Abstract
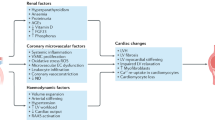
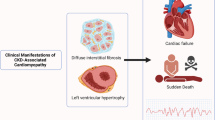
The term uraemic cardiomyopathy refers to the cardiac abnormalities that are seen in patients with chronic kidney disease (CKD). Historically, this term was used to describe a severe cardiomyopathy that was associated with end-stage renal disease and characterized by severe functional abnormalities that could be reversed following renal transplantation. In a modern context, uraemic cardiomyopathy describes the clinical phenotype of cardiac disease that accompanies CKD and is perhaps best characterized as diastolic dysfunction seen in conjunction with left ventricular hypertrophy and fibrosis. A multitude of factors may contribute to the pathogenesis of uraemic cardiomyopathy, and current treatments only modestly improve outcomes. In this Review, we focus on evolving concepts regarding the roles of fibroblast growth factor 23 (FGF23), inflammation and systemic oxidant stress and their interactions with more established mechanisms such as pressure and volume overload resulting from hypertension and anaemia, respectively, activation of the renin–angiotensin and sympathetic nervous systems, activation of the transforming growth factor-β (TGFβ) pathway, abnormal mineral metabolism and increased levels of endogenous cardiotonic steroids.
Key points
-
Patients with chronic kidney disease or end-stage renal disease have an increased risk of cardiovascular disease and mortality.
-
Uraemic cardiomyopathy is characterized by diastolic dysfunction and marked left ventricular hypertrophy with profound ventricular fibrosis.
-
Factors that have been implicated in the development and progression of uraemic cardiomyopathy include haemodynamic overload, alterations in mineral metabolism, insulin resistance, circulating uraemic toxins and endogenous cardiotonic steroids.
-
Oxidative stress seems to have a role in all of the putative molecular pathways that are involved in the pathogenesis of uraemic cardiomyopathy.
-
Treatments that are effective in other cardiomyopathic conditions such as antihypertensive drugs improve clinical outcomes in uraemic cardiomyopathy only modestly at best.
-
The available data suggest that targeting oxidative stress might be a beneficial therapeutic strategy for patients with uraemic cardiomyopathy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Collins, A. J., Foley, R. N., Gilbertson, D. T. & Chen, S. C. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. 5, 2–7 (2015).
Silverberg, D., Wexler, D., Blum, M., Schwartz, D. & Iaina, A. The association between congestive heart failure and chronic renal disease. Curr. Opin. Nephrol. Hypertens. 13, 163–170 (2004).
London, G. M., Pannier, B., Marchais, S. J. & Guerin, A. P. Calcification of the aortic valve in the dialyzed patient. J. Am. Soc. Nephrol. 11, 778–783 (2000).
Dad, T. & Weiner, D. E. Stroke and chronic kidney disease: epidemiology, pathogenesis, and management across kidney disease stages. Semin. Nephrol. 35, 311–322 (2015).
Kulkarni, N., Gukathasan, N., Sartori, S. & Baber, U. Chronic kidney disease and atrial fibrillation: a contemporary overview. J. Atr. Fibrillation 5, 448 (2012).
Whitman, I. R., Feldman, H. I. & Deo, R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J. Am. Soc. Nephrol. 23, 1929–1939 (2012).
Dennis, V. W. Coronary heart disease in patients with chronic kidney disease. J. Am. Soc. Nephrol. 16 (Suppl. 2), 103–106 (2005).
Moe, S. M. & Chen, N. X. Mechanisms of vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 19, 213–216 (2008).
Khan, N. A. et al. Kidney function and mortality among patients with left ventricular systolic dysfunction. J. Am. Soc. Nephrol. 17, 244–253 (2006).
Wang, X., Liu, J., Drummond, C. A. & Shapiro, J. I. Sodium potassium adenosine triphosphatase (Na/K-ATPase) as a therapeutic target for uremic cardiomyopathy. Expert Opin. Ther. Targets 21, 531–541 (2017).
London, G. M. et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J. Am. Soc. Nephrol. 12, 2759–2767 (2001).
Middleton, R. J., Parfrey, P. S. & Foley, R. N. Left ventricular hypertrophy in the renal patient. J. Am. Soc. Nephrol. 12, 1079–1084 (2001).
Zoccali, C. et al. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J. Am. Soc. Nephrol. 15, 1029–1037 (2004).
Kennedy, D. et al. Effect of chronic renal failure on cardiac contractile function, calcium cycling, and gene expression of proteins important for calcium homeostasis in the rat. J. Am. Soc. Nephrol. 14, 90–97 (2003).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 17, 1321–1360 (2016).
Otsuka, T., Suzuki, M., Yoshikawa, H. & Sugi, K. Left ventricular diastolic dysfunction in the early stage of chronic kidney disease. J. Cardiol. 54, 199–204 (2009).
de Almeida, E. A. et al. Diastolic function in several stages of chronic kidney disease in patients with autosomal dominant polycystic kidney disease: a tissue Doppler imaging study. Kidney Blood Press. Res. 30, 234–239 (2007).
Diez, J. Mechanisms of cardiac fibrosis in hypertension. J. Clin. Hypertens. 9, 546–550 (2007).
Lopez, B., Gonzalez, A., Hermida, N., Laviades, C. & Diez, J. Myocardial fibrosis in chronic kidney disease: potential benefits of torasemide. Kidney Int. 74 (Suppl. 111), S19–S23 (2008).
Hung, S. C., Lai, Y. S., Kuo, K. L. & Tarng, D. C. Volume overload and adverse outcomes in chronic kidney disease: clinical observational and animal studies. J. Am. Heart Assoc. 4, e001918 (2015).
Grabner, A. & Faul, C. The role of fibroblast growth factor 23 and Klotho in uremic cardiomyopathy. Curr. Opin. Nephrol. Hypertens. 25, 314–324 (2016).
Shinohara, K. et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J. Am. Soc. Nephrol. 13, 1894–1900 (2002).
Spoto, B., Pisano, A. & Zoccali, C. Insulin resistance in chronic kidney disease: a systematic review. Am. J. Physiol. Renal Physiol. 311, F1087–F1108 (2016).
Hung, S. C., Kuo, K. L., Wu, C. C. & Tarng, D. C. Indoxyl sulfate: a novel cardiovascular risk factor in chronic kidney disease. J. Am. Heart Assoc. 6, e005022 (2017).
Vanholder, R., Schepers, E., Pletinck, A., Nagler, E. V. & Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J. Am. Soc. Nephrol. 25, 1897–1907 (2014).
Kennedy, D. J., Malhotra, D. & Shapiro, J. I. Molecular insights into uremic cardiomyopathy: cardiotonic steroids and Na/K ATPase signaling. Cell. Mol. Biol. 52, 3–14 (2006).
Alhaj, E. et al. Uremic cardiomyopathy: an underdiagnosed disease. Congest. Heart Fail. 19, E40–E45 (2013).
Zoccali, C. et al. Chronic fluid overload and mortality in ESRD. J. Am. Soc. Nephrol. 28, 2491–2497 (2017).
Ruwhof, C. & van der Laarse, A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc. Res. 47, 23–37 (2000).
Sadoshima, J., Jahn, L., Takahashi, T., Kulik, T. J. & Izumo, S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J. Biol. Chem. 267, 10551–10560 (1992).
Kira, Y. et al. Effect of long-term cyclic mechanical load on protein synthesis and morphological changes in cultured myocardial cells from neonatal rat. Cardiovasc. Drugs Ther. 8, 251–262 (1994).
Komuro, I. et al. Stretching cardiac myocytes stimulates protooncogene expression. J. Biol. Chem. 265, 3595–3598 (1990).
Cooper, G. t., Kent, R. L., Uboh, C. E., Thompson, E. W. & Marino, T. A. Hemodynamic versus adrenergic control of cat right ventricular hypertrophy. J. Clin. Invest. 75, 1403–1414 (1985).
Komuro, I., Kurabayashi, M., Takaku, F. & Yazaki, Y. Expression of cellular oncogenes in the myocardium during the developmental stage and pressure-overloaded hypertrophy of the rat heart. Circ. Res. 62, 1075–1079 (1988).
Sadoshima, J., Xu, Y., Slayter, H. S. & Izumo, S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75, 977–984 (1993).
Tamura, K. et al. Activation of angiotensinogen gene in cardiac myocytes by angiotensin II and mechanical stretch. Am. J. Physiol. 275, R1–R9 (1998).
Malhotra, R., Sadoshima, J. & Brosius, F. C. 3rd & Izumo, S. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes in vitro. Circ. Res. 85, 137–146 (1999).
Rockman, H. A. et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl Acad. Sci. USA 88, 8277–8281 (1991).
Fiorillo, C. et al. Cardiac volume overload rapidly induces oxidative stress-mediated myocyte apoptosis and hypertrophy. Biochim. Biophys. Acta 1741, 173–182 (2005).
Kennedy, D. J. et al. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am. J. Physiol. Renal Physiol. 294, F450–F454 (2008).
Kennedy, D. J. et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 47, 488–495 (2006).
Tsujimoto, I. et al. The antioxidant edaravone attenuates pressure overload-induced left ventricular hypertrophy. Hypertension 45, 921–926 (2005).
Hotamisligil, G. S. & Davis, R. J. Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 8, a006072 (2016).
Cohen, P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 7, 353–361 (1997).
Dingar, D. et al. Effect of pressure overload-induced hypertrophy on the expression and localization of p38 MAP kinase isoforms in the mouse heart. Cell. Signal. 22, 1634–1644 (2010).
Ihle, J. N. Cytokine receptor signalling. Nature 377, 591–594 (1995).
Schindler, C. & Darnell, J. E. Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 64, 621–651 (1995).
Pan, J. et al. Role of angiotensin II in activation of the JAK/STAT pathway induced by acute pressure overload in the rat heart. Circ. Res. 81, 611–617 (1997).
Lambers Heerspink, H. J., de Borst, M. H., Bakker, S. J. & Navis, G. J. Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat. Rev. Nephrol. 9, 112–121 (2013).
Franssen, C. F. & Navis, G. Chronic kidney disease: RAAS blockade and diastolic heart failure in chronic kidney disease. Nat. Rev. Nephrol. 9, 190–192 (2013).
Hudlicka, O., Brown, M. & Egginton, S. Angiogenesis in skeletal and cardiac muscle. Physiol. Rev. 72, 369–417 (1992).
Kurdi, M. & Booz, G. W. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension 57, 1034–1038 (2011).
Mehta, P. K. & Griendling, K. K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 292, C82–C97 (2007).
Fernandez-Ruiz, I. Pharmacotherapy: angiotensin II — a new tool in vasodilatory shock. Nat. Rev. Cardiol. 14, 384 (2017).
Taniyama, Y. et al. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 287, C494–C499 (2004).
Pellieux, C. et al. Dilated cardiomyopathy and impaired cardiac hypertrophic response to angiotensin II in mice lacking FGF-2. J. Clin. Invest. 108, 1843–1851 (2001).
Zablocki, D. & Sadoshima, J. Angiotensin II and oxidative stress in the failing heart. Antioxid. Redox Signal. 19, 1095–1109 (2013).
Kawada, N., Imai, E., Karber, A., Welch, W. J. & Wilcox, C. S. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J. Am. Soc. Nephrol. 13, 2860–2868 (2002).
Polizio, A. H. et al. Angiotensin II regulates cardiac hypertrophy via oxidative stress but not antioxidant enzyme activities in experimental renovascular hypertension. Hypertens. Res. 31, 325–334 (2008).
Zhao, Q. D. et al. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkappaB signaling pathways. Circulation 131, 643–655 (2015).
Ferrario, C. M., Chappell, M. C., Dean, R. H. & Iyer, S. N. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J. Am. Soc. Nephrol. 9, 1716–1722 (1998).
Balakumar, P. & Jagadeesh, G. A century old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cell. Signal. 26, 2147–2160 (2014).
Jankowski, V. et al. Angioprotectin: an angiotensin II-like peptide causing vasodilatory effects. FASEB J. 25, 2987–2995 (2011).
Lautner, R. Q. et al. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ. Res. 112, 1104–1111 (2013).
Liu, C. et al. Alamandine attenuates hypertension and cardiac hypertrophy in hypertensive rats. Amino Acids 50, 1071–1081 (2018).
Schlaich, M. P. et al. Sympathetic activation in chronic renal failure. J. Am. Soc. Nephrol. 20, 933–939 (2009).
Park, J. Cardiovascular risk in chronic kidney disease: role of the sympathetic nervous system. Cardiol. Res. Pract. 2012, 319432 (2012).
Fisher, J. P., Young, C. N. & Fadel, P. J. Central sympathetic overactivity: maladies and mechanisms. Auton. Neurosci. 148, 5–15 (2009).
Zoccali, C. et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 105, 1354–1359 (2002).
Grassi, G., Seravalle, G., Dell’Oro, R. & Mancia, G. Sympathetic mechanisms, organ damage, and antihypertensive treatment. Curr. Hypertens. Rep. 13, 303–308 (2011).
Chalothorn, D. et al. Differential cardiovascular regulatory activities of the alpha 1B- and alpha 1D-adrenoceptor subtypes. J. Pharmacol. Exp. Ther. 305, 1045–1053 (2003).
Xu, Q. et al. Myocardial oxidative stress contributes to transgenic beta(2)-adrenoceptor activation-induced cardiomyopathy and heart failure. Br. J. Pharmacol. 162, 1012–1028 (2011).
Cohn, J. N. et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984).
Elias, A. N., Vaziri, N. D. & Maksy, M. Plasma norepinephrine, epinephrine, and dopamine levels in end-stage renal disease. Effect of hemodialysis. Arch. Intern. Med. 145, 1013–1015 (1985).
Patel, M. B. et al. Altered function and structure of the heart in dogs with chronic elevation in plasma norepinephrine. Circulation 84, 2091–2100 (1991).
Barki-Harrington, L., Perrino, C. & Rockman, H. A. Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc. Res. 63, 391–402 (2004).
Vidal, M., Wieland, T., Lohse, M. J. & Lorenz, K. beta-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gbetagamma/Erk-dependent pathway. Cardiovasc. Res. 96, 255–264 (2012).
Moniri, N. H. & Daaka, Y. Agonist-stimulated reactive oxygen species formation regulates beta2-adrenergic receptor signal transduction. Biochem. Pharmacol. 74, 64–73 (2007).
Bovo, E., Lipsius, S. L. & Zima, A. V. Reactive oxygen species contribute to the development of arrhythmogenic Ca(2)(+) waves during beta-adrenergic receptor stimulation in rabbit cardiomyocytes. J. Physiol. 590, 3291–3304 (2012).
Villarreal, F. J. & Dillmann, W. H. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am. J. Physiol. 262, H1861–H1866 (1992).
Lee, A. A., Dillmann, W. H., McCulloch, A. D. & Villarreal, F. J. Angiotensin II stimulates the autocrine production of transforming growth factor-beta 1 in adult rat cardiac fibroblasts. J. Mol. Cell. Cardiol. 27, 2347–2357 (1995).
Murakami, K., Takemura, T., Hino, S. & Yoshioka, K. Urinary transforming growth factor-beta in patients with glomerular diseases. Pediatr. Nephrol. 11, 334–336 (1997).
Bottinger, E. P. & Bitzer, M. TGF-beta signaling in renal disease. J. Am. Soc. Nephrol. 13, 2600–2610 (2002).
Dobaczewski, M., Chen, W. & Frangogiannis, N. G. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 51, 600–606 (2011).
Kuwahara, F. et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106, 130–135 (2002).
Saito, K. et al. Iron chelation and a free radical scavenger suppress angiotensin II-induced upregulation of TGF-beta1 in the heart. Am. J. Physiol. Heart Circ. Physiol. 288, H1836–H1843 (2005).
Elkareh, J. et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension 49, 215–224 (2007).
de Albuquerque Suassuna, P. G., Sanders-Pinheiro, H. & de Paula, R. B. Uremic cardiomyopathy: a new piece in the chronic kidney disease-mineral and bone disorder puzzle. Front. Med. 5, 206 (2018).
London, G. M. et al. Uremic cardiomyopathy: an inadequate left ventricular hypertrophy. Kidney Int. 31, 973–980 (1987).
Goodman, W. G. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin. Dial. 17, 209–216 (2004).
Andersson, P., Rydberg, E. & Willenheimer, R. Primary hyperparathyroidism and heart disease—a review. Eur. Heart J. 25, 1776–1787 (2004).
Rostand, S. G. & Drueke, T. B. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 56, 383–392 (1999).
Lishmanov, A., Dorairajan, S., Pak, Y., Chaudhary, K. & Chockalingam, A. Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease. Int. Urol. Nephrol. 44, 541–547 (2012).
Silver, J. & Naveh-Many, T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat. Rev. Nephrol. 9, 641–649 (2013).
Krajisnik, T. et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 195, 125–131 (2007).
Schluter, K. D. & Piper, H. M. Cardiovascular actions of parathyroid hormone and parathyroid hormone-related peptide. Cardiovasc. Res. 37, 34–41 (1998).
Urena, P. et al. Parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology 133, 617–623 (1993).
Smogorzewski, M., Zayed, M., Zhang, Y. B., Roe, J. & Massry, S. G. Parathyroid hormone increases cytosolic calcium concentration in adult rat cardiac myocytes. Am. J. Physiol. 264, H1998–H2006 (1993).
Yao, L. et al. Parathyroid hormone and the risk of incident hypertension: the Atherosclerosis Risk in Communities study. J. Hypertens. 34, 196–203 (2016).
Craver, L. et al. Mineral metabolism parameters throughout chronic kidney disease stages 1-5—achievement of K/DOQI target ranges. Nephrol. Dial. Transplant. 22, 1171–1176 (2007).
Hruska, K. A., Mathew, S., Lund, R., Qiu, P. & Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 74, 148–157 (2008).
Slinin, Y., Foley, R. N. & Collins, A. J. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J. Am. Soc. Nephrol. 16, 1788–1793 (2005).
Shaman, A. M. & Kowalski, S. R. Hyperphosphatemia management in patients with chronic kidney disease. Saudi Pharm. J. 24, 494–505 (2016).
Vervloet, M. G. et al. The role of phosphate in kidney disease. Nat. Rev. Nephrol. 13, 27–38 (2017).
Yamazaki-Nakazawa, A. et al. Correction of hyperphosphatemia suppresses cardiac remodeling in uremic rats. Clin. Exp. Nephrol. 18, 56–64 (2014).
Rahabi-Layachi, H., Ourouda, R., Boullier, A., Massy, Z. A. & Amant, C. Distinct effects of inorganic phosphate on cell cycle and apoptosis in human vascular smooth muscle cells. J. Cell. Physiol. 230, 347–355 (2015).
Di Marco, G. S. et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Renal Physiol. 294, F1381–F1387 (2008).
Gupta, D., Brietzke, S., Hayden, M. R., Kurukulasuriya, L. R. & Sowers, J. R. Phosphate metabolism in cardiorenal metabolic disease. Cardiorenal Med. 1, 261–270 (2011).
Mehrotra, R. et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 76, 977–983 (2009).
Gluba-Brzozka, A., Franczyk, B., Cialkowska-Rysz, A., Olszewski, R. & Rysz, J. Impact of vitamin D on the cardiovascular system in advanced chronic kidney disease (CKD) and dialysis patients. Nutrients 10, 709 (2018).
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281 (2007).
Jones, G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha, 25-dihydroxyvitamin D(3). Semin. Dial. 20, 316–324 (2007).
Nitsa, A. et al. Vitamin D in cardiovascular disease. In Vivo 32, 977–981 (2018).
Wu, J., Garami, M., Cheng, T. & Gardner, D. G. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J. Clin. Invest. 97, 1577–1588 (1996).
Li, Y. C. et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Invest. 110, 229–238 (2002).
Leifheit-Nestler, M. et al. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol. Dial. Transplant. 32, 1493–1503 (2017).
Benet-Pages, A. et al. FGF23 is processed by proprotein convertases but not by PHEX. Bone 35, 455–462 (2004).
Quarles, L. D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 118, 3820–3828 (2008).
Hu, M. C., Shiizaki, K., Kuro-o, M. & Moe, O. W. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 75, 503–533 (2013).
Leifheit-Nestler, M. & Haffner, D. Paracrine effects of FGF23 on the heart. Front. Endocrinol. 9, 278 (2018).
Tagliabracci, V. S. et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl Acad. Sci. USA 111, 5520–5525 (2014).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Saito, H. et al. Circulating FGF-23 is regulated by 1alpha, 25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 280, 2543–2549 (2005).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Liu, S. et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17, 1305–1315 (2006).
Lopez, I. et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 80, 475–482 (2011).
Rodriguez-Ortiz, M. E. et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 23, 1190–1197 (2012).
Tsuji, K., Maeda, T., Kawane, T., Matsunuma, A. & Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha, 25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J. Bone Miner. Res. 25, 1711–1723 (2010).
Gutierrez, O. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2205–2215 (2005).
Isakova, T. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 (2011).
Isakova, T. et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J. Am. Soc. Nephrol. 19, 615–623 (2008).
Gutierrez, O. M. et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119, 2545–2552 (2009).
Hsu, H. J. & Wu, M. S. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am. J. Med. Sci. 337, 116–122 (2009).
Isakova, T. et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305, 2432–2439 (2011).
Gutierrez, O. M. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 359, 584–592 (2008).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Seeherunvong, W. et al. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr. Nephrol. 27, 2129–2136 (2012).
Leifheit-Nestler, M. et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol. Dial. Transplant. 31, 1088–1099 (2016).
Touchberry, C. D. et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am. J. Physiol. Endocrinol. Metab. 304, E863–E873 (2013).
Faul, C. Fibroblast growth factor 23 and the heart. Curr. Opin. Nephrol. Hypertens. 21, 369–375 (2012).
Grabner, A. et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 22, 1020–1032 (2015).
Grabner, A. et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci. Rep. 7, 1993 (2017).
Moe, S. M. et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 132, 27–39 (2015).
Isakova, T. et al. Associations between fibroblast growth factor 23 and cardiac characteristics in pediatric heart failure. Pediatr. Nephrol. 28, 2035–2042 (2013).
Nehgme, R., Fahey, J. T., Smith, C. & Carpenter, T. O. Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J. Clin. Endocrinol. Metab. 82, 2450–2454 (1997).
Shalhoub, V. et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J. Clin. Invest. 122, 2543–2553 (2012).
Pastor-Arroyo, E. M. et al. The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int. 94, 49–59 (2018).
Chue, C. D. et al. Cardiovascular effects of sevelamer in stage 3 CKD. J. Am. Soc. Nephrol. 24, 842–852 (2013).
Richter, B., Haller, J., Haffner, D. & Leifheit-Nestler, M. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflugers Arch. 468, 1621–1635 (2016).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Ito, S., Fujimori, T., Hayashizaki, Y. & Nabeshima, Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim. Biophys. Acta 1576, 341–345 (2002).
Ogawa, Y. et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl Acad. Sci. USA 104, 7432–7437 (2007).
Fon Tacer, K. et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 24, 2050–2064 (2010).
Barker, S. L. et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol. Dial. Transplant. 30, 223–233 (2015).
Leone, F. et al. Soluble Klotho levels in adult renal transplant recipients are modulated by recombinant human erythropoietin. J. Nephrol. 27, 577–585 (2014).
Ritter, C. S., Zhang, S., Delmez, J., Finch, J. L. & Slatopolsky, E. Differential expression and regulation of Klotho by paricalcitol in the kidney, parathyroid, and aorta of uremic rats. Kidney Int. 87, 1141–1152 (2015).
Lau, W. L. et al. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 82, 1261–1270 (2012).
Moreno, J. A. et al. The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J. Am. Soc. Nephrol. 22, 1315–1325 (2011).
Zhu, H., Gao, Y., Zhu, S., Cui, Q. & Du, J. Klotho improves cardiac function by suppressing reactive oxygen species (ROS) mediated apoptosis by modulating MAPKs/Nrf2 signaling in doxorubicin-induced cardiotoxicity. Med. Sci. Monit. 23, 5283–5293 (2017).
Mitani, H. et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39, 838–843 (2002).
Hu, M. C. et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J. Am. Soc. Nephrol. 26, 1290–1302 (2015).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Corsetti, G. et al. Decreased expression of Klotho in cardiac atria biopsy samples from patients at higher risk of atherosclerotic cardiovascular disease. J. Geriatr. Cardiol. 13, 701–711 (2016).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Couzin, J. Boosting gene extends mouse life span. Science 309, 1310–1311 (2005).
Yamamoto, M. et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 280, 38029–38034 (2005).
Shiraki-Iida, T. et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 424, 6–10 (1998).
Matsumura, Y. et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 242, 626–630 (1998).
Lindberg, K. et al. The kidney is the principal organ mediating klotho effects. J. Am. Soc. Nephrol. 25, 2169–2175 (2014).
Maekawa, Y. et al. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr. Gerontol. Int. 11, 510–516 (2011).
Hui, H. et al. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget 8, 15663–15676 (2017).
Shimamura, Y. et al. Serum levels of soluble secreted alpha-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin. Exp. Nephrol. 16, 722–729 (2012).
Yang, K. et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J. Am. Soc. Nephrol. 26, 2434–2446 (2015).
Xie, J. et al. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat. Commun. 3, 1238 (2012).
Song, S. & Si, L. Y. Klotho ameliorated isoproterenol-induced pathological changes in cardiomyocytes via the regulation of oxidative stress. Life Sci. 135, 118–123 (2015).
Kuwahara, K. et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 116, 3114–3126 (2006).
Wang, Y., Kuro-o, M. & Sun, Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 11, 410–417 (2012).
Fliser, D. et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 53, 1343–1347 (1998).
DeFronzo, R. A. et al. Insulin resistance in uremia. J. Clin. Invest. 67, 563–568 (1981).
Feneberg, R., Schaefer, F. & Veldhuis, J. D. Neuroendocrine adaptations in renal disease. Pediatr. Nephrol. 18, 492–497 (2003).
Walker, B. G., Phear, D. N., Martin, F. I. & Baird, C. W. Inhibition of insulin by acidosis. Lancet 2, 964–965 (1963).
Hotamisligil, G. S. et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271, 665–668 (1996).
Khedr, E. et al. Effect of recombinant human erythropoietin on insulin resistance in hemodialysis patients. Hemodial. Int. 13, 340–346 (2009).
Vaziri, N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 83, 308–315 (2013).
Levin, A. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 71, 31–38 (2007).
Koppe, L. et al. Urea impairs beta cell glycolysis and insulin secretion in chronic kidney disease. J. Clin. Invest. 126, 3598–3612 (2016).
Koppe, L. et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J. Am. Soc. Nephrol. 24, 88–99 (2013).
Xu, H. et al. Clinical correlates of insulin sensitivity and its association with mortality among men with CKD stages 3 and 4. Clin. J. Am. Soc. Nephrol. 9, 690–697 (2014).
Li, Y., Zhang, L., Gu, Y., Hao, C. & Zhu, T. Insulin resistance as a predictor of cardiovascular disease in patients on peritoneal dialysis. Perit. Dial. Int. 33, 411–418 (2013).
Cusi, K. et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105, 311–320 (2000).
Aroor, A. R., Mandavia, C. H. & Sowers, J. R. Insulin resistance and heart failure: molecular mechanisms. Heart Fail. Clin. 8, 609–617 (2012).
Boucher, J., Kleinridders, A. & Kahn, C. R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 6, a009191 (2014).
Matsui, T. & Rosenzweig, A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J. Mol. Cell. Cardiol. 38, 63–71 (2005).
Manning, B. D. & Toker, A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017).
Alessi, D. R. et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 (1996).
Semple, D., Smith, K., Bhandari, S. & Seymour, A. M. Uremic cardiomyopathy and insulin resistance: a critical role for Akt? J. Am. Soc. Nephrol. 22, 207–215 (2011).
McMullen, J. R. et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc. Natl Acad. Sci. USA 100, 12355–12360 (2003).
Samuelsson, A. M. et al. Hyperinsulinemia: effect on cardiac mass/function, angiotensin II receptor expression, and insulin signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 291, H787–H796 (2006).
Cho, H., Thorvaldsen, J. L., Chu, Q., Feng, F. & Birnbaum, M. J. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276, 38349–38352 (2001).
DeBosch, B. et al. Akt1 is required for physiological cardiac growth. Circulation 113, 2097–2104 (2006).
Li, Y. et al. Molecular signaling mediated by angiotensin II type 1A receptor blockade leading to attenuation of renal dysfunction-associated heart failure. J. Card Fail. 13, 155–162 (2007).
Haq, S. et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 103, 670–677 (2001).
Shiojima, I. et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 115, 2108–2118 (2005).
Maillet, M., van Berlo, J. H. & Molkentin, J. D. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 14, 38–48 (2013).
Amann, K. et al. Reduced capillary density in the myocardium of uremic rats—a stereological study. Kidney Int. 42, 1079–1085 (1992).
Amann, K., Breitbach, M., Ritz, E. & Mall, G. Myocyte/capillary mismatch in the heart of uremic patients. J. Am. Soc. Nephrol. 9, 1018–1022 (1998).
Siedlecki, A. M., Jin, X. & Muslin, A. J. Uremic cardiac hypertrophy is reversed by rapamycin but not by lowering of blood pressure. Kidney Int. 75, 800–808 (2009).
Matsui, T. et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J. Biol. Chem. 277, 22896–22901 (2002).
Kataria, A., Trasande, L. & Trachtman, H. The effects of environmental chemicals on renal function. Nat Rev. Nephrol. 11, 610–625 (2015).
Thomas, S. S., Zhang, L. & Mitch, W. E. Molecular mechanisms of insulin resistance in chronic kidney disease. Kidney Int. 88, 1233–1239 (2015).
Borazan, A. & Binici, D. N. Relationship between insulin resistance and inflamation markers in hemodialysis patients. Ren. Fail. 32, 198–202 (2010).
Kursat, S. et al. Relationship of insulin resistance in chronic haemodialysis patients with inflammatory indicators, malnutrition, echocardiographic parameters and 24 hour ambulatory blood pressure monitoring. Scand. J. Urol. Nephrol. 44, 257–264 (2010).
Martins, C. et al. Insulin resistance is associated with circulating fibrinogen levels in nondiabetic patients receiving peritoneal dialysis. J. Ren. Nutr. 17, 132–137 (2007).
Campa, C. C., Ciraolo, E., Ghigo, A., Germena, G. & Hirsch, E. Crossroads of PI3K and Rac pathways. Small GTPases 6, 71–80 (2015).
Trirogoff, M. L., Shintani, A., Himmelfarb, J. & Ikizler, T. A. Body mass index and fat mass are the primary correlates of insulin resistance in nondiabetic stage 3–4 chronic kidney disease patients. Am. J. Clin. Nutr. 86, 1642–1648 (2007).
Mahadev, K. et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 24, 1844–1854 (2004).
Morino, K., Petersen, K. F. & Shulman, G. I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55 (Suppl. 2), 9–15 (2006).
Fujii, H., Goto, S. & Fukagawa, M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins 10, 202 (2018).
Dobre, M., Meyer, T. W. & Hostetter, T. H. Searching for uremic toxins. Clin. J. Am. Soc. Nephrol. 8, 322–327 (2013).
Neirynck, N. et al. An update on uremic toxins. Int. Urol. Nephrol. 45, 139–150 (2013).
Koppe, L. & Fouque, D. Microbiota and prebiotics modulation of uremic toxin generation. Panminerva Med. 59, 173–187 (2017).
Vanholder, R. et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 63, 1934–1943 (2003).
Duranton, F. et al. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 23, 1258–1270 (2012).
Zoccali, C. et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet 358, 2113–2117 (2001).
Tumur, Z. & Niwa, T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am. J. Nephrol. 29, 551–557 (2009).
Huang, C. Y. et al. Effects of pamidronate and calcitriol on the set point of the parathyroid gland in postmenopausal hemodialysis patients with secondary hyperparathyroidism. Nephron Clin. Pract. 122, 93–101 (2012).
Fujii, H. et al. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol. Dial. Transplant. 24, 2089–2095 (2009).
Sibal, L., Agarwal, S. C., Home, P. D. & Boger, R. H. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr. Cardiol. Rev. 6, 82–90 (2010).
Zoccali, C. et al. Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int. 62, 339–345 (2002).
Elesber, A. A. et al. Coronary endothelial dysfunction is associated with erectile dysfunction and elevated asymmetric dimethylarginine in patients with early atherosclerosis. Eur. Heart J. 27, 824–831 (2006).
Wu, I. W. et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 26, 938–947 (2011).
Barreto, F. C. et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 4, 1551–1558 (2009).
Cao, X. S. et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 10, 111–119 (2015).
Wu, C. C. et al. Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J. Am. Soc. Nephrol. 27, 1254–1264 (2016).
Yang, K. et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol. Lett. 234, 110–119 (2015).
Lekawanvijit, S. et al. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLOS ONE 7, e41281 (2012).
Stockler-Pinto, M. B., Fouque, D., Soulage, C. O., Croze, M. & Mafra, D. Indoxyl sulfate and p-cresyl sulfate in chronic kidney disease. Could these toxins modulate the antioxidant Nrf2-Keap1 pathway? J. Ren. Nutr. 24, 286–291 (2014).
Bolati, D., Shimizu, H., Yisireyili, M., Nishijima, F. & Niwa, T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-kappaB. BMC Nephrol. 14, 56 (2013).
Chin, L. H. et al. The regulation of NLRP3 inflammasome expression during the development of cardiac contractile dysfunction in chronic kidney disease. Oncotarget 8, 113303–113317 (2017).
Vilaysane, A. et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 21, 1732–1744 (2010).
Muteliefu, G., Enomoto, A. & Niwa, T. Indoxyl sulfate promotes proliferation of human aortic smooth muscle cells by inducing oxidative stress. J. Ren. Nutr. 19, 29–32 (2009).
Yamamoto, H. et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 69, 1780–1785 (2006).
Bartlett, D. E. et al. Uremic toxins activates Na/K-ATPase oxidant amplification loop causing phenotypic changes in adipocytes in in vitro models. Int. J. Mol. Sci. 19, E2685 (2018).
Zhao, L. et al. Deletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunction. Circ. Res. 118, 1918–1929 (2016).
Sriramula, S. & Francis, J. Tumor necrosis factor-alpha is essential for angiotensin II-induced ventricular remodeling: role for oxidative stress. PLOS ONE 10, e0138372 (2015).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 (2004).
Ramos, L. F., Shintani, A., Ikizler, T. A. & Himmelfarb, J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J. Am. Soc. Nephrol. 19, 593–599 (2008).
Viaene, L. et al. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm. Drug Dispos. 34, 165–175 (2013).
Meijers, B. K. et al. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 73, 1174–1180 (2008).
Han, H. et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J. Am. Heart Assoc. 4, e001852 (2015).
Manunta, P. et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension 34, 450–456 (1999).
Kennedy, D. J. et al. Elevated plasma marinobufagenin, an endogenous cardiotonic steroid, is associated with right ventricular dysfunction and nitrative stress in heart failure. Circ. Heart Fail. 8, 1068–1076 (2015).
Komiyama, Y. et al. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 38, 36–45 (2005).
Bagrov, A. Y. et al. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension 31, 1097–1103 (1998).
Kolmakova, E. V. et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrol. Dial. Transplant. 26, 2912–2919 (2011).
Hamlyn, J. M. & Manunta, P. Endogenous cardiotonic steroids in kidney failure: a review and an hypothesis. Adv. Chron. Kidney Dis. 22, 232–244 (2015).
Haller, S. T. et al. Monoclonal antibody against marinobufagenin reverses cardiac fibrosis in rats with chronic renal failure. Am. J. Hypertens. 25, 690–696 (2012).
Tian, J. et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension 54, 1313–1320 (2009).
Haller, S. T. et al. Rapamycin attenuates cardiac fibrosis in experimental uremic cardiomyopathy by reducing marinobufagenin levels and inhibiting downstream pro-fibrotic signaling. J. Am. Heart Assoc. 5, e004106 (2016).
Haller, S. T. et al. Passive immunization against marinobufagenin attenuates renal fibrosis and improves renal function in experimental renal disease. Am. J. Hypertens. 27, 603–609 (2014).
Arnon, A., Hamlyn, J. M. & Blaustein, M. P. Ouabain augments Ca(2+) transients in arterial smooth muscle without raising cytosolic Na(+). Am. J. Physiol. Heart Circ. Physiol. 279, H679–H691 (2000).
Skou, J. C. The identification of the sodium pump. Biosci. Rep. 24, 436–451 (2004).
Tian, J. et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell 17, 317–326 (2006).
Haas, M., Wang, H., Tian, J. & Xie, Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 277, 18694–18702 (2002).
Liu, J. et al. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 66, 227–241 (2004).
Tian, J., Gong, X. & Xie, Z. Signal-transducing function of Na+-K+-ATPase is essential for ouabain’s effect on [Ca2+]i in rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 281, H1899–H1907 (2001).
Wansapura, A. N., Lasko, V. M., Lingrel, J. B. & Lorenz, J. N. Mice expressing ouabain-sensitive alpha1-Na, K-ATPase have increased susceptibility to pressure overload-induced cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 300, H347–H355 (2011).
Drummond, C. A. et al. Reduction of Na/K-ATPase affects cardiac remodeling and increases c-kit cell abundance in partial nephrectomized mice. Am. J. Physiol. Heart Circ. Physiol. 306, H1631–H1643 (2014).
Xie, Z. et al. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J. Biol. Chem. 274, 19323–19328 (1999).
Liu, J. et al. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 275, 27838–27844 (2000).
Yan, Y. et al. Protein carbonylation of an amino acid residue of the Na/K-ATPase alpha1 subunit determines Na/K-ATPase signaling and sodium transport in renal proximal tubular cells. J. Am. Heart Assoc. 5, e003675 (2016).
Liu, J. et al. Attenuation of Na/K-ATPase mediated oxidant amplification with pNaKtide ameliorates experimental uremic cardiomyopathy. Sci. Rep. 6, 34592 (2016).
Yan, Y. et al. Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J. Biol. Chem. 288, 34249–34258 (2013).
Chen, Y. et al. Oxidized LDL-bound CD36 recruits an Na+/K+-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci. Signal. 8, ra91 (2015).
Kennedy, D. J. et al. CD36 and Na/K-ATPase-alpha1 form a proinflammatory signaling loop in kidney. Hypertension 61, 216–224 (2013).
Mhatre, K. N. et al. Crosstalk between FGF23- and angiotensin II-mediated Ca(2+) signaling in pathological cardiac hypertrophy. Cell. Mol. Life Sci. 75, 4403–4416 (2018).
Raeisi, S. et al. Effects of angiotensin II receptor blockade on soluble Klotho and oxidative stress in calcineurin inhibitor nephrotoxicity in rats. Iran. J. Kidney Dis. 10, 358–363 (2016).
Karalliedde, J., Maltese, G., Hill, B., Viberti, G. & Gnudi, L. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin. J. Am. Soc. Nephrol. 8, 1899–1905 (2013).
Shimizu, H. et al. Indoxyl sulfate enhances angiotensin II signaling through upregulation of epidermal growth factor receptor expression in vascular smooth muscle cells. Life Sci. 91, 172–177 (2012).
Lin, C. J. et al. Association of indoxyl sulfate with fibroblast growth factor 23 in patients with advanced chronic kidney disease. Am. J. Med. Sci. 347, 370–376 (2014).
Taylor, D., Bhandari, S. & Seymour, A. M. Mitochondrial dysfunction in uremic cardiomyopathy. Am. J. Physiol. Renal Physiol. 308, F579–F587 (2015).
Burgoyne, J. R., Mongue-Din, H., Eaton, P. & Shah, A. M. Redox signaling in cardiac physiology and pathology. Circ. Res. 111, 1091–1106 (2012).
Annuk, M., Zilmer, M., Lind, L., Linde, T. & Fellstrom, B. Oxidative stress and endothelial function in chronic renal failure. J. Am. Soc. Nephrol. 12, 2747–2752 (2001).
Ruggenenti, P., Cravedi, P. & Remuzzi, G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 23, 1917–1928 (2012).
Balamuthusamy, S. et al. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. Am. Heart J. 155, 791–805 (2008).
Perkovic, V. et al. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the PROGRESS study. J. Am. Soc. Nephrol. 18, 2766–2772 (2007).
Mann, J. F., Gerstein, H. C., Pogue, J., Bosch, J. & Yusuf, S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann. Intern. Med. 134, 629–636 (2001).
Zannad, F. et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 70, 1318–1324 (2006).
Marquez, D. F., Ruiz-Hurtado, G., Ruilope, L. M. & Segura, J. An update of the blockade of the renin angiotensin aldosterone system in clinical practice. Expert Opin. Pharmacother. 16, 2283–2292 (2015).
Juurlink, D. N. et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N. Engl. J. Med. 351, 543–551 (2004).
Frankenfield, D. L. et al. Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare Part D. Am. J. Kidney Dis. 59, 670–681 (2012).
Cice, G. et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J. Am. Coll. Cardiol. 41, 1438–1444 (2003).
Cohen-Solal, A. et al. Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur. J. Heart Fail. 11, 872–880 (2009).
Badve, S. V. et al. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 58, 1152–1161 (2011).
Agarwal, R., Sinha, A. D., Pappas, M. K., Abraham, T. N. & Tegegne, G. G. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol. Dial. Transplant. 29, 672–681 (2014).
Kitchlu, A. et al. Beta-blockers and cardiovascular outcomes in dialysis patients: a cohort study in Ontario, Canada. Nephrol. Dial. Transplant. 27, 1591–1598 (2012).
Koizumi, M., Komaba, H., Nakanishi, S., Fujimori, A. & Fukagawa, M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol. Dial. Transplant. 27, 784–790 (2012).
Greeviroj, P. et al. Cinacalcet for treatment of chronic kidney disease-mineral and bone disorder: a meta-analysis of randomized controlled trials. Nephron 139, 197–210 (2018).
Brunelli, S. M., Thadhani, R., Ikizler, T. A. & Feldman, H. I. Thiazolidinedione use is associated with better survival in hemodialysis patients with non-insulin dependent diabetes. Kidney Int. 75, 961–968 (2009).
Ramirez, S. P. et al. Rosiglitazone is associated with mortality in chronic hemodialysis patients. J. Am. Soc. Nephrol. 20, 1094–1101 (2009).
Hatakeyama, S. et al. Effect of an oral adsorbent, AST-120, on dialysis initiation and survival in patients with chronic kidney disease. Int. J. Nephrol. 2012, 376128 (2012).
Schulman, G. et al. Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J. Am. Soc. Nephrol. 26, 1732–1746 (2015).
Drechsler, C. et al. Protein carbamylation is associated with heart failure and mortality in diabetic patients with end-stage renal disease. Kidney Int. 87, 1201–1208 (2015).
Drozdz, D. et al. Oxidative stress biomarkers and left ventricular hypertrophy in children with chronic kidney disease. Oxid. Med. Cell. Longev. 2016, 7520231 (2016).
Himmelfarb, J. et al. Provision of antioxidant therapy in hemodialysis (PATH): a randomized clinical trial. J. Am. Soc. Nephrol. 25, 623–633 (2014).
Bolignano, D. et al. Antioxidant agents for delaying diabetic kidney disease progression: a systematic review and meta-analysis. PLOS ONE 12, e0178699 (2017).
Cheitlin, M. D. et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. J. Am. Soc. Echocardiogr. 16, 1091–1110 (2003).
Marwick, T. H. et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J. Am. Soc. Echocardiogr. 28, 727–754 (2015).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1–39 (2015).
Khouri, S. J., Maly, G. T., Suh, D. D. & Walsh, T. E. A practical approach to the echocardiographic evaluation of diastolic function. J. Am. Soc. Echocardiogr. 17, 290–297 (2004).
Sodhi, K. et al. pNaKtide attenuates steatohepatitis and atherosclerosis by blocking Na/K-ATPase/ROS amplification in C57Bl6 and ApoE knockout mice fed a Western diet. Sci. Rep. 7, 193 (2017).
Sodhi, K. et al. pNaKtide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis. Sci. Adv. 1, e1500781 (2015).
Acknowledgements
The authors’ work was supported by US National Institutes of Health grants HL109015, HL071556 and HL105649; by the Brickstreet Foundation; and by the Huntington Foundation, Inc.
Reviewer information
Nature Reviews Nephrology thanks C. Faul, J. Jankowski and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched the data discussed in this article, discussed the content, wrote the article and reviewed or edited the text before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Glossary
- Endogenous cardiotonic steroids
-
A class of steroid hormones with important roles in health and disease. Endogenous cardiotonic steroids such as cardenolide and bufadienolide signal through the Na+/K+-ATPase.
- Pressure overload
-
Refers to the pathological state of cardiac muscle in which it has to contract against excessive pressure.
- Volume overload
-
Refers to the pathological state of the heart in which an abnormally large volume of blood must be pumped.
- Hypervolaemia
-
Also known as fluid overload, hypervolaemia is a pathological condition in which there is too much fluid in the blood. Hypervolaemia is common in the setting of renal failure.
- Aortocaval fistula
-
A surgically created arteriovenous fistula between the abdominal aorta and inferior vena cava, distal to the origin of the renal arteries. Aortocaval fistula is used as an experimental model of volume overload.
- Secondary hyperparathyroidism
-
Refers to excessive secretion of parathyroid hormone by the parathyroid gland in response to low serum calcium level and high phosphorus level in the setting of renal failure.
- Inflammasomes
-
A multiprotein intracellular complex that detects pathogenic microorganisms and activates inflammatory responses via the activation of pro-inflammatory cytokines such as IL-1β and IL-18.
Rights and permissions
About this article
Cite this article
Wang, X., Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol 15, 159–175 (2019). https://doi.org/10.1038/s41581-018-0101-8
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0101-8
This article is cited by
-
Factors associated with changes in echocardiographic parameters following kidney transplantation
Clinical Research in Cardiology (2024)
-
Factors to consider during anesthesia in patients undergoing preemptive kidney transplantation: a propensity-score matched analysis
BMC Anesthesiology (2023)
-
Structural basis for FGF hormone signalling
Nature (2023)
-
Hypertension and cardiomyopathy associated with chronic kidney disease: epidemiology, pathogenesis and treatment considerations
Journal of Human Hypertension (2023)
-
Implications of uremic cardiomyopathy for the practicing clinician: an educational review
Heart Failure Reviews (2023)