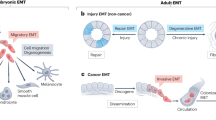
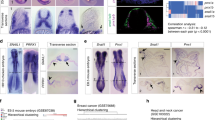
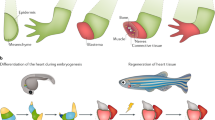
Abstract
Embryonic genetic programs are reactivated in response to various types of tissue damage, providing cell plasticity for tissue regeneration or disease progression. In acute conditions, these programs remedy the damage and then halt to allow a return to homeostasis. In chronic situations, including inflammatory diseases, fibrosis and cancer, prolonged activation of embryonic programs leads to disease progression and tissue deterioration. Induction of progenitor identity and cell plasticity, for example, epithelial–mesenchymal plasticity, are critical outcomes of reactivated embryonic programs. In this Review, we describe molecular players governing reactivated embryonic genetic programs, their role during disease progression, their similarities and differences and lineage reversion in pathology and discuss associated therapeutics and drug-resistance mechanisms across many organs. We also discuss the diversity of reactivated programs in different disease contexts. A comprehensive overview of commonalities between development and disease will provide better understanding of the biology and therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Virchow, R. L. K. Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre (A. Hirschwald, 1859).
Fazilaty, H. Restoration of embryonic gene expression patterns in tissue regeneration and disease. Nat. Rev. Mol. Cell Biol. 24, 375–376 (2023).
Peluffo, A. E. The ‘genetic program’: behind the genesis of an influential metaphor. Genetics 200, 685–696 (2015).
Malta, T. M. et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 173, 338–354 (2018).
Hnisz, D. et al. Convergence of developmental and oncogenic signaling pathways at transcriptional super-enhancers. Mol. Cell 58, 362–370 (2015).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Bala, P. et al. Aberrant cell state plasticity mediated by developmental reprogramming precedes colorectal cancer initiation. Sci. Adv. 9, eadf0927 (2023).
Fazilaty, H. et al. Tracing colonic embryonic transcriptional profiles and their reactivation upon intestinal damage. Cell Rep. 36, 109484 (2021).
Varum, S. et al. Yin yang 1 orchestrates a metabolic program required for both neural crest development and melanoma formation. Cell Stem Cell 24, 637–653 (2019).
Rehman, S. K. et al. Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. Cell 184, 226–242 (2021).
Dhimolea, E. et al. An embryonic diapause-like adaptation with suppressed Myc activity enables tumor treatment persistence. Cancer Cell 39, 240–256 (2021).
Jatiani, S. S. et al. SOX11 inhibitors are cytotoxic in mantle cell lymphoma. Clin. Cancer Res. 27, 4652–4663 (2021).
Grande, M. T. et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 21, 989–997 (2015).
Burdziak, C. et al. Epigenetic plasticity cooperates with cell–cell interactions to direct pancreatic tumorigenesis. Science 380, eadd5327 (2023).
Moorman, A. R. et al. Progressive plasticity during colorectal cancer metastasis. Preprint at bioRxiv https://doi.org/10.1101/2023.08.18.553925 (2023).
Li, Y. et al. Mutant Kras co-opts a proto-oncogenic enhancer network in inflammation-induced metaplastic progenitor cells to initiate pancreatic cancer. Nat. Cancer 2, 49–65 (2021).
Hill, W. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616, 159–167 (2023).
Salgia, R. & Kulkarni, P. The genetic/non-genetic duality of drug ‘resistance’ in cancer. Trends Cancer 4, 110–118 (2018).
Shivdasani, R. A., Clevers, H. & de Sauvage, F. J. Tissue regeneration: reserve or reverse? Science 371, 784–786 (2021).
Sun, Q. et al. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature 616, 774–782 (2023).
Bhartiya, D. Adult tissue-resident stem cells—fact or fiction? Stem Cell Res. Ther. 12, 73 (2021).
Yui, S. et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22, 35–49 (2018).
Del Poggetto, E. et al. Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science 373, eabj0486 (2021).
Nusse, Y. M. et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 559, 109–113 (2018).
Mustata, R. C. et al. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 5, 421–432 (2013).
Househam, J. et al. Phenotypic plasticity and genetic control in colorectal cancer evolution. Nature 611, 744–753 (2022).
Laughney, A. M. et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat. Med. 26, 259–269 (2020).
Perino, M. & Veenstra, G. J. C. Chromatin control of developmental dynamics and plasticity. Dev. Cell 38, 610–620 (2016).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Hayashi, A., Hong, J. & Iacobuzio-Donahue, C. A. The pancreatic cancer genome revisited. Nat. Rev. Gastroenterol. Hepatol. 18, 469–481 (2021).
Dagogo-Jack, I. et al. Clinicopathologic characteristics of BRG1-deficient NSCLC. J. Thorac. Oncol. 15, 766–776 (2020).
Kleppe, A. et al. Chromatin organisation and cancer prognosis: a pan-cancer study. Lancet Oncol. 19, 356–369 (2018).
Kim, Y. J. et al. Melanoma dedifferentiation induced by IFN-γ epigenetic remodeling in response to anti-PD-1 therapy. J. Clin. Invest. 131, e145859 (2021).
Yang, J. et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Thiery, J. P., Acloque, H., Huang, R. Y. J. & Nieto, M. A. Epithelial–mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Nieto, M. A., Huang, R. Y.-J., Jackson, R. A. & Thiery, J. P. EMT: 2016. Cell 166, 21–45 (2016).
Brabletz, T., Kalluri, R., Nieto, M. A. & Weinberg, R. A. EMT in cancer. Nat. Rev. Cancer 18, 128–134 (2018).
Fazilaty, H. et al. A gene regulatory network to control EMT programs in development and disease. Nat. Commun. 10, 5115 (2019).
Zhang, Y. et al. Genome-wide CRISPR screen identifies PRC2 and KMT2D-COMPASS as regulators of distinct EMT trajectories that contribute differentially to metastasis. Nat. Cell Biol. 24, 554–564 (2022).
Lambert, A. W. & Weinberg, R. A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 21, 325–338 (2021).
Shaw, T. J. & Martin, P. Wound repair: a showcase for cell plasticity and migration. Curr. Opin. Cell Biol. 42, 29–37 (2016).
Frangogiannis, N. G. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 217, e20190103 (2020).
Scharl, M. et al. Hallmarks of epithelial to mesenchymal transition are detectable in Crohn’s disease associated intestinal fibrosis. Clin. Transl. Med. 4, 1 (2015).
Scharl, M. & Rogler, G. Pathophysiology of fistula formation in Crohn’s disease. World J. Gastrointest. Pathophysiol. 5, 205–212 (2014).
Rout-Pitt, N., Farrow, N., Parsons, D. & Donnelley, M. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 19, 136 (2018).
Su, J. et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 577, 566–571 (2020).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Lüönd, F. et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 56, 3203–3221 (2021).
Nieto, M. A. Context-specific roles of EMT programmes in cancer cell dissemination. Nat. Cell Biol. 19, 416–418 (2017).
Pastushenko, I. et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 589, 448–455 (2021).
Lehmann, W. et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 7, 10498 (2016).
Sreekumar, R. et al. Assessment of nuclear ZEB2 as a biomarker for colorectal cancer outcome and TNM risk stratification. JAMA Netw. Open 1, e183115 (2018).
Marie, K. L. et al. Melanoblast transcriptome analysis reveals pathways promoting melanoma metastasis. Nat. Commun. 11, 333 (2020).
Soldatov, R. et al. Spatiotemporal structure of cell fate decisions in murine neural crest. Science 364, eaas9536 (2019).
Karras, P. et al. A cellular hierarchy in melanoma uncouples growth and metastasis. Nature 610, 190–198 (2022).
Semaan, A. et al. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br. J. Cancer 124, 1970–1977 (2021).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Donato, C. et al. Hypoxia triggers the intravasation of clustered circulating tumor cells. Cell Rep. 32, 108105 (2020).
Bonnomet, A. et al. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene 31, 3741–3753 (2012).
Ting, D. T. et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 8, 1905–1918 (2014).
Ruscetti, M., Quach, B., Dadashian, E. L., Mulholland, D. J. & Wu, H. Tracking and functional characterization of epithelial–mesenchymal transition and mesenchymal tumor cells during prostate cancer metastasis. Cancer Res. 75, 2749–2759 (2015).
Mani, S. A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Fazilaty, H., Gardaneh, M., Akbari, P., Zekri, A. & Behnam, B. SLUG and SOX9 cooperatively regulate tumor initiating niche factors in breast cancer. Cancer Microenviron. 9, 71–74 (2016).
Celià-Terrassa, T. et al. Epithelial–mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J. Clin. Invest. 122, 1849–1868 (2012).
Ocaña, O. H. et al. Metastatic colonization requires the repression of the epithelial–mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 (2012).
Pastushenko, I. et al. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468 (2018).
Bartel, D. P. Metazoan microRNAs. Cell 173, 20–51 (2018).
Cano, A. & Nieto, M. A. Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol. 18, 357–359 (2008).
Rago, L. et al. MicroRNAs establish the right-handed dominance of the heart laterality pathway in vertebrates. Dev. Cell 51, 446–459 (2019).
Díaz-López, A., Moreno-Bueno, G. & Cano, A. Role of microRNA in epithelial to mesenchymal transition and metastasis and clinical perspectives. Cancer Manag. Res. 6, 205–216 (2014).
Monzo, M. et al. Overlapping expression of microRNAs in human embryonic colon and colorectal cancer. Cell Res. 18, 823–833 (2008).
Saghafinia, S. et al. Cancer cells retrace a stepwise differentiation program during malignant progression. Cancer Discov. 11, 2638–2657 (2021).
Lee, J. H. & Massagué, J. TGF-β in developmental and fibrogenic EMTs. Semin. Cancer Biol. 86, 136–145 (2022).
Rim, E. Y., Clevers, H. & Nusse, R. The Wnt pathway: from signaling mechanisms to synthetic modulators. Annu. Rev. Biochem. 91, 571–598 (2022).
Gregorieff, A., Liu, Y., Inanlou, M. R., Khomchuk, Y. & Wrana, J. L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526, 715–718 (2015).
Solé, L. et al. p53 wild-type colorectal cancer cells that express a fetal gene signature are associated with metastasis and poor prognosis. Nat. Commun. 13, 2866 (2022).
Jacquemin, G. et al. Paracrine signalling between intestinal epithelial and tumour cells induces a regenerative programme. eLife 11, e76541 (2022).
Er, E. E. et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol. 20, 966–978 (2018).
Ganesh, K. et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 1, 28–45 (2020).
Altevogt, P. et al. Recent insights into the role of L1CAM in cancer initiation and progression. Int. J. Cancer 147, 3292–3296 (2020).
Zhang, W. et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci. Signal. 7, ra42 (2014).
Wu, B.-K., Mei, S.-C., Chen, E. H., Zheng, Y. & Pan, D. YAP induces an oncogenic transcriptional program through TET1-mediated epigenetic remodeling in liver growth and tumorigenesis. Nat. Genet. 54, 1202–1213 (2022).
Kowalczyk, W. et al. Hippo signaling instructs ectopic but not normal organ growth. Science 378, eabg3679 (2022).
Tiana, M. et al. Pluripotency factors regulate the onset of Hox cluster activation in the early embryo. Sci. Adv. 8, eabo3583 (2022).
Li, M. & Belmonte, J. C. I. Ground rules of the pluripotency gene regulatory network. Nat. Rev. Genet. 18, 180–191 (2017).
Blassberg, R. et al. Sox2 levels regulate the chromatin occupancy of WNT mediators in epiblast progenitors responsible for vertebrate body formation. Nat. Cell Biol. 24, 633–644 (2022).
Ben-Porath, I. et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 40, 499–507 (2008).
Yong, K. J. et al. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N. Engl. J. Med. 368, 2266–2276 (2013).
Hepburn, A. C. et al. The induction of core pluripotency master regulators in cancers defines poor clinical outcomes and treatment resistance. Oncogene 38, 4412–4424 (2019).
Gkountela, S. et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112 (2019).
Lee, T. K. W. et al. CD24+ liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 9, 50–63 (2011).
Kim, J. et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 143, 313–324 (2010).
Dhanasekaran, R. et al. The MYC oncogene — the grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 19, 23–36 (2022).
Sodir, N. M. et al. MYC instructs and maintains pancreatic adenocarcinoma phenotype. Cancer Discov. 10, 588–607 (2020).
Kortlever, R. M. et al. Myc cooperates with Ras by programming inflammation and immune suppression. Cell 171, 1301–1315 (2017).
Sodir, N. M. et al. Reversible Myc hypomorphism identifies a key Myc-dependency in early cancer evolution. Nat. Commun. 13, 6782 (2022).
Lee-Six, H. et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature 574, 532–537 (2019).
Jo, A. et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 1, 149–161 (2014).
Campana, L., Esser, H., Huch, M. & Forbes, S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat. Rev. Mol. Cell Biol. 22, 608–624 (2021).
Ge, Y. et al. Stem cell lineage infidelity drives wound repair and cancer. Cell 169, 636–650 (2017).
Drubbel, A. V. et al. Reactivation of the Hedgehog pathway in esophageal progenitors turns on an embryonic-like program to initiate columnar metaplasia. Cell Stem Cell 28, 1411–1427 (2021).
Guo, W. et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148, 1015–1028 (2012).
Fazilaty, H., Gardaneh, M., Bahrami, T., Salmaninejad, A. & Behnam, B. Crosstalk between breast cancer stem cells and metastatic niche: emerging molecular metastasis pathway? Tumour Biol. 34, 2019–2030 (2013).
Hanieh, H., Ahmed, E. A., Vishnubalaji, R. & Alajez, N. M. SOX4: epigenetic regulation and role in tumorigenesis. Semin. Cancer Biol. 67, 91–104 (2020).
Dravis, C. et al. Epigenetic and transcriptomic profiling of mammary gland development and tumor models disclose regulators of cell state plasticity. Cancer Cell 34, 466–482 (2018).
Reynolds, G. et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 371, eaba6500 (2021).
Sharma, A. et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell 183, 377–394 (2020).
Chen, Y. et al. Type-I collagen produced by distinct fibroblast lineages reveals specific function during embryogenesis and osteogenesis imperfecta. Nat. Commun. 12, 7199 (2021).
Ramadan, R. et al. The extracellular matrix controls stem cell specification and crypt morphology in the developing and adult mouse gut. Biology Open 11, bio059544 (2022).
Chen, Y. et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell 40, 818–834 (2022).
Wei, S. C. et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17, 678–688 (2015).
Brügger, M. D., Valenta, T., Fazilaty, H., Hausmann, G. & Basler, K. Distinct populations of crypt-associated fibroblasts act as signaling hubs to control colon homeostasis. PLoS Biol. 18, e3001032 (2020).
Degirmenci, B., Valenta, T., Dimitrieva, S., Hausmann, G. & Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558, 449–453 (2018).
Kwon, O.-J., Zhang, L., Ittmann, M. M. & Xin, L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl Acad. Sci. USA 111, E592–E600 (2014).
Qin, H. et al. SOX9 in prostate cancer is upregulated by cancer-associated fibroblasts to promote tumor progression through HGF/c-Met–FRA1 signaling. FEBS J. 288, 5406–5429 (2021).
Lee, K.-W. et al. PRRX1 is a master transcription factor of stromal fibroblasts for myofibroblastic lineage progression. Nat. Commun. 13, 2793 (2022).
Arumi-Planas, M. et al. Microenvironmental Snail1-induced immunosuppression promotes melanoma growth. Oncogene 42, 2659–2672 (2023).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576 (2017).
Giraddi, R. R. et al. Single-cell transcriptomes distinguish stem cell state changes and lineage specification programs in early mammary gland development. Cell Rep. 24, 1653–1666 (2018).
Pal, B. et al. Construction of developmental lineage relationships in the mouse mammary gland by single-cell RNA profiling. Nat. Commun. 8, 1627 (2017).
Liu, Y. & Guo, W. SOX factors as cell-state regulators in the mammary gland and breast cancer. Semin. Cell Dev. Biol. 114, 126–133 (2021).
Wuidart, A. et al. Early lineage segregation of multipotent embryonic mammary gland progenitors. Nat. Cell Biol. 20, 666–676 (2018).
Chakrabarti, R. et al. ΔNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat. Cell Biol. 16, 1004–1015 (2014).
Bagati, A. et al. Integrin αvβ6–TGFβ–SOX4 pathway drives immune evasion in triple-negative breast cancer. Cancer Cell 39, 54–67 (2021).
Roukens, M. G. et al. Regulation of a progenitor gene program by SOX4 is essential for mammary tumor proliferation. Oncogene 40, 6343–6353 (2021).
Tiwari, N. et al. Sox4 is a master regulator of epithelial–mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell 23, 768–783 (2013).
Oliemuller, E. et al. SOX11 promotes invasive growth and ductal carcinoma in situ progression: SOX11 promotes invasion and DCIS progression. J. Pathol. 243, 193–207 (2017).
Zvelebil, M. et al. Embryonic mammary signature subsets are activated in Brca1−/− and basal-like breast cancers. Breast Cancer Res. 15, R25 (2013).
Wen, W. et al. Genetic variations of DNA bindings of FOXA1 and co-factors in breast cancer susceptibility. Nat. Commun. 12, 5318 (2021).
Hurtado, A., Holmes, K. A., Ross-Innes, C. S., Schmidt, D. & Carroll, J. S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat. Genet. 43, 27–33 (2011).
Fu, X. et al. FOXA1 upregulation promotes enhancer and transcriptional reprogramming in endocrine-resistant breast cancer. Proc. Natl Acad. Sci. USA 116, 26823–26834 (2019).
Sherwood, R. I., Chen, T.-Y. A. & Melton, D. A. Transcriptional dynamics of endodermal organ formation. Dev. Dyn. 238, 29–42 (2009).
Francis, R. et al. Gastrointestinal transcription factors drive lineage-specific developmental programs in organ specification and cancer. Sci. Adv. 5, eaax8898 (2019).
Salari, K. et al. CDX2 is an amplified lineage-survival oncogene in colorectal cancer. Proc. Natl Acad. Sci. USA 109, E3196–E3205 (2012).
Gold, P. & Freedman, S. O. Specific carcinoembryonic antigens of the human digestive system. J. Exp. Med. 122, 467–481 (1965).
Banwo, O., Versey, J. & Hobbs, J. R. New oncofetal antigen for human pancreas. Lancet 303, 643–645 (1974).
Pattabiraman, D. R. et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 351, aad3680 (2016).
Cassier, P. A. et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 620, 409–416 (2023).
Lengrand, J. et al. Pharmacological targeting of netrin-1 inhibits EMT in cancer. Nature 620, 402–408 (2023).
Ishay-Ronen, D. et al. Gain fat—lose metastasis: converting invasive breast cancer cells into adipocytes inhibits cancer metastasis. Cancer Cell 35, 17–32 (2019).
Terry, S. et al. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 11, 824–846 (2017).
Dongre, A. et al. Direct and indirect regulators of epithelial–mesenchymal transition-mediated immunosuppression in breast carcinomas. Cancer Discov. 11, 1286–1305 (2021).
Hu, J. et al. STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature 616, 806–813 (2023).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Milanovic, M., Yu, Y. & Schmitt, C. A. The senescence–stemness alliance — a cancer-hijacked regeneration principle. Trends Cell Biol. 28, 1049–1061 (2018).
Hüser, L., Novak, D., Umansky, V., Altevogt, P. & Utikal, J. Targeting SOX2 in anticancer therapy. Expert Opin. Ther. Targets 22, 983–991 (2018).
Luo, Z. et al. Human fetal cerebellar cell atlas informs medulloblastoma origin and oncogenesis. Nature 612, 787–794 (2022).
Chen, Y. et al. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science 373, 1537–1540 (2021).
Chen, J., Ju, H. L., Yuan, X. Y., Wang, T. J. & Lai, B. Q. SOX4 is a potential prognostic factor in human cancers: a systematic review and meta-analysis. Clin. Transl. Oncol. 18, 65–72 (2016).
Pomerantz, M. M. et al. Prostate cancer reactivates developmental epigenomic programs during metastatic progression. Nat. Genet. 52, 790–799 (2020).
Ma, F. et al. SOX9 drives WNT pathway activation in prostate cancer. J. Clin. Invest. 126, 1745–1758 (2016).
Mu, P. et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 (2017).
Rudin, C. M. et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 44, 1111–1116 (2012).
Tanaka, H. et al. Lineage-specific dependency of lung adenocarcinomas on the lung development regulator TTF-1. Cancer Res. 67, 6007–6011 (2007).
Jing, X., Wang, T., Huang, S., Glorioso, J. C. & Albers, K. M. The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a. Exp. Neurol. 233, 221–232 (2012).
Hamon, A., Roger, J. E., Yang, X.-J. & Perron, M. Müller glial cell-dependent regeneration of the neural retina: an overview across vertebrate model systems. Dev. Dyn. 245, 727–738 (2016).
Decaesteker, B. et al. SOX11 regulates SWI/SNF complex components as member of the adrenergic neuroblastoma core regulatory circuitry. Nat. Commun. 14, 1267 (2023).
Angelozzi, M., da Silva, R. P., Gonzalez, M. V. & Lefebvre, V. Single-cell atlas of craniogenesis uncovers SOXC-dependent, highly proliferative, and myofibroblast-like osteodermal progenitors. Cell Rep. 40, 111045 (2022).
Storz, P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 14, 296–304 (2017).
Dassaye, R., Naidoo, S. & Cerf, M. E. Transcription factor regulation of pancreatic organogenesis, differentiation and maturation. Islets 8, 13–34 (2015).
Patel, S. A. et al. The renal lineage factor PAX8 controls oncogenic signalling in kidney cancer. Nature 606, 999–1006 (2022).
Imgrund, M. et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice. Kidney Int. 56, 1423–1431 (1999).
Kimura, S. Thyroid-specific transcription factors and their roles in thyroid cancer. J. Thyroid Res. 2011, e710213 (2011).
Vu-Phan, D. et al. The thyroid cancer PAX8–PPARG fusion protein activates Wnt/TCF-responsive cells that have a transformed phenotype. Endocr. Relat. Cancer 20, 725–739 (2013).
Shakhova, O. et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat. Cell Biol. 14, 882–890 (2012).
Garraway, L. A. et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122 (2005).
Miao, Q. et al. SOX11 and SOX4 drive the reactivation of an embryonic gene program during murine wound repair. Nat. Commun. 10, 4042 (2019).
Limaye, P. B. et al. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Lab. Invest. 88, 865–872 (2008).
Sun, R. et al. SOX4 contributes to the progression of cervical cancer and the resistance to the chemotherapeutic drug through ABCG2. Cell Death Dis. 6, e1990 (2015).
Mills, J. C., Stanger, B. Z. & Sander, M. Nomenclature for cellular plasticity: are the terms as plastic as the cells themselves? EMBO J. 38, e103148 (2019).
Acknowledgements
We thank E. Brunner and T. Dalessi for discussions as well as other members of the Basler laboratory including M. D. Brügger, G. Hausmann, T. Valenta, D. King, G. Moro, A. F. Guthörl and L. F. Rago for critical input on the manuscript. Considering the broad topic of this Review and limited space, we were not able to include all essential studies and therefore apologize to authors whose work we did not cite. H.F. was supported by the Forschungskredit of the University of Zürich (grant FK-21-119) and a grant from the University and Medical Faculty of Zürich and the Comprehensive Cancer Center Zürich. K.B. is supported by Swiss National Science Foundation (grants 192475 and 207594) project funding in biology and medicine.
Author information
Authors and Affiliations
Contributions
H.F. conceived and wrote the paper. K.B. provided support and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Joan Massague, Joseph Taube and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fazilaty, H., Basler, K. Reactivation of embryonic genetic programs in tissue regeneration and disease. Nat Genet 55, 1792–1806 (2023). https://doi.org/10.1038/s41588-023-01526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01526-4