Abstract
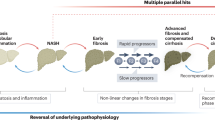
The current approaches to the management of patients with decompensated cirrhosis are based on targeted strategies aimed at preventing or treating specific complications of the disease. The improved knowledge of the pathophysiological background of advanced cirrhosis, represented by a sustained systemic inflammation strictly linked to a circulatory dysfunction, provides a novel paradigm for the management of these patients, with the ambitious target of modifying the course of the disease by preventing the onset of complications and multiorgan failure; these interventions will eventually improve patients’ quality of life, prolong survival and reduce health-care costs. Besides aetiological treatments, these goals could be achieved by persistently antagonizing key pathophysiological events, such as portal hypertension, abnormal bacterial translocation from the gut, liver damage, systemic inflammation, circulatory dysfunction and altered immunological responses. Interestingly, in addition to strategies based on new therapeutic agents, these targets can be tackled by employing drugs that are already used in patients with cirrhosis for different indications or in other clinical settings, including non-absorbable oral antibiotics, non-selective β-blockers, human albumin and statins. The scope of the present Review includes reporting updated information on the treatments that promise to influence the course of advanced cirrhosis and thus act as disease-modifying agents.
Key points
-
The management of decompensated cirrhosis currently addresses the prevention or treatment of specific complications; disease-modifying agents able to modify the course of decompensated cirrhosis still represent an unmet need.
-
Removing aetiological factors can halt the progression of chronic liver disease, yet a substantial portion of patients with decompensated cirrhosis do not benefit from even successful aetiological treatments.
-
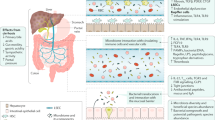
Portal hypertension, abnormal translocation of bacterial products from the gut (‘upstream’ events) and the consequent systemic inflammation and circulatory dysfunction (‘downstream’ events) represent the main targets for mechanistic approaches.
-
Transjugular portosystemic shunt and non-selective β-blockers can be seen as upstream treatments, as they lower portal hypertension and might prevent bacterial translocation.
-
Bacterial translocation can be antagonized by antibiotic-based and non-antibiotic-based interventions, but the former entail the risk of antibiotic resistance, whereas the efficacy of the latter still needs to be demonstrated.
-
Human albumin and statins, which are able to simultaneously target several downstream pathophysiological mechanisms, represent promising disease-modifying agents, as they have proved their efficacy in prospective randomized trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
D’Amico, G., Garcia-Tsao, G. & Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J. Hepatol. 44, 217–231 (2006).
Ginés, P., Fernández, J., Durand, F. & Saliba, F. Management of critically-ill cirrhotic patients. J. Hepatol. 56 (Suppl. 1), S13–S24 (2012).
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144, 1426–1437 (2013).
Albillos, A., Lario, M. & Alvarez-Mon, M. Cirrhosis-associated immune dysfunction: distinct features and clinical relevance. J. Hepatol. 61, 1385–1396 (2014).
Schrier, R. W. et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 8, 1151–1157 (1988).
García-Pagán, J. C., Gracia-Sancho, J. & Bosch, J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J. Hepatol. 57, 458–461 (2012).
Wiese, S., Hove, J. D., Bendtsen, F. & Møller, S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 11, 177–186 (2014).
Arroyo, V., Terra, C. & Ginés, P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J. Hepatol. 46, 935–946 (2007).
Iwakiri, Y. & Groszmannm, R. J. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 43 (2 Suppl. 1), S121–S131 (2006).
Bernardi, M., Moreau, R., Angeli, P., Schnabl, B. & Arroyo, V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 63, 1272–1284 (2015).
Wiest, R., Lawson, M. & Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 60, 197–209 (2014).
Sriskandan, S. & Altmann, D. M. The immunology of sepsis. J. Pathol. 214, 211–223 (2008).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Hotchkiss, R. S. et al. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2, 16045 (2016).
Clària, J. et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 64, 1249–1264 (2016).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. https://doi.org/10.1016/j.jhep.2018.03.024 (2018).
O’Brien, J., Triantos, C. & Burroughs, A. K. Management of varices in patients with cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 402–412 (2013).
Fagiuoli, S. et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig. Liver Dis. 49, 121–137 (2017).
Prakash, R. & Mullen, K. D. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat. Rev. Gastroenterol. Hepatol. 7, 515–525 (2010).
Fernández, J., Tandon, P., Mensa, J. & Garcia-Tsao, G. Antibiotic prophylaxis in cirrhosis: good and bad. Hepatology 63, 2019–2031 (2016).
Böttcher, K. & Pinzani, M. Pathophysiology of liver fibrosis and the methodological barriers to the development of anti-fibrogenic agents. Adv. Drug. Deliv. Rev. 121, 3–8 (2017).
Marcellin, P. et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381, 468–475 (2013).
Di Marco, V. et al. Effects of eradicating hepatitis C virus infection in patients with cirrhosis differ with stage of portal hypertension. Gastroenterology 151, 130–139 (2016).
Nahon, P. et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 152, 142–156 (2017).
Takahashi, H., Shigefuku, R., Maeyama, S. & Suzuki, M. Cirrhosis improvement to alcoholic liver fibrosis after passive abstinence. BMJ Case Rep. 2014, bcr2013201618 (2014).
Lok, A. S. et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 63, 284–306 (2016).
Curry, M. P. et al. Sofosbuvir and Velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 373, 2618–2628 (2015).
Foster, G. R. et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 64, 1224–1231 (2016).
Cheung, M. C. et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 65, 741–747 (2016).
Martini, S. et al. The Italian compassionate use of sofosbuvir in HCV patients waitlisted for liver transplantation: a national real-life experience. Liver Int. 38, 733–741 (2017).
Veldt, B. J. et al. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J. Hepatol. 36, 93–98 (2002).
Carrillo, C. F. et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: analysis of data from the Hepa-C Registry. Hepatology 65, 1810–1822 (2017).
Pascasio, J. M. et al. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J. Hepatol. 67, 1168–1176 (2017).
Shim, J. H. et al. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J. Hepatol. 52, 176–182 (2010).
Alvarez, M. A. et al. Long-term clinical course of decompensated alcoholic cirrhosis. A prospective study of 165 patients. J. Clin. Gastroenterol. 45, 906–911 (2011).
Trebicka, J. et al. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur. J. Gastroenterol. Hepatol. 23, 1218–1225 (2011).
Trebicka, J. Emergency TIPS in a Child-Pugh B patient: when does the window of opportunity open and close? J. Hepatol. 66, 442–450 (2017).
Berres, M. L. et al. CXCL9 is a prognostic marker in patients with liver cirrhosis receiving transjugular intrahepatic portosystemic shunt insertion. J. Hepatol. 62, 332–339 (2015).
Halabi, S. A. et al. Early TIPS versus endoscopic therapy for secondary prophylaxis after management of acute esophageal variceal bleeding in cirrhotic patients: a meta-analysis of randomized controlled trials. J. Gastroenterol. Hepatol. 31, 1519–1526 (2016).
Salerno, F., Cammà, C., Enea, M., Rössle, M. & Wong, F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology 133, 825–834 (2007).
Chen, R. P. et al. Prophylactic use of transjugular intrahepatic portosystemic shunt aids in the treatment of refractory ascites: metaregression and trial sequential meta-analysis. J. Clin. Gastroenterol. 48, 290–299 (2014).
Bureau, C. et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 152, 157–163 (2017).
De Franchis, R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J. Hepatol. 63, 743–752 (2015).
Ohnishi, K. et al. Effects of propranolol on portal hemodynamics in patients with chronic liver disease. Am. J. Gastroenterol. 80, 132–135 (1985).
Bosch, J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology 51, 2214–2218 (2010).
Straub, R. H., Wiest, R., Strauch, U. G., Härle, P. & Schölmerich, J. The role of the sympathetic nervous system in intestinal inflammation. Gut 55, 1640–1649 (2006).
Madsen, B. S., Havelund, T. & Krag, A. Targeting the gut-liver axis in cirrhosis: antibiotics and non-selective β-blockers. Adv. Ther. 30, 659–670 (2013).
Perez-Paramo, M. et al. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology 31, 43–48 (2000).
Worlicek, M. et al. Splanchnic sympathectomy prevents translocation and spreading of E coli but not S aureus in liver cirrhosis. Gut 59, 1127–1134 (2010).
Reiberger, T. et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J. Hepatol. 58, 911–921 (2013).
Senzolo, M. et al. Beta-blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int. 29, 1189–1193 (2009).
Merli, M. et al. The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int. 35, 362–369 (2015).
Sersté, T. et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 52, 1017–1022 (2010).
Mandorfer, M. et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 146, 1680–1690 (2014).
Mookerjee, R. P. et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J. Hepatol. 64, 574–582 (2016).
Shah, N. et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J. Hepatol. 56, 1047–1053 (2012).
Kainth, S. et al. Efficacy and safety of Carvedilol in patients of acute-on-chronic liver failure with small or no esophageal varices. A placebo control open label randomised trial. Hepatology 66 (Suppl. 1), 83A (2017).
Jalan, R. et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J. Hepatol. 60, 1310–1324 (2014).
Runyon, B. A. et al. Effect of selective bowel decontamination with norfloxacin on spontaneous bacterial peritonitis, translocation, and survival in an animal model of cirrhosis. Hepatology 21, 1719–1724 (1995).
Llovet, J. M. et al. Selective intestinal decontamination with norfloxacin reduces bacterial translocation in ascitic cirrhotic rats exposed to hemorrhagic shock. Hepatology 23, 781–787 (1996).
Albillos, A. et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 37, 208–217 (2003).
Soriano, G. et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage. Gastroenterology 103, 1267–1272 (1992).
Bernard, B. et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with ascites: a meta-analysis. Digestion 59 (Suppl. 2), 54–57 (1998).
Loomba, R., Wesley, R., Bain, A., Csako, G. & Pucino, F. Role of fluoroquinolones in the primary prophylaxis of spontaneous bacterial peritonitis: meta-analysis. Clin. Gastroenterol. Hepatol. 7, 487–493 (2009).
Fernández, J. et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 133, 818–824 (2007).
Moreau, R. et al. A randomized trial of 6-month norfloxacin therapy in patients with Child-Pugh class C cirrhosis. J. Hepatol. 66 (Suppl. 1), S1 (2017).
Campillo, B., Dupeyron, C., Richardet, J. P., Mangeney, N. & Leluan, G. Epidemiology of severe hospital-acquired infections in patients with liver cirrhosis: effect of long-term administration of norfloxacin. Clin. Infect. Dis. 26, 1066–1070 (1998).
Fernández, J. et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 55, 1551–1561 (2012).
Adachi, J. A. & Dupont, H. L. Rifaximin: a novel nonabsorbed rifamycin for gastrointestinal disorders. Clin. Infect. Dis. 42, 541–547 (2006).
Reigadas, E. et al. Rifaximin-resistant Clostridium difficile strains isolated from symptomatic patients. Anaerobe 48, 269–272 (2017).
Jiang, Z. D., Ke, S. & Dupont, H. L. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int. J. Antimicrob. Agents 35, 278–281 (2010).
Brown, E. L., Xue, Q., Jiang, Z. D., Xu, Y. & Dupont, H. L. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob. Agents Chemother. 54, 388–396 (2010).
Bajaj, J. S. et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLOS ONE 8, e60042 (2013).
Fiorucci, S. et al. Inhibition of intestinal bacterial translocation with rifaximin modulates lamina propria monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion 66, 246–256 (2002).
Kalambokis, G. N. et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin. Gastroenterol. Hepatol. 10, 815–818 (2012).
Kimer, N. et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: A randomized, double-blind, placebo-controlled trial. Hepatology 65, 592–603 (2017).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Bass, N. M. et al. Rifaximin treatment in hepatic encephalopathy. N. Engl. J. Med. 362, 1071–1081 (2010).
Hanouneh, M. A. et al. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J. Clin. Gastroenterol. 46, 709–715 (2012).
Vlachogiannakos, J. et al. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J. Gastroenterol. Hepatol. 28, 450–455 (2013).
Dong, T., Aronsohn, A., Gautham Reddy, K. & Te, H. S. Rifaximin decreases the incidence and severity of acute kidney injury and hepatorenal syndrome in cirrhosis. Dig. Dis. Sci. 61, 3621–3626 (2016).
Lutz, P. et al. Impact of rifaximin on the frequency and characteristics of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. PLOS ONE 9, e93909 (2014).
Hanafy, A. S. & Hassaneen, A. M. Rifaximin and midodrine improve clinical outcome in refractory ascites including renal function, weight loss, and short-term survival. Eur. J. Gastroenterol. Hepatol. 28, 1455–1461 (2016).
Bajaj, J. S. et al. Oral rifaximin soluble solid dispersion immediate-release 40 mg prevents development of cirrhosis-related complications: a phase 2, randomized, multicenter, double-blind, placebo-controlled trial. Hepatology 64 (Suppl. 1), 1027A (2016).
Kamal, F. et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 29, 1109–1117 (2017).
Gómez-Hurtado, I. et al. Norfloxacin is more effective than rifaximin in avoiding bacterial translocation in an animal model of cirrhosis. Liver Int. 38, 295–302 (2018).
Kimer, N. et al. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: a randomized trial. J. Gastroenterol. Hepatol. 33, 307–314 (2018).
Villa, E. et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 143, 1253–1260 (2012).
Cerini, F. et al. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J. Hepatol. 64, 834–842 (2016).
Fortea, J. I. et al. Enoxaparin does not ameliorate liver fibrosis or portal hypertension in rats with advanced cirrhosis. Liver Int. 38, 102–112 (2018).
Copple, B. L. & Li, T. Pharmacology of bile acid receptors: evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 104, 9–21 (2016).
Distrutti, E. et al. Bile acid activated receptors are targets for regulation of integrity of gastrointestinal mucosa. J. Gastroenterol. 50, 707–719 (2015).
Verbeke, L. et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am. J. Pathol. 185, 409–419 (2015).
Ubeda, M. et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J. Hepatol. 64, 1049–1057 (2016).
Lutz, P. et al. A farnesoid X receptor polymorphism predisposes to spontaneous bacterial peritonitis. Dig. Liver Dis. 46, 1047–1050 (2014).
Verbeke, L. et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 59, 2286–2298 (2014).
Nevens, F. et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 375, 631–643 (2016).
Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 60, 940–947 (2014).
Fukui, H. Gut microbiome-based therapeutics in liver cirrhosis: basic consideration for the next step. J. Clin. Transl Hepatol. 5, 249–260 (2017).
Wiest, R. Albillos, A., Trauner, M., Bajaj, J. S. & Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103 (2017).
Saab, S. et al. Probiotics are helpful in hepatic encephalopathy: a meta-analysis of randomized trials. Liver Int. 36, 986–993 (2016).
Viramontes Hörner, D., Avery, A. & Stow, R. The effects of probiotics and symbiotics on risk factors for hepatic encephalopathy: a systematic review. J. Clin. Gastroenterol. 51, 312–323 (2017).
Woodhouse, C. A., Patel, V. C., Singanayagam, A. & Shawcross, D. L. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 47, 192–202 (2017).
Tousoulis, D. et al. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J. Am. Coll. Cardiol. 63, 2491–2502 (2014).
Trebicka, J. et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology 46, 242–253 (2007).
Abraldes, J. G. et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology 136, 1651–1658 (2009).
Pollo-Flores, P. et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig. Liver. Dis. 47, 957–963 (2015).
Zafra, C. et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology 126, 749–755 (2004).
Abraldes, J. G. et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 150, 1160–1170 (2016).
Yang, Y. H. et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J. Hepatol. 63, 1111–1117 (2015).
Huang, Y. W. et al. Statins Reduce the risk of cirrhosis and its decompensation in chronic hepatitis B patients: a nationwide cohort study. Am. J. Gastroenterol. 111, 976–985 (2016).
Simon, T. G., Bonilla, H., Yan, P., Chung, R. T. & Butt, A. A. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: results from ERCHIVES. Hepatology 64, 47–57 (2016).
Mohanty, A., Tate, J. P. & Garcia-Tsao, G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology 150, 430–440 (2016).
Bang, U. C., Benfield, T. & Bendtsen, F. Reduced risk of decompensation and death associated with use of statins in patients with alcoholic cirrhosis. A nationwide case-cohort study. Aliment. Pharmacol. Ther. 46, 673–680 (2017).
Chang, F. M. et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: a population-based study. Hepatology 66, 896–907 (2017).
Weersink, R. A., Drenth, J. P. & Borgsteede, S. D. Altered pharmacokinetics of statins explain increased risk of rhabdomyolysis in advanced cirrhosis. Gastroenterology 151, 1036 (2016).
Rodríguez, S. et al. A nitric oxide-donating statin decreases portal pressure with a better toxicity profile than conventional statins in cirrhotic rats. Sci. Rep. 7, 40461 (2017).
Sort, P. et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 341, 403–409 (1999).
Bernardi, M., Caraceni, P., Navickis, R. J. & Wilkes, M. M. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology 55, 1172–1181 (2012).
Ortega, R. et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology 36, 941–948 (2002).
Garcia-Martinez, R. et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 58, 1836–1846 (2013).
Bernardi, M., Ricci, C. S. & Zaccherini, G. Role of human albumin in the management of complications of liver cirrhosis. J. Clin. Exp. Hepatol. 4, 302–311 (2014).
Oettl, K. et al. Oxidative albumin damage in chronic liver failure: relation to albumin binding capacity, liver dysfunction and survival. J. Hepatol. 59, 978–983 (2013).
Domenicali, M. et al. Posttranscriptional changes of serum albumin: clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology 60, 1851–1860 (2014).
Giannone, F. A. et al. Ischaemia-modified albumin: a marker of bacterial infection in hospitalized patients with cirrhosis. Liver Int. 35, 2425–2432 (2015).
Baldassarre, M. et al. Albumin homodimers in patients with cirrhosis: clinical and prognostic relevance of a novel identified structural alteration of the molecule. Sci. Rep. 6, 35987 (2016).
Bortoluzzi, A. et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology 57, 266–276 (2013).
Fernández, J. et al. A randomized unblinded pilot study comparing albumin vs. hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology 42, 627–634 (2005).
O’Brien, A. J. et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat. Med. 20, 518–523 (2014).
Gentilini, P. et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J. Hepatol. 30, 639–645 (1999).
Romanelli, R. G. et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World. J. Gastroenterol. 12, 1403–1407 (2006).
Caraceni, P. et al. Long-term albumin administration in decompensated cirrhosis: an open label randomized trial. Lancet 391, 2417–2429 (2018).
Solà, E. et al. Midodrine and albumin for prevention of complications of cirrhosis in patients in the waiting list for liver transplantation. A randomized, multicenter, double-blind, placebo-controlled trial. J. Hepatol. 66 (Suppl. 1), S11 (2017).
Kedarisetty, C. K. et al. Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology 148, 1362–1370 (2015).
Verma, N. et al. Outcomes after multiple courses of granulocyte-colony stimulating factor and growth hormone in decompensated cirrhosis: randomized trial. Hepatology. https://doi.org/10.1002/hep.29763 (2017).
Suk, K. T. et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology 64, 2185–2197 (2016).
Newsome, P. N. et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 3, 25–36 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03202498 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03150459 (2018).
Kalafately, M. et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J. Cachexia Sarcopenia Muscle 8, 113–121 (2017).
Montano-Loza, A. et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 10, 166–173 (2012).
Lai, J. C. et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 66, 564–574 (2017).
Acknowledgements
The authors thank M. Baldassare for figure editing and A. P. Collins for editing the manuscript.
Reviewer information
Nature Reviews Gastroenterology and Hepatology thanks J. Abraldes, M. Peck-Radosavljevic and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all the aspects of this article.
Corresponding author
Ethics declarations
Competing interests
M.B. has been a scientific consultant for Baxalta, CSL Behring and Grifols and speaker for AbbVie Italia, Baxalta, CSL Behring, Gilead Science, Grifols and Octapharma. P.C. has been a speaker for Baxalta, Grifols, Kedrion and Octapharma. He also participates in advisory boards for Grifols and has been a scientific consultant for Kedrion. He is also the recipient of a research grant from Octapharma.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Protein Data Bank: http://www.rcsb.org/pdb/home/home.do
Rights and permissions
About this article
Cite this article
Bernardi, M., Caraceni, P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol 15, 753–764 (2018). https://doi.org/10.1038/s41575-018-0045-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0045-2
This article is cited by
-
Gut liver brain axis in diseases: the implications for therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
How non-alcoholic fatty liver disease and cirrhosis affect the heart
Hepatology International (2023)
-
Successful treatment of acute-on-chronic liver failure secondary to alcoholic cirrhosis with glucocorticoids and albumin: a case report
DARU Journal of Pharmaceutical Sciences (2022)
-
Pathogenese und Progression der Leberzirrhose: aktuelle Perspektiven
Der Gastroenterologe (2021)
-
SUMOylation inhibitors synergize with FXR agonists in combating liver fibrosis
Nature Communications (2020)