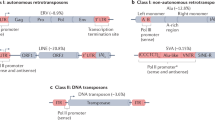
Abstract
Transposable elements (TEs) are mobile DNA elements that comprise almost 50% of mammalian genomic sequence. TEs are capable of making additional copies of themselves that integrate into new positions in host genomes. This unique property has had an important impact on mammalian genome evolution and on the regulation of gene expression because TE-derived sequences can function as cis-regulatory elements such as enhancers, promoters and silencers. Now, advances in our ability to identify and characterize TEs have revealed that TE-derived sequences also regulate gene expression by both maintaining and shaping 3D genome architecture. Studies are revealing how TEs contribute raw sequence that can give rise to the structures that shape chromatin organization, and thus gene expression, allowing for species-specific genome innovation and evolutionary novelty.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McClintock, B. The origin and behavior of mutable loci in maize. Proc. Natl Acad. Sci. USA 36, 344–355 (1950).
Finnegan, D. J. Eukaryotic transposable elements and genome evolution. Trends Genet. 5, 103–107 (1989).
Fueyo, R., Judd, J., Feschotte, C. & Wysocka, J. Roles of transposable elements in the regulation of mammalian transcription. Nat. Rev. Mol. Cell Biol. 23, 481–497 (2022).
Sultana, T., Zamborlini, A., Cristofari, G. & Lesage, P. Integration site selection by retroviruses and transposable elements in eukaryotes. Nat. Rev. Genet. 18, 292–308 (2017).
Cheung, S., Manhas, S. & Measday, V. Retrotransposon targeting to RNA polymerase III-transcribed genes. Mob. DNA https://doi.org/10.1186/s13100-018-0119-2 (2018).
Wagstaff, B. J. et al. Rescuing Alu: recovery of new inserts shows LINE-1 preserves Alu activity through A-tail expansion. PLoS Genet. 8, e1002842 (2012).
Lander, E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Ichiyanagi, K. Regulating Pol III transcription to change Pol II transcriptome. Cell Cycle 13, 3625–3626 (2014).
Lu, J. Y. et al. Homotypic clustering of L1 and B1/Alu repeats compartmentalizes the 3D genome. Cell Res. 31, 613–630 (2021). This study shows that TEs cluster in compartments and form distinct, segregating domains.
Campos-Sanchez, R., Cremona, M. A., Pini, A., Chiaromonte, F. & Makova, K. D. Integration and fixation preferences of human and mouse endogenous retroviruses uncovered with functional data analysis. PLoS Comput. Biol. 12, e1004956 (2016).
Kvikstad, E. M. & Makova, K. D. The (r)evolution of SINE versus LINE distributions in primate genomes: sex chromosomes are important. Genome Res. 20, 600–613 (2010).
Zhou, W. D., Liang, G. N., Molloy, P. L. & Jones, P. A. DNA methylation enables transposable element-driven genome expansion. Proc. Natl Acad. Sci. USA 117, 19359–19366 (2020).
Molaro, A. & Malik, H. S. Hide and seek: how chromatin-based pathways silence retroelements in the mammalian germline. Curr. Opin. Genet. Dev. 37, 51–58 (2016).
Modzelewski, A. J., Gan Chong, J., Wang, T. & He, L. Mammalian genome innovation through transposon domestication. Nat. Cell Biol. 24, 1332–1340 (2022).
Schmitz, J. & Brosius, J. Exonization of transposed elements: a challenge and opportunity for evolution. Biochimie 93, 1928–1934 (2011).
van de Lagemaat, L. N., Landry, J. R., Mager, D. L. & Medstrand, P. Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions. Trends Genet. 19, 530–536 (2003).
Feschotte, C. & Pritham, E. J. DNA transposons and the evolution of eukaryotic genomes. Annu. Rev. Genet. 41, 331–368 (2007).
Agren, J. A. & Wright, S. I. Co-evolution between transposable elements and their hosts: a major factor in genome size evolution? Chromosome Res. 19, 777–786 (2011).
Bogutz, A. B. et al. Evolution of imprinting via lineage-specific insertion of retroviral promoters. Nat. Commun. 10, 5674 (2019).
Chuong, E. B., Elde, N. C. & Feschotte, C. Regulatory activities of transposable elements: from conflicts to benefits. Nat. Rev. Genet. 18, 71–86 (2017).
Choudhary, M. N. et al. Co-opted transposons help perpetuate conserved higher-order chromosomal structures. Genome Biol. 21, 16 (2020). This study shows that TEs provide redundant CTCF motifs that maintain chromatin organization over evolutionary time.
Kentepozidou, E. et al. Clustered CTCF binding is an evolutionary mechanism to maintain topologically associating domains. Genome Biol. 21, 5 (2020).
Choudhary, M. N. K., Quaid, K., Xing, X., Schmidt, H. & Wang, T. Widespread contribution of transposable elements to the rewiring of mammalian 3D genomes. Nat. Commun. 14, 634 (2023).
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Cremer, T. & Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2, 292–301 (2001).
Dietzel, S. et al. Separate and variably shaped chromosome arm domains are disclosed by chromosome arm painting in human cell nuclei. Chromosome Res. 6, 25–33 (1998).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009).
Rowley, M. J. & Corces, V. G. Organizational principles of 3D genome architecture. Nat. Rev. Genet. 19, 789–800 (2018).
Larson, A. G. et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547, 236-240 (2017).
Oudelaar, A. M. & Higgs, D. R. The relationship between genome structure and function. Nat. Rev. Genet. 22, 154–168 (2021).
Sikorska, N. & Sexton, T. Defining functionally relevant spatial chromatin domains: it is a TAD complicated. J. Mol. Biol. 432, 653–664 (2020).
Krietenstein, N. et al. Ultrastructural details of mammalian chromosome architecture. Mol. Cell 78, 554–565.e7 (2020).
Phillips-Cremins, J. E. et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 153, 1281–1295 (2013).
Byrd, K. & Corces, V. G. Visualization of chromatin domains created by the gypsy insulator of Drosophila. J. Cell Biol. 162, 565–574 (2003).
Sexton, T. et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148, 458–472 (2012).
Marsano, R. M., Giordano, E., Messina, G. & Dimitri, P. A new portrait of constitutive heterochromatin: lessons from Drosophila melanogaster. Trends Genet. 35, 615–631 (2019).
Sun, L. H. et al. Heat stress-induced transposon activation correlates with 3D chromatin organization rearrangement in Arabidopsis. Nat. Commun. 11, 1886 (2020).
Liu, Y. L. et al. Back-spliced RNA from retrotransposon binds to centromere and regulates centromeric chromatin loops in maize. PLoS Biol. 18, e3000582 (2020).
Grob, S., Schmid, M. W. & Grossniklaus, U. Hi-C analysis in Arabidopsis identifies the KNOT, a structure with similarities to the flamenco locus of Drosophila. Mol. Cell 55, 678–693 (2014).
Kumar, S., Kaur, S., Seem, K., Kumar, S. & Mohapatra, T. Understanding 3D genome organization and its effect on transcriptional gene regulation under environmental stress in plant: a chromatin perspective. Front. Cell Dev. Biol. 9, 774719 (2021).
Kempfer, R. & Pombo, A. Methods for mapping 3D chromosome architecture. Nat. Rev. Genet. 21, 207–226 (2020).
Ghavi-Helm, Y. et al. Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression. Nat. Genet. 51, 1272–1282 (2019).
Williamson, I. et al. Developmentally regulated Shh expression is robust to TAD perturbations. Development 146, dev179523 (2019).
Hanssen, L. L. P. et al. Tissue-specific CTCF–cohesin-mediated chromatin architecture delimits enhancer interactions and function in vivo. Nat. Cell Biol. 19, 952–961 (2017).
Bourque, G. et al. Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Res. 18, 1752–1762 (2008).
Lunyak, V. V. et al. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science 317, 248–251 (2007).
Schmidt, D. et al. Waves of retrotransposon expansion remodel genome organization and CTCF binding in multiple mammalian lineages. Cell 148, 335–348 (2012).
Ringel, A. R. et al. Repression and 3D-restructuring resolves regulatory conflicts in evolutionarily rearranged genomes. Cell 185, 3689–3704.e21 (2022).
Vian, L. et al. The energetics and physiological impact of cohesin extrusion. Cell 173, 1165–1178.e20 (2018).
Barrington, C. et al. Enhancer accessibility and CTCF occupancy underlie asymmetric TAD architecture and cell type specific genome topology. Nat. Commun. 10, 2908 (2019).
Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016).
Chang, L. H., Ghosh, S. & Noordermeer, D. TADs and their borders: free movement or building a wall? J. Mol. Biol. 432, 643–652 (2020).
Lupianez, D. G. et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene–enhancer interactions. Cell 161, 1012–1025 (2015).
Despang, A. et al. Functional dissection of the Sox9–Kcnj2 locus identifies nonessential and instructive roles of TAD architecture. Nat. Genet. 51, 1263-1271 (2019).
Chen, H. T. et al. Dynamic interplay between enhancer–promoter topology and gene activity. Nat. Genet. 50, 1296-1303 (2018).
Matthews, B. J. & Waxman, D. J. Computational prediction of CTCF/cohesin-based intra-TAD loops that insulate chromatin contacts and gene expression in mouse liver. eLife https://doi.org/10.7554/eLife.34077 (2018).
Diehl, A. G., Ouyang, N. & Boyle, A. P. Transposable elements contribute to cell and species-specific chromatin looping and gene regulation in mammalian genomes. Nat. Commun. 11, 1796 (2020). This study shows that differential TE exaptations between human and mouse contribute to differential looping that is associated with species-specific gene expression.
Lleres, D. et al. CTCF modulates allele-specific sub-TAD organization and imprinted gene activity at the mouse Dlk1-Dio3 and Igf2-H19 domains. Genome Biol. 20, 272 (2019).
Beagan, J. A. & Phillips-Cremins, J. E. On the existence and functionality of topologically associating domains. Nat. Genet. 52, 8–16 (2020).
Wang, J. et al. MIR retrotransposon sequences provide insulators to the human genome. Proc. Natl Acad. Sci. USA 112, E4428–E4437 (2015).
Zhang, Y. et al. Transcriptionally active HERV-H retrotransposons demarcate topologically associating domains in human pluripotent stem cells. Nat. Genet. 51, 1380–1388 (2019). This study shows that HERV-H family TEs serve as both TAD boundary elements and enhancers during pluripotency.
Harmston, N. et al. Topologically associating domains are ancient features that coincide with Metazoan clusters of extreme noncoding conservation. Nat. Commun. 8, 441 (2017).
Cournac, A., Koszul, R. & Mozziconacci, J. The 3D folding of metazoan genomes correlates with the association of similar repetitive elements. Nucleic Acids Res. 44, 245–255 (2016).
Kaaij, L. J. T., Mohn, F., van der Weide, R. H., de Wit, E. & Buhler, M. The ChAHP complex counteracts chromatin looping at CTCF sites that emerged from SINE expansions in mouse. Cell 178, 1437–1451.e14 (2019).
Rao, S. S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Kvon, E. Z., Waymack, R., Gad, M. & Wunderlich, Z. Enhancer redundancy in development and disease. Nat. Rev. Genet. 22, 324–336 (2021).
Lin, X. et al. Nested epistasis enhancer networks for robust genome regulation. Science 377, 1077–1085 (2022).
Nora, E. P. et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell 169, 930–944 e922 (2017). This study shows that CTCF is required to maintain chromatin organization and proper transcriptional activity.
Narendra, V. et al. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science 347, 1017–1021 (2015).
Symmons, O. et al. The Shh topological domain facilitates the action of remote enhancers by reducing the effects of genomic distances. Dev. Cell 39, 529–543 (2016).
Paliou, C. et al. Preformed chromatin topology assists transcriptional robustness of Shh during limb development. Proc. Natl Acad. Sci. USA 116, 12390–12399 (2019).
Ichiyanagi, T. et al. B2 SINE copies serve as a transposable boundary of DNA methylation and histone modifications in the mouse. Mol. Biol. Evol. 38, 2380–2395 (2021).
Kruse, K. et al. Transposable elements drive reorganization of 3D chromatin during early embryogenesis. Preprint at bioRxiv https://doi.org/10.1101/523712 (2019).
Chen, X. et al. Key role for CTCF in establishing chromatin structure in human embryos. Nature 576, 306–310 (2019).
Symmons, O. et al. Functional and topological characteristics of mammalian regulatory domains. Genome Res. 24, 390–400 (2014).
Bourque, G. et al. Ten things you should know about transposable elements. Genome Biol. 19, 199 (2018).
Glinsky, G. V. Contribution of transposable elements and distal enhancers to evolution of human-specific features of interphase chromatin architecture in embryonic stem cells. Chromosome Res. 26, 61–84 (2018).
Sundaram, V. & Wysocka, J. Transposable elements as a potent source of diverse cis-regulatory sequences in mammalian genomes. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190347 (2020).
Davidson, E. H. & Britten, R. J. Regulation of gene-expression — possible role of repetitive sequences. Science 204, 1052–1059 (1979).
Sundaram, V. et al. Widespread contribution of transposable elements to the innovation of gene regulatory networks. Genome Res. 24, 1963–1976 (2014).
Kunarso, G. et al. Transposable elements have rewired the core regulatory network of human embryonic stem cells. Nat. Genet. 42, 631–634 (2010).
Chuong, E. B., Elde, N. C. & Feschotte, C. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 351, 1083–1087 (2016).
Lynch, V. J., Leclerc, R. D., May, G. & Wagner, G. P. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat. Genet. 43, 1154–1159 (2011).
Modzelewski, A. J. et al. A mouse-specific retrotransposon drives a conserved Cdk2ap1 isoform essential for development. Cell 184, 5541–5558.e22 (2021).
Huda, A., Bowen, N. J., Conley, A. B. & Jordan, I. K. Epigenetic regulation of transposable element derived human gene promoters. Gene 475, 39–48 (2011).
Franke, V. et al. Long terminal repeats power evolution of genes and gene expression programs in mammalian oocytes and zygotes. Genome Res. 27, 1384–1394 (2017).
Pasquesi, G. I. M. et al. Vertebrate lineages exhibit diverse patterns of transposable element regulation and expression across tissues. Genome Biol. Evol. 12, 506–521 (2020).
Miao, B. et al. Tissue-specific usage of transposable element-derived promoters in mouse development. Genome Biol. 21, 255 (2020).
Morgan, H. D., Sutherland, H. G., Martin, D. I. & Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 23, 314–318 (1999).
Beyer, U., Moll-Rocek, J., Moll, U. M. & Dobbelstein, M. Endogenous retrovirus drives hitherto unknown proapoptotic p63 isoforms in the male germ line of humans and great apes. Proc. Natl Acad. Sci. USA 108, 3624–3629 (2011).
Notwell, J. H., Chung, T., Heavner, W. & Bejerano, G. A family of transposable elements co-opted into developmental enhancers in the mouse neocortex. Nat. Commun. 6, 6644 (2015).
Bejerano, G. et al. A distal enhancer and an ultraconserved exon are derived from a novel retroposon. Nature 441, 87–90 (2006).
Nishihara, H. et al. Coordinately co-opted multiple transposable elements constitute an enhancer for wnt5a expression in the mammalian secondary palate. PLoS Genet. 12, e1006380 (2016).
Nishihara, H., Smit, A. F. & Okada, N. Functional noncoding sequences derived from SINEs in the mammalian genome. Genome Res. 16, 864–874 (2006).
Prescott, S. L. et al. Enhancer divergence and cis-regulatory evolution in the human and chimp neural crest. Cell 163, 68–83 (2015).
Judd, J., Sanderson, H. & Feschotte, C. Evolution of mouse circadian enhancers from transposable elements. Genome Biol. 22, 193 (2021).
Song, M. et al. Cell-type-specific 3D epigenomes in the developing human cortex. Nature 587, 644–649 (2020). This study shows that TE-mediated formation of promotors may hold together a transcriptional hub that shapes chromatin organization in the developing brain.
Cao, Y. et al. Widespread roles of enhancer-like transposable elements in cell identity and long-range genomic interactions. Genome Res. 29, 40–52 (2019). This study shows that TEs have a widespread role as enhancer-like sequences that contribute to both cell and lineage specificity, and describes the long-range interactions of the MIR and LINE-2 TE families.
Misteli, T. The self-organizing genome: principles of genome architecture and function. Cell 183, 28–45 (2020).
Paulsen, J. et al. Long-range interactions between topologically associating domains shape the four-dimensional genome during differentiation. Nat. Genet. 51, 835–843 (2019).
He, J. et al. Identifying transposable element expression dynamics and heterogeneity during development at the single-cell level with a processing pipeline scTE. Nat. Commun. 12, 1456 (2021).
Consortium, E. P. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Roadmap Epigenomics Consortium et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Burns, K. H. Repetitive DNA in disease. Science 376, 353–354 (2022).
Burns, K. H. Our conflict with transposable elements and its implications for human disease. Annu. Rev. Pathol.-Mech. 15, 51–70 (2020).
Thomas, C. A. et al. Modeling of TREX1-dependent autoimmune disease using human stem cells highlights L1 accumulation as a source of neuroinflammation. Cell Stem Cell 21, 319-331.e8 (2017).
Haws, S. A., Simandi, Z., Barnett, R. J. & Phillips-Cremins, J. E. 3D genome, on repeat: higher-order folding principles of the heterochromatinized repetitive genome. Cell 185, 2690–2707 (2022).
Saksouk, N., Simboeck, E. & Dejardin, J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin. 8, 3 (2015).
Janssen, A., Colmenares, S. U. & Karpen, G. H. Heterochromatin: guardian of the genome. Annu. Rev. Cell Dev. Biol. 34, 265–288 (2018).
Marsano, R. M. & Dimitri, P. Constitutive heterochromatin in eukaryotic genomes: a mine of transposable elements. Cells https://doi.org/10.3390/cells11050761 (2022).
Pimpinelli, S. et al. Transposable elements are stable structural components of Drosophila melanogaster heterochromatin. Proc. Natl Acad. Sci. USA 92, 3804–3808 (1995).
Zamudio, N. & Bourc’his, D. Transposable elements in the mammalian germline: a comfortable niche or a deadly trap? Heredity 105, 92–104 (2010).
Musselman, C. A., Lalonde, M. E., Cote, J. & Kutateladze, T. G. Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 19, 1218–1227 (2012).
Risca, V. I., Denny, S. K., Straight, A. F. & Greenleaf, W. J. Variable chromatin structure revealed by in situ spatially correlated DNA cleavage mapping. Nature 541, 237 (2017).
Oh, I., Choi, S., Jung, Y. & Kim, J. S. Entropic effect of macromolecular crowding enhances binding between nucleosome clutches in heterochromatin, but not in euchromatin. Sci. Rep. 8, 5469 (2018).
Singh, P. B. & Newman, A. G. On the relations of phase separation and Hi-C maps to epigenetics. Roy. Soc. Open Sci. 7, 191976 (2020).
Trono, D. Transposable elements, polydactyl proteins, and the genesis of human-specific transcription networks. Cold Spring Harb. Symp. Quant. Biol. 80, 281–288 (2015).
Spracklin, G. et al. Diverse silent chromatin states modulate genome compartmentalization and loop extrusion barriers. Nat. Struct. Mol. Biol. 30, 38–51 (2023).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Chojnowski, A. et al. Heterochromatin loss as a determinant of progerin-induced DNA damage in Hutchinson–Gilford progeria. Aging Cell 19, e13108 (2020).
Cecco, M. et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell 12, 247–256 (2013).
Zhang, X. L. et al. The loss of heterochromatin is associated with multiscale three-dimensional genome reorganization and aberrant transcription during cellular senescence. Genome Res. 31, 1121–1135 (2021).
Della Valle, F. et al. LINE-1 RNA causes heterochromatin erosion and is a target for amelioration of senescent phenotypes in progeroid syndromes. Sci. Transl. Med. 14, eabl6057 (2022).
Zhou, T., Zhang, R. & Ma, J. The 3D genome structure of single cells. Annu. Rev. Biomed. Data Sci. 4, 21–41 (2021).
Galitsyna, A. A. & Gelfand, M. S. Single-cell Hi-C data analysis: safety in numbers. Brief. Bioinform. https://doi.org/10.1093/bib/bbab316 (2021).
Hoyt, S. J. et al. From telomere to telomere: the transcriptional and epigenetic state of human repeat elements. Science 376, 57 (2022).
Wang, T. et al. The Human Pangenome Project: a global resource to map genomic diversity. Nature 604, 437–446 (2022).
Taberlay, P. C. et al. Three-dimensional disorganization of the cancer genome occurs coincident with long-range genetic and epigenetic alterations. Genome Res. 26, 719–731 (2016).
Jang, H. S. et al. Transposable elements drive widespread expression of oncogenes in human cancers. Nat. Genet. 51, 611–617 (2019).
O’Neill, K., Brocks, D. & Hammell, M. G. Mobile genomics: tools and techniques for tackling transposons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190345 (2020).
Goerner-Potvin, P. & Bourque, G. Computational tools to unmask transposable elements. Nat. Rev. Genet. 19, 688–704 (2018).
Bao, W., Kojima, K. K. & Kohany, O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mob. DNA 6, 11 (2015).
Smit, A. F., Hubley, R. & Green, P. RepeatMasker Open-4.0. Institute for Systems Biology http://www.repeatmasker.org (2015).
Bao, Z. & Eddy, S. R. Automated de novo identification of repeat sequence families in sequenced genomes. Genome Res. 12, 1269–1276 (2002).
Smit, A. F. & Hubley, R. RepeatModeler 1.0.11. Institute for Systems Biology http://www.repeatmasker.org/RepeatModeler/ (2018).
Novak, P., Neumann, P., Pech, J., Steinhaisl, J. & Macas, J. RepeatExplorer: a Galaxy-based web server for genome-wide characterization of eukaryotic repetitive elements from next-generation sequence reads. Bioinformatics 29, 792–793 (2013).
Taylor, D. & Branco, M. R. Inferring protein–DNA binding profiles at interspersed repeats using HiChIP and PAtChER. Methods Mol. Biol. 2607, 199–214 (2023).
Acknowledgements
The authors thank members of the Wang laboratory for helpful discussions related to the project.
Author information
Authors and Affiliations
Contributions
All authors researched data for the manuscript and reviewed/edited the manuscript before submission. H.A.L and T.W discussed the content and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Juan Vaquerizas, who co-reviewed with Maria Rigau; Kenji Ichiyanagi; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- A and B compartments
-
Spatial compartments in which chromosomes are organized. The A compartment broadly correlates with transcriptionally active euchromatic regions, whereas the B compartment broadly correlates with transcriptionally inactive heterochromatic regions.
- CCCT-C binding factor
-
(CTCF). A DNA binding protein that is essential for many cellular processes, including chromatin organization.
- Chromosomal territories
-
Distinct regions of a cell’s nuclear volume that are occupied by each chromosome.
- Cohesin
-
A multiprotein complex that is integral for loop extrusion and formation of topologically associating domain (TAD) boundaries.
- DNA transposons
-
Transposable elements (TEs) that replicate through a cut-and-paste mechanism whereby the element is excised and moved to a different genomic location. DNA transposons are referred to as class II TEs.
- Exaptation
-
When a trait or sequence evolves to function in a manner that is different from the function it originally served.
- Insulator elements
-
DNA sequence motifs that either prevent enhancers from acting on a corresponding promoter or shield genes from the spread of heterochromatin that silences gene expression. In mammals, insulators frequently bind CTCF, often in association with the cohesin complex, and help organize distinct chromatin domains.
- Long terminal repeat
-
(LTR). A class I retrotransposon that is characterized by LTRs flanking an internal coding sequence. LTRs comprise 8% of the human genome.
- Phase separation
-
A mechanism by which genomic compartments with distinct chromatin states are formed. Phase separation refers to the formation of two distinct phases of a solution.
- Retrotransposons
-
Transposable elements (TEs), including long terminal repeats (LTRs), long interspersed nuclear elements (LINEs) and short interspersed nuclear elements (SINEs), that replicate through a copy-and-paste mechanism: an RNA intermediate is reverse transcribed into cDNA and integrated into the genome. Retrotransposons are referred to as class I TEs.
- Topologically associating domains
-
(TADs). Structures that are delimited by boundary elements, and that contain sequences that preferentially interact with themselves rather than with other genomic regions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lawson, H.A., Liang, Y. & Wang, T. Transposable elements in mammalian chromatin organization. Nat Rev Genet 24, 712–723 (2023). https://doi.org/10.1038/s41576-023-00609-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-023-00609-6
This article is cited by
-
Towards targeting transposable elements for cancer therapy
Nature Reviews Cancer (2024)
-
Epigenomic insights into common human disease pathology
Cellular and Molecular Life Sciences (2024)
-
Hi-C sequencing unravels dynamic three-dimensional chromatin interactions in muntjac lineage: insights from chromosome fusions in Fea’s muntjac genome
Chromosome Research (2023)
-
Vitamin C activates young LINE-1 elements in mouse embryonic stem cells via H3K9me3 demethylation
Epigenetics & Chromatin (2023)