in maintaining renal epithelial barrier function in uremic conditions. Int. J. Mol. Sci. 18, 2531 (2017).
Giménez, I. & Forbush, B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J. Biol. Chem. 278, 26946–26951 (2003).
Weber, S. et al. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur. J. Hum. Genet. 8, 414–422 (2000).
Müller, D. et al. Unusual clinical presentation and possible rescue of a novel claudin-16 mutation. J. Clin. Endocrinol. Metab. 91, 3076–3079 (2006).
Bardet, C. et al. Claudin-16 deficiency impairs tight junction function in ameloblasts, leading to abnormal enamel formation. J. Bone Min. Res. 31, 498–513 (2016).
Vall-Palomar, M., Madariaga, L. & Ariceta, G. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Pediatr. Nephrol. 36, 3045–3055 (2021).
Godron, A. et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: phenotype-genotype correlation and outcome in 32 patients with CLDN16 or CLDN19 mutations. Clin. J. Am. Soc. Nephrol. 7, 801–809 (2012).
Yamaguti, P. M. et al. Amelogenesis imperfecta in familial hypomagnesaemia and hypercalciuria with nephrocalcinosis caused by CLDN19 gene mutations. J. Med. Genet. 54, 26–37 (2017).
Ye, M. et al. Glomerular localization and expression of Angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 17, 3067–3075 (2006).
Gurley, S. B. et al. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 13, 469–475 (2011).
Kanai, M. et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 50, 390–400 (2018).
Graham, S. E. et al. Sex-specific and pleiotropic effects underlying kidney function identified from GWAS meta-analysis. Nat. Commun. 10, 1847 (2019).
Acknowledgements
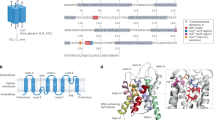
The authors thank J. Piontek for providing Fig. 1a image and M. Fromm and M. A. Knepper for valuable discussion. The work of the authors is funded by the Deutsche Forschungsgemeinschaft (grant ID 318374368).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Katalin Szaszi and Pascal Houillier for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Kidney Cell Explorer: https://cello.shinyapps.io/kidneycellexplorer/
Kidney Interactive Transcriptomics: https://humphreyslab.com/SingleCell/
Kidney Tubules Expression Atlas: https://esbl.nhlbi.nih.gov/KTEA/
Mouse Renal Epithelial Cell Atlas: https://esbl.nhlbi.nih.gov/MRECA/Nephron/
Nephroseq: https://www.nephroseq.org/resource/login.html
OMIM: https://omim.org
RNA-seq Analysis of Microdissected Rat Kidney Tubule Segments: https://esbl.nhlbi.nih.gov/helixweb/Database/NephronRNAseq/All_transcripts.html
REC dehydration database: https://endotheliomics.shinyapps.io/rec_dehydration/
Single-cell transcriptomics: https://shiny.mdc-berlin.de/humAKI/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meoli, L., Günzel, D. The role of claudins in homeostasis. Nat Rev Nephrol 19, 587–603 (2023). https://doi.org/10.1038/s41581-023-00731-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00731-y
This article is cited by
-
Calcium signalling and transport in the kidney
Nature Reviews Nephrology (2024)
-
Convergent gene losses and pseudogenizations in multiple lineages of stomachless fishes
Communications Biology (2024)
-
Gut liver brain axis in diseases: the implications for therapeutic interventions
Signal Transduction and Targeted Therapy (2023)