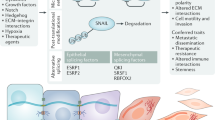
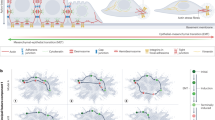
Abstract
Epithelial–mesenchymal transitions (EMTs) are the epitome of cell plasticity in embryonic development and cancer; during EMT, epithelial cells undergo dramatic phenotypic changes and become able to migrate to form different tissues or give rise to metastases, respectively. The importance of EMTs in other contexts, such as tissue repair and fibrosis in the adult, has become increasingly recognized and studied. In this Review, we discuss the function of EMT in the adult after tissue damage and compare features of embryonic and adult EMT. Whereas sustained EMT leads to adult tissue degeneration, fibrosis and organ failure, its transient activation, which confers phenotypic and functional plasticity on somatic cells, promotes tissue repair after damage. Understanding the mechanisms and temporal regulation of different EMTs provides insight into how some tissues heal and has the potential to open new therapeutic avenues to promote repair or regeneration of tissue damage that is currently irreversible. We also discuss therapeutic strategies that modulate EMT that hold clinical promise in ameliorating fibrosis, and how precise EMT activation could be harnessed to enhance tissue repair.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nieto, M. A., Huang, R. Y.-J., Jackson, R. A. & Thiery, J. P. EMT: 2016. Cell 166, 21–45 (2016).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 (2019).
Stone, R. C. et al. Epithelial–mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 365, 495–506 (2016).
Yang, J. et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Pastushenko, I. & Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 29, 212–226 (2019).
Neumann, D. P., Goodall, G. J. & Gregory, P. A. Regulation of splicing and circularisation of RNA in epithelial mesenchymal plasticity. Semin. Cell Dev. Biol. 75, 50–60 (2018).
Skrypek, N., Goossens, S., De Smedt, E., Vandamme, N. & Berx, G. Epithelial-to-mesenchymal transition: epigenetic reprogramming driving cellular plasticity. Trends Genet. 33, 943–959 (2017).
Sample, R. A., Nogueira, M. F., Mitra, R. D. & Puram, S. V. Epigenetic regulation of hybrid epithelial–mesenchymal cell states in cancer. Oncogene 42, 2237–2248 (2023).
Morin, C., Moyret-Lalle, C., Mertani, H. C., Diaz, J.-J. & Marcel, V. Heterogeneity and dynamic of EMT through the plasticity of ribosome and mRNA translation. Biochim. Biophys. Acta Rev. Cancer 1877, 188718 (2022).
Cano, A. et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000).
Batlle, E. et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84–89 (2000).
Stanisavljevic, J., Porta-de-la-Riva, M., Batlle, R., de Herreros, A. G. & Baulida, J. The p65 subunit of NF-κB and PARP1 assist Snail1 in activating fibronectin transcription. J. Cell Sci. 124, 4161–4171 (2011).
Hsu, D. S.-S. et al. Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell 26, 534–548 (2014).
Aghdassi, A. et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 61, 439–448 (2012).
Lehmann, W. et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 7, 10498 (2016).
Feldker, N. et al. Genome-wide cooperation of EMT transcription factor ZEB1 with YAP and AP-1 in breast cancer. EMBO J. 39, e103209 (2020).
Leptin, M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5, 1568–1576 (1991).
Chen, Z. F. & Behringer, R. R. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 9, 686–699 (1995).
Ocaña, O. H. et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 (2012).
Yeo, S.-Y. et al. A positive feedback loop bi-stably activates fibroblasts. Nat. Commun. 9, 3016 (2018).
Nieto, M. A. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342, 1234850 (2013).
Probst, S. et al. Spatiotemporal sequence of mesoderm and endoderm lineage segregation during mouse gastrulation. Development 148, dev193789 (2021).
Burtscher, I. & Lickert, H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 136, 1029–1038 (2009).
Nowotschin, S., Hadjantonakis, A.-K. & Campbell, K. The endoderm: a divergent cell lineage with many commonalities. Development 146, dev150920 (2019).
Scheibner, K. et al. Epithelial cell plasticity drives endoderm formation during gastrulation. Nat. Cell Biol. 23, 692–703 (2021).
Jägle, S. et al. SNAIL1-mediated downregulation of FOXA proteins facilitates the inactivation of transcriptional enhancer elements at key epithelial genes in colorectal cancer cells. PLoS Genet. 13, e1007109 (2017).
Piacentino, M. L., Li, Y. & Bronner, M. E. Epithelial-to-mesenchymal transition and different migration strategies as viewed from the neural crest. Curr. Opin. Cell Biol. 66, 43–50 (2020).
Li, Y. et al. In vivo quantitative imaging provides insights into trunk neural crest migration. Cell Rep. 26, 1489–1500.e3 (2019).
E Davies, J. et al. Epithelial-mesenchymal transition during extravillous trophoblast differentiation. Cell Adh. Migr. 10, 310–321 (2016).
Turco, M. Y. & Moffett, A. Development of the human placenta. Development 146, dev163428 (2019).
DaSilva-Arnold, S., James, J. L., Al-Khan, A., Zamudio, S. & Illsley, N. P. Differentiation of first trimester cytotrophoblast to extravillous trophoblast involves an epithelial-mesenchymal transition. Placenta 36, 1412–1418 (2015).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353 (2018).
Greenbaum, S. et al. A spatially resolved timeline of the human maternal–fetal interface. Nature 619, 595–605 (2023).
Kudo-Saito, C., Shirako, H., Takeuchi, T. & Kawakami, Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15, 195–206 (2009).
Shibue, T. & Weinberg, R. A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629 (2017).
Du, L. et al. Mesenchymal-to-epithelial transition in the placental tissues of patients with preeclampsia. Hypertens. Res. 40, 67–72 (2017).
Harmon, A. C. et al. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 130, 409–419 (2016).
Simões-Costa, M. & Bronner, M. E. Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257 (2015).
Baggiolini, A. et al. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell 16, 314–322 (2015).
Tang, W., Li, Y., Li, A. & Bronner, M. E. Clonal analysis and dynamic imaging identify multipotency of individual Gallus gallus caudal hindbrain neural crest cells toward cardiac and enteric fates. Nat. Commun. 12, 1894 (2021).
Buitrago-Delgado, E., Nordin, K., Rao, A., Geary, L. & LaBonne, C. NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science 348, 1332–1335 (2015).
Zalc, A. et al. Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science 371, eabb4776 (2021).
Soldatov, R. et al. Spatiotemporal structure of cell fate decisions in murine neural crest. Science 364, eaas9536 (2019).
Fan, X. et al. TWIST1 and chromatin regulatory proteins interact to guide neural crest cell differentiation. eLife 10, e62873 (2021).
Acloque, H. et al. Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev. Cell 21, 546–558 (2011).
Thiery, J. P., Acloque, H., Huang, R. Y. J. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Kovacic, J. C., Mercader, N., Torres, M., Boehm, M. & Fuster, V. Epithelial- and endothelial- to mesenchymal transition: from cardiovascular development to disease. Circulation 125, 1795–1808 (2012).
Nakajima, Y., Yamagishi, T., Hokari, S. & Nakamura, H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat. Rec. 258, 119–127 (2000).
Haensel, D. & Dai, X. Epithelial-to-mesenchymal transition in cutaneous wound healing: where we are and where we are heading. Dev. Dyn. 247, 473–480 (2018).
Savagner, P. et al. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J. Cell. Physiol. 202, 858–866 (2005).
Hudson, L. G. et al. Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2). J. Dermatol. Sci. 56, 19–26 (2009).
Arnoux, V., Nassour, M., L’Helgoualc’h, A., Hipskind, R. A. & Savagner, P. Erk5 controls Slug expression and keratinocyte activation during wound healing. Mol. Biol. Cell 19, 4738–4749 (2008).
Shaw, T. J. & Martin, P. Wound repair: a showcase for cell plasticity and migration. Curr. Opin. Cell Biol. 42, 29–37 (2016).
Nunan, R. et al. Ephrin-Bs drive junctional downregulation and actin stress fiber disassembly to enable wound re-epithelialization. Cell Rep. 13, 1380–1395 (2015).
Vega, S. et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 18, 1131–1143 (2004).
Shimizu, H. et al. Senescence and dysfunction of proximal tubular cells are associated with activated p53 expression by indoxyl sulfate. Am. J. Physiol. Cell Physiol. 299, C1110–C1117 (2010).
Liu, L. et al. p53 upregulated by HIF-1α promotes hypoxia-induced G2/M arrest and renal fibrosis in vitro and in vivo. J. Mol. Cell Biol. 11, 371–382 (2019).
Tang, C. et al. p53 in kidney injury and repair: mechanism and therapeutic potentials. Pharmacol. Ther. 195, 5–12 (2019).
Yao, C. et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 203, 707–717 (2021).
Wu, W.-S. et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing Puma. Cell 123, 641–653 (2005).
Rodríguez-Aznar, E. & Nieto, M. A. Repression of puma by scratch2 is required for neuronal survival during embryonic development. Cell Death Differ. 18, 1196–1207 (2011).
Kurrey, N. K. et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 27, 2059–2068 (2009).
Escrivà, M. et al. Repression of PTEN phosphatase by snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol. Cell. Biol. 28, 1528–1540 (2008).
Calcinotto, A. et al. Cellular senescence: aging, cancer, and injury. Physiol. Rev. 99, 1047–1078 (2019).
Ansieau, S. et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14, 79–89 (2008).
Cakouros, D. et al. Twist-1 induces Ezh2 recruitment regulating histone methylation along the Ink4A/Arf locus in mesenchymal stem cells. Mol. Cell. Biol. 32, 1433–1441 (2012).
Lee, S.-H. et al. Blocking of p53-Snail binding, promoted by oncogenic K-Ras, recovers p53 expression and function. Neoplasia 11, 22–31 (2009).
Siemens, H. et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial–mesenchymal transitions. Cell Cycle 10, 4256–4271 (2011).
Shetty, S. K. et al. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am. J. Pathol. 187, 1016–1034 (2017).
Kim, J., Lee, J., Kim, U., Park, J.-K. & Um, H.-D. Slug promotes p53 and p21 protein degradation by inducing Mdm2 expression in HCT116 colon cancer cells. Oncol. Lett. 22, 681 (2021).
Zhu, P. et al. The transcription factor Slug represses p16Ink4a and regulates murine muscle stem cell aging. Nat. Commun. 10, 2568 (2019).
Poss, K. D. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11, 710–722 (2010).
Jopling, C., Boue, S. & Izpisua Belmonte, J. C. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat. Rev. Mol. Cell Biol. 12, 79–89 (2011).
Merrell, A. J. & Stanger, B. Z. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 17, 413–425 (2016).
Peter, M. E. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle 8, 843–852 (2009).
Boyerinas, B., Park, S.-M., Hau, A., Murmann, A. E. & Peter, M. E. The role of let-7 in cell differentiation and cancer. Endocr. Relat. Cancer 17, F19–F36 (2010).
Cimadamore, F., Amador-Arjona, A., Chen, C., Huang, C.-T. & Terskikh, A. V. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc. Natl Acad. Sci. USA 110, E3017–E3026 (2013).
Chua, H. L. et al. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26, 711–724 (2007).
Díaz-López, A., Moreno-Bueno, G. & Cano, A. Role of microRNA in epithelial to mesenchymal transition and metastasis and clinical perspectives. Cancer Manag. Res. 6, 205–216 (2014).
Gill, J. G. et al. Snail and the microRNA-200 family act in opposition to regulate epithelial-to-mesenchymal transition and germ layer fate restriction in differentiating ESCs. Stem Cells 29, 764–776 (2011).
Brabletz, S. & Brabletz, T. The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep. 11, 670–677 (2010).
Zander, M. A., Cancino, G. I., Gridley, T., Kaplan, D. R. & Miller, F. D. The Snail transcription factor regulates the numbers of neural precursor cells and newborn neurons throughout mammalian life. PLoS ONE 9, e104767 (2014).
Gupta, B. et al. The transcription factor ZEB1 regulates stem cell self-renewal and cell fate in the adult hippocampus. Cell Rep. 36, 109588 (2021).
Shimozaki, K., Clemenson, G. D. & Gage, F. H. Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. J. Neurosci. 33, 4066–4075 (2013).
García-Arrarás, J. E. et al. Cell dedifferentiation and epithelial to mesenchymal transitions during intestinal regeneration in H. glaberrima. BMC Dev. Biol. 11, 61 (2011).
Tanaka, E. M. & Reddien, P. W. The cellular basis for animal regeneration. Dev. Cell 21, 172–185 (2011).
Tanaka, E. M. The molecular and cellular choreography of appendage regeneration. Cell 165, 1598–1608 (2016).
Joven, A., Elewa, A. & Simon, A. Model systems for regeneration: salamanders. Development 146, dev167700 (2019).
Gerber, T. et al. Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science 362, eaaq0681 (2018).
Lin, T.-Y. et al. Fibroblast dedifferentiation as a determinant of successful regeneration. Dev. Cell 56, 1541–1551.e6 (2021).
Kusaba, T., Lalli, M., Kramann, R., Kobayashi, A. & Humphreys, B. D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl Acad. Sci. USA 111, 1527–1532 (2014).
Berger, K. et al. Origin of regenerating tubular cells after acute kidney injury. Proc. Natl Acad. Sci. USA 111, 1533–1538 (2014).
Chang-Panesso, M. et al. FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J. Clin. Invest. 129, 5501–5517 (2019).
Yu, C.-P. et al. FoxM1 promotes epithelial-mesenchymal transition of hepatocellular carcinoma by targeting Snai1. Mol. Med. Rep. 16, 5181–5188 (2017).
Wang, X., Kiyokawa, H., Dennewitz, M. B. & Costa, R. H. The forkhead box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc. Natl Acad. Sci. USA 99, 16881–16886 (2002).
Grande, M. T. et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 21, 989–997 (2015).
Oh, S.-H., Swiderska-Syn, M., Jewell, M. L., Premont, R. T. & Diehl, A. M. Liver regeneration requires Yap1-TGFβ-dependent epithelial–mesenchymal transition in hepatocytes. J. Hepatol. 69, 359–367 (2018).
Volckaert, T. et al. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Invest. 121, 4409–4419 (2011).
Chen, P., Piao, X. & Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 130, 605–618 (2015).
Gaudet, A. D., Popovich, P. G. & Ramer, M. S. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 8, 110 (2011).
Cattin, A.-L. et al. Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 162, 1127–1139 (2015).
Parfejevs, V., Antunes, A. T. & Sommer, L. Injury and stress responses of adult neural crest-derived cells. Dev. Biol. 444, S356–S365 (2018).
Jessen, K. R. & Mirsky, R. The success and failure of the schwann cell response to nerve injury. Front. Cell. Neurosci. 13, 33 (2019).
Clements, M. P. et al. The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron 96, 98–114.e7 (2017).
Parfejevs, V. et al. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 9, 236 (2018).
Jessen, K. R. & Arthur-Farraj, P. Repair Schwann cell update: adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 67, 421–437 (2019).
Johnston, A. P. W. et al. Dedifferentiated Schwann cell precursors secreting paracrine factors are required for regeneration of the mammalian digit tip. Cell Stem Cell 19, 433–448 (2016).
Klatt Shaw, D. et al. Localized EMT reprograms glial progenitors to promote spinal cord repair. Dev. Cell 56, 613–626.e7 (2021).
Mokalled, M. H. et al. Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 354, 630–634 (2016).
Tang, W. J., Watson, C. J., Olmstead, T., Allan, C. H. & Kwon, R. Y. Single-cell resolution of MET- and EMT-like programs in osteoblasts during zebrafish fin regeneration. iScience 25, 103784 (2022).
Chen, F. et al. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 26, 27–33.e4 (2020).
Katsuno, Y. et al. Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci. Signal. 12, eaau8544 (2019).
Takeichi, M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 15, 397–410 (2014).
Ferguson, M. W. J. & O’Kane, S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Phil. Trans. R. Soc. Lond. B 359, 839–850 (2004).
Larson, B. J., Longaker, M. T. & Lorenz, H. P. Scarless fetal wound healing: a basic science review. Plast. Reconstr. Surg. 126, 1172–1180 (2010).
Darby, I. A., Laverdet, B., Bonté, F. & Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 7, 301–311 (2014).
Estes, J. M. et al. Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differ. Res. Biol. Divers. 56, 173–181 (1994).
Cass, D. L. et al. Wound size and gestational age modulate scar formation in fetal wound repair. J. Pediatr. Surg. 32, 411–415 (1997).
Walmsley, G. G. et al. Scarless wound healing: chasing the holy grail. Plast. Reconstr. Surg. 135, 907–917 (2015).
Moore, A. L. et al. Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip. Rev. Dev. Biol. 7, wdev.309 (2018).
Xue, M. & Jackson, C. J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 4, 119–136 (2015).
Longaker, M. T. et al. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann. Surg. 213, 292–296 (1991).
West, D. C., Shaw, D. M., Lorenz, P., Adzick, N. S. & Longaker, M. T. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int. J. Biochem. Cell Biol. 29, 201–210 (1997).
Beanes, S. R. et al. Confocal microscopic analysis of scarless repair in the fetal rat: defining the transition. Plast. Reconstr. Surg. 109, 160–170 (2002).
Koudouna, E. et al. Recapitulation of normal collagen architecture in embryonic wounded corneas. Sci. Rep. 10, 13815 (2020).
Dang, C. M. et al. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast. Reconstr. Surg. 111, 2273–2285 (2003).
Hinz, B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 47, 54–65 (2015).
Wei, S. C. & Yang, J. Forcing through tumor metastasis: the interplay between tissue rigidity and epithelial-mesenchymal transition. Trends Cell Biol. 26, 111–120 (2016).
Rice, A. J. et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6, e352 (2017).
Cowin, A. J., Brosnan, M. P., Holmes, T. M. & Ferguson, M. W. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev. Dyn. 212, 385–393 (1998).
Liechty, K. W., Kim, H. B., Adzick, N. S. & Crombleholme, T. M. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J. Pediatr. Surg. 35, 866–872 (2000).
Liechty, K. W., Adzick, N. S. & Crombleholme, T. M. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine 12, 671–676 (2000).
Occleston, N. L., Laverty, H. G., O’Kane, S. & Ferguson, M. W. J. Prevention and reduction of scarring in the skin by transforming growth factor beta 3 (TGFβ3): from laboratory discovery to clinical pharmaceutical. J. Biomater. Sci. Polym. Ed. 19, 1047–1063 (2008).
Gordon, A. et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 16, 70–79 (2008).
Peranteau, W. H. et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J. Invest. Dermatol. 128, 1852–1860 (2008).
Meng, X.-M., Nikolic-Paterson, D. J. & Lan, H. Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 10, 493–503 (2014).
López-Novoa, J. M. & Nieto, M. A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 1, 303–314 (2009).
Warburg, O., Wind, F. & Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 (1927).
Burns, J. S. & Manda, G. Metabolic pathways of the warburg effect in health and disease: perspectives of choice, chain or chance. Int. J. Mol. Sci. 18, 2755 (2017).
Chen, Z., Liu, M., Li, L. & Chen, L. Involvement of the Warburg effect in non-tumor diseases processes. J. Cell. Physiol. 233, 2839–2849 (2018).
Youssef, K. K. & Nieto, M. A. Glucose metabolism takes center stage in epithelial-mesenchymal plasticity. Dev. Cell 53, 133–135 (2020).
Bhattacharya, D., Azambuja, A. P. & Simoes-Costa, M. Metabolic reprogramming promotes neural crest migration via Yap/Tead signaling. Dev. Cell 53, 199–211.e6 (2020).
Oginuma, M. et al. A gradient of glycolytic activity coordinates fgf and wnt signaling during elongation of the body axis in amniote embryos. Dev. Cell 40, 342–353.e10 (2017).
Bulusu, V. et al. Spatiotemporal analysis of a glycolytic activity gradient linked to mouse embryo mesoderm development. Dev. Cell 40, 331–341.e4 (2017).
Luengo, A. et al. Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol. Cell 81, 691–707.e6 (2021).
Enzo, E. et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 34, 1349–1370 (2015).
Haensel, D. et al. Defining epidermal basal cell states during skin homeostasis and wound healing using single-cell transcriptomics. Cell Rep. 30, 3932–3947.e6 (2020).
Scott, C. A., Carney, T. J. & Amaya, E. Aerobic glycolysis is important for zebrafish larval wound closure and tail regeneration. Wound Repair Regen. 30, 665–680 (2022).
Sinclair, J. W. et al. The Warburg effect is necessary to promote glycosylation in the blastema during zebrafish tail regeneration. NPJ Regen. Med. 6, 55 (2021).
Fukuda, R. et al. Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish. EMBO Rep. 21, e49752 (2020).
Honkoop, H. et al. Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. eLife 8, e50163 (2019).
Dong, C. et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 23, 316–331 (2013).
Liu, M., Quek, L.-E., Sultani, G. & Turner, N. Epithelial-mesenchymal transition induction is associated with augmented glucose uptake and lactate production in pancreatic ductal adenocarcinoma. Cancer Metab. 4, 19 (2016).
Krebs, A. M. et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518–529 (2017).
Liu, Y. et al. Insulin/Snail1 axis ameliorates fatty liver disease by epigenetically suppressing lipogenesis. Nat. Commun. 9, 2751 (2018).
Liu, Y. et al. Hepatic Slug epigenetically promotes liver lipogenesis, fatty liver disease, and type 2 diabetes. J. Clin. Invest. 130, 2992–3004 (2020).
Brabletz, S., Schuhwerk, H., Brabletz, T. & Stemmler, M. P. Dynamic EMT: a multi-tool for tumor progression. EMBO J. 40, e108647 (2021).
Kalluri, R. & Weinberg, R. A. The basics of epithelial–mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Wang, J., Liu, S., Heallen, T. & Martin, J. F. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 15, 672–684 (2018).
Panciera, T. et al. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19, 725–737 (2016).
Moya, I. M. & Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Patel, S. H., Camargo, F. D. & Yimlamai, D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology 152, 533–545 (2017).
Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618 (2014).
Noce, V. et al. YAP integrates the regulatory Snail/HNF4α circuitry controlling epithelial/hepatocyte differentiation. Cell Death Dis. 10, 768 (2019).
Xu, J. et al. Involvement of the Hippo pathway in regeneration and fibrogenesis after ischaemic acute kidney injury: YAP is the key effector. Clin. Sci. 130, 349–363 (2016).
McNeill, H. & Reginensi, A. Lats1/2 regulate Yap/Taz to control nephron progenitor epithelialization and inhibit myofibroblast formation. J. Am. Soc. Nephrol. 28, 852–861 (2017).
Rockey, D. C., Bell, P. D. & Hill, J. A. Fibrosis–a common pathway to organ injury and failure. N. Engl. J. Med. 372, 1138–1149 (2015).
Rock, J. R. et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl Acad. Sci. USA 108, E1475–E1483 (2011).
Zepp, J. A. et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170, 1134–1148.e10 (2017).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Chu, A. S. et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53, 1685–1695 (2011).
Yang, W. et al. Single-cell transcriptomic analysis reveals a hepatic stellate cell-activation roadmap and myofibroblast origin during liver fibrosis in mice. Hepatology 74, 2774–2790 (2021).
Boutet, A. et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 25, 5603–5613 (2006).
Lovisa, S. et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 21, 998–1009 (2015).
Borges, F. T. et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 24, 385–392 (2013).
Szeto, S. G. et al. YAP/TAZ are mechanoregulators of TGF-β-smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. 27, 3117–3128 (2016).
Seo, E. et al. The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci. Rep. 6, 31931 (2016).
Anorga, S. et al. Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype. FASEB J. 32, 2644–2657 (2018).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Qi, R. et al. Snai1-induced partial epithelial-mesenchymal transition orchestrates p53-p21-mediated G2/M arrest in the progression of renal fibrosis via NF-κB-mediated inflammation. Cell Death Dis. 12, 44 (2021).
Mederacke, I. et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 4, 2823 (2013).
Rowe, R. G. et al. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol. Cell. Biol. 31, 2392–2403 (2011).
Mooring, M. et al. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis. Hepatology 71, 1813–1830 (2020).
Sabin, K. Z., Jiang, P., Gearhart, M. D., Stewart, R. & Echeverri, K. AP-1cFos/JunB/miR-200a regulate the pro-regenerative glial cell response during axolotl spinal cord regeneration. Commun. Biol. 2, 91 (2019).
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Solanas, G. et al. E-cadherin controls beta-catenin and NF-kappaB transcriptional activity in mesenchymal gene expression. J. Cell Sci. 121, 2224–2234 (2008).
Peiró, S. et al. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 34, 2077–2084 (2006).
Duffield, J. S., Lupher, M., Thannickal, V. J. & Wynn, T. A. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 8, 241–276 (2013).
Wei, S. C. et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17, 678–688 (2015).
Fattet, L. et al. Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex. Dev. Cell 54, 302–316.e7 (2020).
Zheng, Z. et al. Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death Dis. 12, 754 (2021).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Seifert, A. W. et al. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561–565 (2012).
Meng, X.-M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Walton, K. L., Johnson, K. E. & Harrison, C. A. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 8, 461 (2017).
Györfi, A. H., Matei, A.-E. & Distler, J. H. W. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 68–69, 8–27 (2018).
Shull, M. M. et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359, 693–699 (1992).
Li, J. H. et al. Smad7 inhibits fibrotic effect of TGF-beta on renal tubular epithelial cells by blocking Smad2 activation. J. Am. Soc. Nephrol. 13, 1464–1472 (2002).
Chung, A. C. K. et al. Smad7 suppresses renal fibrosis via altering expression of TGF-β/Smad3-regulated microRNAs. Mol. Ther. 21, 388–398 (2013).
Hamzavi, J. et al. Disruption of the Smad7 gene enhances CCI4-dependent liver damage and fibrogenesis in mice. J. Cell. Mol. Med. 12, 2130–2144 (2008).
Rinaldi, C. & Wood, M. J. A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 14, 9–21 (2018).
Allison, S. J. Fibrosis: targeting EMT to reverse renal fibrosis. Nat. Rev. Nephrol. 11, 565 (2015).
Feng, Y.-L., Chen, D.-Q., Vaziri, N. D., Guo, Y. & Zhao, Y.-Y. Small molecule inhibitors of epithelial-mesenchymal transition for the treatment of cancer and fibrosis. Med. Res. Rev. 40, 54–78 (2020).
Yi, H. et al. Metformin attenuates renal fibrosis in a mouse model of adenine-induced renal injury through inhibiting TGF-β1 signaling pathways. Front. Cell Dev. Biol. 9, 603802 (2021).
Wang, Y., Wu, Z. & Hu, L. The regulatory effects of metformin on the [SNAIL/miR-34]:[ZEB/miR-200] system in the epithelial-mesenchymal transition (EMT) for colorectal cancer (CRC). Eur. J. Pharmacol. 834, 45–53 (2018).
Oba, S. et al. miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS ONE 5, e13614 (2010).
Xiong, M. et al. The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am. J. Physiol. Renal Physiol. 302, F369–F379 (2012).
Brennan, E. P. et al. Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFβR1. J. Am. Soc. Nephrol. 24, 627–637 (2013).
Wang, B. et al. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 24, 1290–1301 (2016).
Yang, S. et al. Participation of miR-200 in pulmonary fibrosis. Am. J. Pathol. 180, 484–493 (2012).
Yue, H.-Y. et al. Hepatocyte nuclear factor 4alpha attenuates hepatic fibrosis in rats. Gut 59, 236–246 (2010).
Nishikawa, T. et al. Resetting the transcription factor network reverses terminal chronic hepatic failure. J. Clin. Invest. 125, 1533–1544 (2015).
Kirita, Y., Wu, H., Uchimura, K., Wilson, P. C. & Humphreys, B. D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl Acad. Sci. USA 117, 15874–15883 (2020).
Cicchini, C. et al. Molecular mechanisms controlling the phenotype and the EMT/MET dynamics of hepatocyte. Liver Int. 35, 302–310 (2015).
Morimoto, A. et al. An HNF4α-microRNA-194/192 signaling axis maintains hepatic cell function. J. Biol. Chem. 292, 10574–10585 (2017).
Cicchini, C. et al. Epigenetic control of EMT/MET dynamics: HNF4α impacts DNMT3s through miRs-29. Biochim. Biophys. Acta Gene Regul. Mech. 1849, 919–929 (2015).
Dey, A., Varelas, X. & Guan, K.-L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 19, 480–494 (2020).
Jin, J. et al. Inhibition of yes-associated protein by verteporfin ameliorates unilateral ureteral obstruction-induced renal tubulointerstitial inflammation and fibrosis. Int. J. Mol. Sci. 21, 8184 (2020).
Wang, X. et al. A therapeutic silencing RNA targeting hepatocyte TAZ prevents and reverses fibrosis in nonalcoholic steatohepatitis in mice. Hepatol. Commun. 3, 1221–1234 (2019).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Hangauer, M. J. et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017).
Ide, S. et al. Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. eLife 10, e68603 (2021).
Huber, M. A. et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 114, 569–581 (2004).
Wu, Y. et al. Stabilization of snail by NF-κB is required for inflammation-induced cell migration and invasion. Cancer Cell 15, 416–428 (2009).
Liu, H. et al. High uric acid-induced epithelial-mesenchymal transition of renal tubular epithelial cells via the TLR4/NF-kB signaling pathway. Am. J. Nephrol. 46, 333–342 (2017).
Peng, L. et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 11, 978 (2020).
Alyaseer, A. A. A., de Lima, M. H. S. & Braga, T. T. The role of NLRP3 inflammasome activation in the epithelial to mesenchymal transition process during the fibrosis. Front. Immunol. 11, 883 (2020).
Wang, W. et al. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J. Immunol. 190, 1239–1249 (2013).
Zhang, L.-L. et al. Angiotensin(1-7) attenuated angiotensin II-induced hepatocyte EMT by inhibiting NOX-derived H2O2-activated NLRP3 inflammasome/IL-1β/Smad circuit. Free Radic. Biol. Med. 97, 531–543 (2016).
Kang, H., Kim, H., Lee, S., Youn, H. & Youn, B. Role of metabolic reprogramming in epithelial−mesenchymal transition (EMT). Int. J. Mol. Sci. 20, 2042 (2019).
Zhao, X., Kwan, J. Y. Y., Yip, K., Liu, P. P. & Liu, F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 19, 57–75 (2020).
Li, J. et al. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight 5, e129034 (2020).
Ding, H. et al. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am. J. Physiol. Renal Physiol. 313, F561–F575 (2017).
Xie, N. et al. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am. J. Respir. Crit. Care Med. 192, 1462–1474 (2015).
Soto-Heredero, G., Gómez de Las Heras, M. M., Gabandé-Rodríguez, E., Oller, J. & Mittelbrunn, M. Glycolysis – a key player in the inflammatory response. FEBS J. 287, 3350–3369 (2020).
Storer, M. A. et al. Acquisition of a unique mesenchymal precursor-like blastema state underlies successful adult mammalian digit tip regeneration. Dev. Cell 52, 509–524.e9 (2020).
Reginelli, A. D., Wang, Y. Q., Sassoon, D. & Muneoka, K. Digit tip regeneration correlates with regions of Msx1 (Hox 7) expression in fetal and newborn mice. Development 121, 1065–1076 (1995).
Han, M., Yang, X., Farrington, J. E. & Muneoka, K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development 130, 5123–5132 (2003).
Maden, M. & Varholick, J. A. Model systems for regeneration: the spiny mouse, Acomys cahirinus. Development 147, dev167718 (2020).
Okamura, D. M. et al. Spiny mice activate unique transcriptional programs after severe kidney injury regenerating organ function without fibrosis. iScience 24, 103269 (2021).
Gawriluk, T. R. et al. Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat. Commun. 7, 11164 (2016).
Bedelbaeva, K. et al. Epithelial–mesenchymal transition: an organizing principle of mammalian regeneration. Front. Cell Dev. Biol. 11, 1101480 (2023).
Strunz, M. et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 11, 3559 (2020).
Lake, B. B. et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature 619, 585–594 (2023).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
Xin, M. et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl Acad. Sci. USA 110, 13839–13844 (2013).
D’Uva, G. et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638 (2015).
Aharonov, A. et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 22, 1346–1356 (2020).
González-Iglesias, A. & Nieto, M. A. Proliferation and EMT trigger heart repair. Nat. Cell Biol. 22, 1291–1292 (2020).
Goldman, D. Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 15, 431–442 (2014).
Sharma, P. et al. Oct4 mediates Müller glia reprogramming and cell cycle exit during retina regeneration in zebrafish. Life Sci. Alliance 2, e201900548 (2019).
Sharma, P. et al. Biphasic role of TGF-β signaling during Müller glia reprogramming and retinal regeneration in zebrafish. iScience 23, 100817 (2020).
Ramachandran, R., Fausett, B. V. & Goldman, D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat. Cell Biol. 12, 1101–1107 (2010).
Ueki, Y. et al. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc. Natl Acad. Sci. USA 112, 13717–13722 (2015).
Jorstad, N. L. et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103–107 (2017).
Fernández-Ruiz, I. ERBB2–YAP crosstalk mediates cardiac regeneration in mice. Nat. Rev. Cardiol. 18, 4 (2021).
Emiliani, V. et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Primers 2, 55 (2022).
Yesbolatova, A. et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat. Commun. 11, 5701 (2020).
Blanpain, C. & Fuchs, E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281 (2014).
Li, L. & Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010).
de Sousa, E., Melo, F. & de Sauvage, F. J. Cellular plasticity in intestinal homeostasis and disease. Cell Stem Cell 24, 54–64 (2019).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Mahmoudi, S., Xu, L. & Brunet, A. Turning back time with emerging rejuvenation strategies. Nat. Cell Biol. 21, 32–43 (2019).
Beauséjour, C. M. et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 22, 4212–4222 (2003).
Ocampo, A. et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167, 1719–1733.e12 (2016).
Chondronasiou, D. et al. Multi-omic rejuvenation of naturally aged tissues by a single cycle of transient reprogramming. Aging Cell 21, e13578 (2022).
Gavish, A. et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618, 598–606 (2023).
van Dijk, D. et al. Recovering gene interactions from single-cell data using data diffusion. Cell 174, 716–729.e27 (2018).
Alonso-Curbelo, D. et al. A gene–environment-induced epigenetic program initiates tumorigenesis. Nature 590, 642–648 (2021).
Shahbazi, M. N. & Zernicka-Goetz, M. Deconstructing and reconstructing the mouse and human early embryo. Nat. Cell Biol. 20, 878–887 (2018).
Pei, D., Shu, X., Gassama-Diagne, A. & Thiery, J. P. Mesenchymal–epithelial transition in development and reprogramming. Nat. Cell Biol. 21, 44–53 (2019).
Hamidi, S. et al. Mesenchymal-epithelial transition regulates initiation of pluripotency exit before gastrulation. Development 147, dev184960 (2020).
Ismagulov, G., Hamidi, S. & Sheng, G. Epithelial-mesenchymal transition drives three-dimensional morphogenesis in mammalian early development. Front. Cell Dev. Biol. 9, 639244 (2021).
Chen, A. F. et al. GRHL2-dependent enhancer switching maintains a pluripotent stem cell transcriptional subnetwork after exit from naive pluripotency. Cell Stem Cell 23, 226–238.e4 (2018).
Vallier, L., Alexander, M. & Pedersen, R. A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 118, 4495–4509 (2005).
Xu, R.-H. et al. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell 3, 196–206 (2008).
Dias, A. et al. A Tgfbr1/Snai1-dependent developmental module at the core of vertebrate axial elongation. eLife 9, e56615 (2020).
Dong, J. et al. Single-cell RNA-seq analysis unveils a prevalent epithelial/mesenchymal hybrid state during mouse organogenesis. Genome Biol. 19, 31 (2018).
Rohm, T. V., Meier, D. T., Olefsky, J. M. & Donath, M. Y. Inflammation in obesity, diabetes, and related disorders. Immunity 55, 31–55 (2022).
Younossi, Z. et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69, 2672–2682 (2019).
Kwapisz, O. et al. Fatty acids and a high-fat diet induce epithelial-mesenchymal transition by activating tgfβ and β-catenin in liver cells. Int. J. Mol. Sci. 22, 1272 (2021).
Loft, A. et al. Liver-fibrosis-activated transcriptional networks govern hepatocyte reprogramming and intra-hepatic communication. Cell Metab. 33, 1685–1700.e9 (2021).
Subbalakshmi, A. R. et al. The ELF3 transcription factor is associated with an epithelial phenotype and represses epithelial-mesenchymal transition. J. Biol. Eng. 17, 17 (2023).
Beaven, S. W. et al. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab. 18, 106–117 (2013).
Ganesan, R., Mallets, E. & Gomez-Cambronero, J. The transcription factors Slug (SNAI2) and Snail (SNAI1) regulate phospholipase D (PLD) promoter in opposite ways towards cancer cell invasion. Mol. Oncol. 10, 663–676 (2016).
Boor, P. & Floege, J. Renal allograft fibrosis: biology and therapeutic targets. Am. J. Transplant. 15, 863–886 (2015).
Bedi, S., Vidyasagar, A. & Djamali, A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant. Rev. 22, 1–5 (2008).
Vongwiwatana, A., Tasanarong, A., Rayner, D. C., Melk, A. & Halloran, P. F. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am. J. Transplant. 5, 1367–1374 (2005).
Robertson, H., Ali, S., McDonnell, B. J., Burt, A. D. & Kirby, J. A. Chronic renal allograft dysfunction: the role of T cell-mediated tubular epithelial to mesenchymal cell transition. J. Am. Soc. Nephrol. 15, 390–397 (2004).
Rygiel, K. A. et al. T cell-mediated biliary epithelial-to-mesenchymal transition in liver allograft rejection. Liver Transplant. 16, 567–576 (2010).
Straub, J. M. et al. Radiation-induced fibrosis: mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 141, 1985–1994 (2015).
Almeida, C. et al. The role of alveolar epithelium in radiation-induced lung injury. PLoS ONE 8, e53628 (2013).
Nagarajan, D., Melo, T., Deng, Z., Almeida, C. & Zhao, W. ERK/GSK3β/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition. Free Radic. Biol. Med. 52, 983–992 (2012).
Choi, S.-H. et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis. Clin. Cancer Res. 21, 3716–3726 (2015).
Sleijfer, S. Bleomycin-induced pneumonitis. Chest 120, 617–624 (2001).
Della Latta, V., Cecchettini, A., Del Ry, S. & Morales, M. A. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol. Res. 97, 122–130 (2015).
Acknowledgements
The authors thank all members of the Nieto laboratory for discussion and comments on the manuscript. This work was supported by grants from the Spanish Ministries of Science and Innovation (MCI PID2021-125682NB-I00), from the AECC Scientific Foundation (FC_AECC PROYE19073NIE), Instituto de Salud Carlos III (CIBERER, CB19/07/00038 to M.A.N.) and Generalitat Valenciana (Prometeo 2021/45) to M.A.N., who also acknowledges financial support from the Spanish State Research Agency, through the Severo Ochoa Program for Centres of Excellence in R&D (CEX2021-001165-S). K.K.Y. holds an investigator contract from the AECC Scientific Foundation (Ayudas AECC investigador 2022).
Author information
Authors and Affiliations
Contributions
Both authors wrote the manuscript and generated the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Guojun Sheng, Jing Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Apicobasal polarity
-
A term that alludes to the polarization of epithelial cells into a basal pole that faces and adheres to the basal membrane and an apical pole that faces the outside of the body or the lumen of internal cavities.
- Blastema
-
Mass of proliferative and undifferentiated progenitor-like cells that appears in response to injury to initiate tissue repair.
- Cell senescence
-
Irreversible cell cycle arrest.
- Clara cells
-
Population of non-ciliated secretory cells in the lung bronchiolar epithelium that have an essential role in repair after injury.
- Embryo tail bud
-
Mass of embryonic undifferentiated, multipotent and proliferative cells that resides in the most caudal part of chordate embryos.
- Ephrin B1
-
Ephrin B1, encoded by the EFNB1 gene, is a member of the ephrin family, ligands of EPH-related receptor tyrosine kinases.
- Epimorphosis
-
The dedifferentiation of somatic adult cells into phenotypes reminiscent of those that occur during embryonic development in response to injury and their extensive cell proliferation before undergoing redifferentiation to replace the damaged tissues.
- Ferroptosis
-
Iron-dependent programmed cell death induced by the accumulation of lipid peroxides at the cell membrane.
- Genetic fate mapping
-
Stable and inheritable labelling of a cell (or group of cells). Genetic fate mapping is used in embryonic development to define cell lineages and to define the cells of origin in cancer evolution.
- Inflammasome
-
Cytoplasmic multiprotein complex that senses both endogenous and exogenous stimuli and, as a response, mediates proteolytic cleavage and secretion of pro-inflammatory cytokines.
- Leader cells
-
Term used to define cells located at the edge of neural crest or other migratory streams, which can instruct migration directionality.
- Myofibroblasts
-
Specialized fibroblasts with high contractile capacity. Myofibroblasts initially form to protect adult tissues after stress and damage, but during chronic damage they accumulate and cause excessive extracellular matrix deposition, scarring and tissue degeneration.
- Octamer-binding transcription factor 4
-
(OCT4). A transcription factor that promotes self-renewal and the maintenance of pluripotency in embryonic stem cells or multipotency in some progenitors. It is also one of the four ‘Yamaka factors’, used for somatic cell reprogramming into induced pluripotent stem cells.
- Pluripotency
-
The ability of given cells to give rise to multiple cell types. It is commonly used to refer to the ability of a cell to give rise to cells of the three primary germ cell layers: ectoderm, mesoderm and endoderm.
- Pre-eclampsia
-
A disorder characterized by persistent high blood pressure during pregnancy, leading to multiple organ dysfunction. Untreated or severe pre-eclampsia can lead to serious complications to the mother and fetus.
- Primitive streak
-
A structure that forms in the early embryo amniotes in which multiple epiblast cells ingress to allow gastrulation and formation of the three germ layers.
- Trophoblasts
-
Cells that form the outer layer of the blastocyst, which has an essential role in embryo implantation and placenta formation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Youssef, K.K., Nieto, M.A. Epithelial–mesenchymal transition in tissue repair and degeneration. Nat Rev Mol Cell Biol (2024). https://doi.org/10.1038/s41580-024-00733-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41580-024-00733-z