Abstract
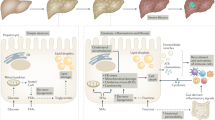
Nonalcoholic steatohepatitis (NASH) is considered the progressive form of nonalcoholic fatty liver disease (NAFLD) and is characterized by liver steatosis, inflammation, hepatocellular injury and different degrees of fibrosis. A central issue in this field relates to the identification of those factors that trigger inflammation, thus fuelling the transition from nonalcoholic fatty liver to NASH. These triggers of liver inflammation might have their origins outside the liver (such as in adipose tissue or the gut) as well as inside the organ (for instance, lipotoxicity, innate immune responses, cell death pathways, mitochondrial dysfunction and endoplasmic reticulum stress), both of which contribute to NASH development. In this Review, we summarize the currently available information on the key upstream triggers of inflammation in NASH. We further delineate the mechanisms by which liver inflammation is resolved and the implications of a defective pro-resolution process. A better knowledge of these mechanisms should help to design targeted therapies able to halt or reverse disease progression.
Key points
-
Triggers of hepatic inflammation have their origins outside as well as inside the liver, and both pathways contribute to the transition from isolated steatosis to NASH.
-
Adipose tissue dysfunction and the hepatic inflammatory response have a fundamental role during NASH development.
-
Cellular and molecular response mechanisms also promote liver inflammation in the absence of a fatty liver by inducing a chronic inflammatory response that results in hepatocyte damage.
-
System biology approaches that integrate metabolomics, proteomics and epigenetic analysis are needed to unravel the molecular signatures of NASH.
-
Abrogation of liver inflammation could be achieved by exploiting active, physiological pro-resolving mechanisms (a ‘pushing for’ strategy) instead of the classical passive blockade of pro-inflammatory mediators (the ‘push back’ strategy).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brunt, E. M. et al. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 1, 15080 (2015).
Bedossa, P. Pathology of non-alcoholic fatty liver disease. Liver Int. 37 (Suppl. 1), 85–89 (2017).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Marengo, A., Jouness, R. I. & Bugianesi, E. Progression and natural history of nonalcoholic fatty liver disease in adults. Clin. Liver Dis. 20, 313–324 (2016).
Goh, G. B. & McCullough, A. J. Natural history of nonalcoholic fatty liver disease. Dig. Dis. Sci. 61, 1226–1233 (2016).
Hardy, T., Oakley, F., Anstee, Q. M. & Day, C. P. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu. Rev. Pathol. 11, 451–496 (2016).
Machado, M. V. & Diehl, A. M. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology 150, 1769–1777 (2016).
Koyama, Y. & Brenner, D. A. Liver inflammation and fibrosis. J. Clin. Invest. 127, 55–64 (2017).
Lee, Y. A., Wallace, M. C. & Friedman, S. L. Pathobiology of liver fibrosis: a translational success story. Gut 64, 830–841 (2015).
Argo, C. K., Northup, P. G., Al-Osaimi, A. M. & Caldwell, S. H. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J. Hepatol. 51, 371–379 (2009).
Hagstrom, H. et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67, 1265–1273 (2017).
Filozof, C. et al. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: facilitating development approaches for an emerging epidemic. Hepatol. Commun. 1, 577–585 (2017).
Kawano, Y. & Cohen, D. E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 48, 434–441 (2013).
Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52, 1836–1846 (2010).
Bray, G. A., Nielsen, S. J. & Popkin, B. M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 79, 537–543 (2004).
Barquera, S. et al. Energy intake from beverages is increasing among Mexican adolescents and adults. J. Nutr. 138, 2454–2461 (2008).
Duffey, K. J. & Popkin, B. M. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity 15, 2739–2747 (2007).
Yang, Z. H., Miyahara, H., Takeo, J. & Katayama, M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab. Syndr. 4, 32 (2012).
Liu, J. et al. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin. Exp. Pharmacol. Physiol. 41, 482–488 (2014).
Crescenzo, R. et al. Increased hepatic de novo lipogenesis and mitochondrial efficiency in a model of obesity induced by diets rich in fructose. Eur. J. Nutr. 52, 537–545 (2013).
Rebollo, A. et al. Liquid fructose downregulates Sirt1 expression and activity and impairs the oxidation of fatty acids in rat and human liver cells. Biochim. Biophys. Acta 1841, 514–524 (2014).
Teff, K. L. et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 89, 2963–2972 (2004).
Charlton, M. et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G825–G834 (2011).
Dupas, J. et al. Progressive induction of type 2 diabetes: effects of a reality-like fructose enriched diet in young wistar rats. PLOS ONE 11, e0146821 (2016).
Kawasaki, T. et al. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J. Nutr. 139, 2067–2071 (2009).
Ren, L. P. et al. Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance. PLOS ONE 7, e30816 (2012).
Sapp, V., Gaffney, L., EauClaire, S. F. & Matthews, R. P. Fructose leads to hepatic steatosis in zebrafish that is reversed by mechanistic target of rapamycin (mTOR) inhibition. Hepatology 60, 1581–1592 (2014).
Spruss, A. et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 50, 1094–1104 (2009).
Rosas-Villegas, A. et al. Differential effect of sucrose and fructose in combination with a high fat diet on intestinal microbiota and kidney oxidative stress. Nutrients 9, pii: E393 (2017).
Kavanagh, K. et al. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am. J. Clin. Nutr. 98, 349–357 (2013).
Aigner, E., Weiss, G. & Datz, C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver. World J. Hepatol. 7, 177–188 (2015).
Aigner, E. et al. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology 135, 680–688 (2008).
Nelson, J. E., Klintworth, H. & Kowdley, K. V. Iron metabolism in nonalcoholic fatty liver disease. Curr. Gastroenterol. Rep. 14, 8–16 (2012).
Ceccarelli, D., Gallesi, D., Giovannini, F., Ferrali, M. & Masini, A. Relationship between free iron level and rat liver mitochondrial dysfunction in experimental dietary iron overload. Biochem. Biophys. Res. Commun. 209, 53–59 (1995).
Galaris, D. & Pantopoulos, K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit. Rev. Clin. Lab Sci. 45, 1–23 (2008).
Dixon, S. J. & Stockwell, B. R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014).
Britton, L. J., Subramaniam, V. N. & Crawford, D. H. Iron and non-alcoholic fatty liver disease. World J. Gastroenterol. 22, 8112–8122 (2016).
al-Othman, A. A., Rosenstein, F. & Lei, K. Y. Copper deficiency alters plasma pool size, percent composition and concentration of lipoprotein components in rats. J. Nutr. 122, 1199–1204 (1992).
al-Othman, A. A., Rosenstein, F. & Lei, K. Y. Copper deficiency increases in vivo hepatic synthesis of fatty acids, triacylglycerols, and phospholipids in rats. Proc. Soc. Exp. Biol. Med. 204, 97–103 (1993).
Stattermayer, A. F. et al. Low hepatic copper content and PNPLA3 polymorphism in non-alcoholic fatty liver disease in patients without metabolic syndrome. J. Trace Elem. Med. Biol. 39, 100–107 (2017).
Aigner, E. et al. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 105, 1978–1985 (2010).
Tallino, S. et al. Nutrigenomics analysis reveals that copper deficiency and dietary sucrose up-regulate inflammation, fibrosis and lipogenic pathways in a mature rat model of nonalcoholic fatty liver disease. J. Nutr. Biochem. 26, 996–1006 (2015).
Leamy, A. K., Egnatchik, R. A. & Young, J. D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 52, 165–174 (2013).
Alisi, A. et al. Relationship between portal chronic inflammation and disease severity in paediatric non-alcoholic fatty liver disease. Dig. Liver Dis. 43, 143–146 (2011).
Enjoji, M., Yasutake, K., Kohjima, M. & Nakamuta, M. Nutrition and nonalcoholic fatty liver disease: the significance of cholesterol. Int. J. Hepatol. 2012, 925807 (2012).
Arguello, G., Balboa, E., Arrese, M. & Zanlungo, S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta 1852, 1765–1778 (2015).
Puri, P. et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46, 1081–1090 (2007).
Min, H. K. et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15, 665–674 (2012).
Al-Rasadi, K., Rizzo, M., Montalto, G. & Berg, G. Nonalcoholic fatty liver disease, cardiovascular risk, and carotid inflammation. Angiology 66, 601–603 (2015).
Arrese, M. & Karpen, S. J. Nuclear receptors, inflammation, and liver disease: insights for cholestatic and fatty liver diseases. Clin. Pharmacol. Ther. 87, 473–478 (2010).
Asrih, M. & Jornayvaz, F. R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J. Endocrinol. 218, R25–R36 (2013).
Wouters, K. et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 48, 474–486 (2008).
Das, U. N. Biological significance of essential fatty acids. J. Assoc. Physicians India 54, 309–319 (2006).
Seki, H., Tani, Y. & Arita, M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E. Prostaglandins Other Lipid Mediat. 89, 126–130 (2009).
Araya, J. et al. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 106, 635–643 (2004).
Argo, C. K. et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J. Hepatol. 62, 190–197 (2015).
He, X. X. et al. Effectiveness of Omega-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. PLOS ONE 11, e0162368 (2016).
Feldstein, A. E. et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Lipid Res. 51, 3046–3054 (2010).
Santoro, N. et al. Oxidized fatty acids: a potential pathogenic link between fatty liver and type 2 diabetes in obese adolescents? Antioxid. Redox Signal 20, 383–389 (2014).
Dongiovanni, P., Rametta, R., Meroni, M. & Valenti, L. The role of insulin resistance in nonalcoholic steatohepatitis and liver disease development — a potential therapeutic target? Expert Rev. Gastroenterol. Hepatol. 10, 229–242 (2016).
Masarone, M. et al. Liver biopsy in type 2 diabetes mellitus: steatohepatitis represents the sole feature of liver damage. PLOS ONE 12, e0178473 (2017).
Bieghs, V. et al. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLOS ONE 7, e30668 (2012).
Cortez-Pinto, H., Camilo, M. E., Baptista, A., De Oliveira, A. G. & De Moura, M. C. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin. Nutr. 18, 353–358 (1999).
Balato, N. et al. Nonalcoholic fatty liver disease, spleen and psoriasis: new aspects of low-grade chronic inflammation. World J. Gastroenterol. 21, 6892–6897 (2015).
van der Poorten, D. & George, J. Disease-specific mechanisms of fibrosis: hepatitis C virus and nonalcoholic steatohepatitis. Clin. Liver Dis. 12, 805–824, ix (2008).
Stanton, M. C. et al. Inflammatory signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm 8, 8 (2011).
Cancello, R. et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 55, 1554–1561 (2006).
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J. & Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105, 141–150 (2014).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Lanthier, N. et al. Kupffer cell depletion prevents but has no therapeutic effect on metabolic and inflammatory changes induced by a high-fat diet. FASEB J 25, 4301–4311 (2011).
Panee, J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 60, 1–12 (2012).
Maximos, M. et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology 61, 153–160 (2015).
Lomonaco, R. et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55, 1389–1397 (2012).
Rotman, Y. & Neuschwander-Tetri, B. A. Liver fat accumulation as a barometer of insulin responsiveness again points to adipose tissue as the culprit. Hepatology 65, 1088–1090 (2017).
Bugianesi, E. et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 48, 634–642 (2005).
Fabbrini, E. et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 139, 448–455 (2010).
Adolph, T. E., Grander, C., Grabherr, F. & Tilg, H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int. J. Mol. Sci. 18, E1649 (2017).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Adipokines in nonalcoholic fatty liver disease. Metabolism 65, 1062–1079 (2016).
Buechler, C., Wanninger, J. & Neumeier, M. Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 17, 2801–2811 (2011).
Hui, J. M. et al. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 40, 46–54 (2004).
Wieckowska, A. et al. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 103, 1372–1379 (2008).
Tsochatzis, E., Papatheodoridis, G. V. & Archimandritis, A. J. The evolving role of leptin and adiponectin in chronic liver diseases. Am. J. Gastroenterol. 101, 2629–2640 (2006).
Hendy, O. M. et al. Evaluation of circulating zonulin as a potential marker in the pathogenesis of nonalcoholic fatty liver disease. APMIS 125, 607–613 (2017).
Polyzos, S. A. et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 59, 30–43 (2016).
Procaccini, C. et al. Leptin: the prototypic adipocytokine and its role in NAFLD. Curr. Pharm. Des. 16, 1902–1912 (2010).
Abu-Tair, L., Doron, S., Mahamid, M., Amer, J. & Safadi, R. Leptin modulates lymphocytes’ adherence to hepatic stellate cells is associated with oxidative status alterations. Mitochondrion 13, 473–480 (2013).
Brandl, K. & Schnabl, B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 33, 128–133 (2017).
Boursier, J. et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63, 764–775 (2016).
Farhadi, A. et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 28, 1026–1033 (2008).
Csak, T. et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G433–G441 (2011).
Etienne-Mesmin, L., Vijay-Kumar, M., Gewirtz, A. T. & Chassaing, B. Hepatocyte toll-like receptor 5 promotes bacterial clearance and protects mice against high-fat diet-induced liver disease. Cell. Mol. Gastroenterol. Hepatol. 2, 584–604 (2016).
Schneider, K. M. et al. CX3CR1 is a gatekeeper for intestinal barrier integrity in mice: limiting steatohepatitis by maintaining intestinal homeostasis. Hepatology 62, 1405–1416 (2015).
Rahman, K. et al. Loss of junctional adhesion molecule A promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology 151, 733–746.e12 (2016).
Jiang, W. et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 5, 8096 (2015).
Luck, H. et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 21, 527–542 (2015).
du Plessis, J. et al. Pro-inflammatory cytokines but not endotoxin-related parameters associate with disease severity in patients with NAFLD. PLOS ONE 11, e0166048 (2016).
Pang, J. et al. Significant positive association of endotoxemia with histological severity in 237 patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 46, 175–182 (2017).
Kitabatake, H. et al. Association between endotoxemia and histological features of nonalcoholic fatty liver disease. World J. Gastroenterol. 23, 712–722 (2017).
Freudenberg, M. A., Freudenberg, N. & Galanos, C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br. J. Exp. Pathol. 63, 56–65 (1982).
Albenberg, L. G. & Wu, G. D. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology 146, 1564–1572 (2014).
Arab, J. P., Karpen, S. J., Dawson, P. A., Arrese, M. & Trauner, M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 65, 350–362 (2017).
Chow, M. D., Lee, Y. H. & Guo, G. L. The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mol. Aspects Med. 56, 34–44 (2017).
Ridlon, J. M., Kang, D. J., Hylemon, P. B. & Bajaj, J. S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 30, 332–338 (2014).
Jungst, C. et al. Intrahepatic cholestasis in common chronic liver diseases. Eur. J. Clin. Invest. 43, 1069–1083 (2013).
Pizarro, M. et al. Bile secretory function in the obese Zucker rat: evidence of cholestasis and altered canalicular transport function. Gut 53, 1837–1843 (2004).
Aranha, M. M. et al. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur. J. Gastroenterol. Hepatol. 20, 519–525 (2008).
Cai, S. Y. & Boyer, J. L. Studies on the mechanisms of bile acid initiated hepatic inflammation in cholestatic liver injury. Inflamm. Cell Signal 4, pii: e1561 (2017).
Li, M., Cai, S. Y. & Boyer, J. L. Mechanisms of bile acid mediated inflammation in the liver. Mol. Aspects Med. 56, 45–53 (2017).
Puri, P. et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology https://doi.org/10.1002/hep.29359 (2017).
Jahn, D. & Geier, A. Bile acids in NASH: pathophysiological driving force or innocent bystanders? Hepatology 67, 464–466 (2017).
Magee, N., Zou, A. & Zhang, Y. Pathogenesis of nonalcoholic steatohepatitis: interactions between liver parenchymal and nonparenchymal cells. Biomed. Res. Int. 2016, 5170402 (2016).
Ikura, Y. et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology 43, 506–514 (2006).
Terman, A., Kurz, T., Gustafsson, B. & Brunk, U. T. Lysosomal labilization. IUBMB Life 58, 531–539 (2006).
Zhao, G. N. et al. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat. Med. 23, 742–752 (2017).
Krenkel, O. & Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 17, 306–321 (2017).
Chatterjee, S. et al. P2X7 receptor-NADPH oxidase axis mediates protein radical formation and kupffer cell activation in carbon tetrachloride-mediated steatohepatitis in obese mice. Free Radic. Biol. Med. 52, 1666–1679 (2012).
Dunning, S. et al. Superoxide anions and hydrogen peroxide inhibit proliferation of activated rat stellate cells and induce different modes of cell death. Liver Int. 29, 922–932 (2009).
Zhan, S. S. et al. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 43, 435–443 (2006).
Leung, T. M. & Nieto, N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 58, 395–398 (2013).
Choi, S. & Diehl, A. M. Role of inflammation in nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 21, 702–707 (2005).
Chung, H. K. et al. The indole derivative NecroX-7 improves nonalcoholic steatohepatitis in ob/ob mice through suppression of mitochondrial ROS/RNS and inflammation. Liver Int. 35, 1341–1353 (2015).
Wree, A., Broderick, L., Canbay, A., Hoffman, H. M. & Feldstein, A. E. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 10, 627–636 (2013).
Yamaguchi, K. et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45, 1366–1374 (2007).
Li, Z. Z., Berk, M., McIntyre, T. M. & Feldstein, A. E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J. Biol. Chem. 284, 5637–5644 (2009).
Monetti, M. et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6, 69–78 (2007).
Neuschwander-Tetri, B. A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52, 774–788 (2010).
Cynis, H. et al. Inhibition of glutaminyl cyclases alleviates CCL2-mediated inflammation of non-alcoholic fatty liver disease in mice. Int. J. Exp. Pathol. 94, 217–225 (2013).
Da Silva Morais, A. et al. Prevention of steatohepatitis by pioglitazone: implication of adiponectin-dependent inhibition of SREBP-1c and inflammation. J. Hepatol. 50, 489–500 (2009).
Das, S. et al. NADPH oxidase-derived peroxynitrite drives inflammation in mice and human nonalcoholic steatohepatitis via TLR4-lipid raft recruitment. Am. J. Pathol. 185, 1944–1957 (2015).
Chen, H. L. et al. Kefir peptides prevent high-fructose corn syrup-induced non-alcoholic fatty liver disease in a murine model by modulation of inflammation and the JAK2 signaling pathway. Nutr. Diabetes 6, e237 (2016).
Han, M. S. et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 49, 84–97 (2008).
Hirsova, P. et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology 150, 956–967 (2016).
Kakazu, E., Mauer, A. S., Yin, M. & Malhi, H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J. Lipid Res. 57, 233–245 (2016).
Cazanave, S. C. et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J. Biol. Chem. 286, 39336–39348 (2011).
Ibrahim, S. H. et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 63, 731–744 (2016).
Mari, M. et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 4, 185–198 (2006).
Andreozzi, P. et al. [Predictors of liver fibrosis in patients with non-alcoholic fatty liver disease. The role of metabolic syndrome, insulin-resistance and inflammation]. Recenti Prog. Med. 103, 570–574 (2012).
Ioannou, G. N. et al. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J. Lipid Res. 58, 1067–1079 (2017).
Rajamaki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLOS ONE 5, e11765 (2010).
Wree, A. et al. NLRP3 inflammasome driven liver injury and fibrosis: roles of IL- 17 and TNF in mice. Hepatology https://doi.org/10.1002/hep.29523 (2017).
Gan, L. T. et al. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J. Hepatol. 61, 1376–1384 (2014).
Foroughi, M. et al. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv. Biomed. Res. 5, 28 (2016).
Fujii, H. & Kawada, N. Inflammation and fibrogenesis in steatohepatitis. J. Gastroenterol. 47, 215–225 (2012).
Perez-Carreras, M. et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38, 999–1007 (2003).
Rolo, A. P., Teodoro, J. S. & Palmeira, C. M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 52, 59–69 (2012).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Carlos, D. et al. Mitochondrial DNA activates the NLRP3 inflammasome and predisposes to type 1 diabetes in murine model. Front. Immunol. 8, 164 (2017).
Luedde, T., Kaplowitz, N. & Schwabe, R. F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147, 765–783.e4 (2014).
Hirsova, P. & Gores, G. J. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 1, 17–27 (2015).
Iredale, J. P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 (1998).
Wree, A., Mehal, W. Z. & Feldstein, A. E. Targeting cell death and sterile inflammation loop for the treatment of nonalcoholic steatohepatitis. Semin. Liver Dis. 36, 27–36 (2016).
Feldstein, A. E. et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125, 437–443 (2003).
Wang, P. X. et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat. Med. 23, 439–449 (2017).
Feldstein, A. E. et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 40, 185–194 (2004).
Hirsova, P., Ibrahim, S. H., Gores, G. J. & Malhi, H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J. Lipid Res. 57, 1758–1770 (2016).
Canbay, A. et al. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab. Invest. 83, 655–663 (2003).
Watanabe, A. et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology 46, 1509–1518 (2007).
Hirsova, P. et al. TRAIL deletion prevents liver inflammation but not adipose tissue inflammation during murine diet-induced obesity. Hepatol. Commun. 1, 648–662 (2017).
Idrissova, L. et al. TRAIL receptor deletion in mice suppresses the inflammation of nutrient excess. J. Hepatol. 62, 1156–1163 (2015).
Canbay, A. et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 38, 1188–1198 (2003).
Xiang, M. et al. Targeting hepatic TRAF1-ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J. Hepatol. 64, 1365–1377 (2016).
Ha, H., Han, D. & Choi, Y. TRAF-mediated TNFR-family signaling. Curr. Protoc. Immunol. https://doi.org/10.1002/0471142735.im1109ds87 (2009).
Zhang, P. et al. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat. Med. 24, 84–94 (2018).
Machado, M. V. et al. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut 64, 1148–1157 (2015).
Johnson, E. S. et al. Metabolomic profiling reveals a role for caspase-2 in lipoapoptosis. J. Biol. Chem. 288, 14463–14475 (2013).
Machado, M. V. et al. Caspase-2 promotes obesity, the metabolic syndrome and nonalcoholic fatty liver disease. Cell Death Dis. 7, e2096 (2016).
Linkermann, A. & Green, D. R. Necroptosis. N. Engl. J. Med. 370, 455–465 (2014).
Dara, L., Liu, Z.-X. & Kaplowitz, N. Questions and controversies: the role of necroptosis in liver disease. Cell Death Discov. 2, 16089 (2016).
Afonso, M. B. et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin. Sci. 129, 721–739 (2015).
Gautheron, J. et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol. Med. 6, 1062–1074 (2014).
Hatting, M. et al. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology 57, 2189–2201 (2013).
Wang, Z., Jiang, H., Chen, S., Du, F. & Wang, X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148, 228–243 (2012).
Vince, J. E. et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 (2012).
Cookson, B. T. & Brennan, M. A. Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001).
Alegre, F., Pelegrin, P. & Feldstein, A. E. Inflammasomes in liver fibrosis. Semin. Liver Dis. 37, 119–127 (2017).
Jo, E. K., Kim, J. K., Shin, D. M. & Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 13, 148–159 (2016).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153 (2016).
Wree, A. et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59, 898–910 (2014).
Wree, A. et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. 92, 1069–1082 (2014).
Mridha, A. R. et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 66, 1037–1046 (2017).
Baroja-Mazo, A. et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 15, 738–748 (2014).
Franklin, B. S. et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 15, 727–737 (2014).
Fontana, L. et al. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology 57, 995–1004 (2013).
Yang, L., Li, P., Fu, S., Calay, E. S. & Hotamisligil, G. S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 (2010).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Amir, M. & Czaja, M. J. Autophagy in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 5, 159–166 (2011).
Kwanten, W. J. et al. Hepatocellular autophagy modulates the unfolded protein response and fasting-induced steatosis in mice. Am J. Physiol. Gastrointest. Liver Physiol. 311, G599–G609 (2016).
Lemasters, J. J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5 (2005).
Wagner, M., Zollner, G. & Trauner, M. Nuclear receptors in liver disease. Hepatology 53, 1023–1034 (2011).
Schmitz, G. & Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47, 147–155 (2008).
Larter, C. Z. et al. Activation of peroxisome proliferator-activated receptor alpha by dietary fish oil attenuates steatosis, but does not prevent experimental steatohepatitis because of hepatic lipoperoxide accumulation. J. Gastroenterol. Hepatol. 23, 267–275 (2008).
Shan, W. et al. Peroxisome proliferator-activated receptor-beta/delta protects against chemically induced liver toxicity in mice. Hepatology 47, 225–235 (2008).
Bouhlel, M. A. et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 (2007).
Luo, W., Xu, Q., Wang, Q., Wu, H. & Hua, J. Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci. Rep. 7, 44612 (2017).
Griffett, K. et al. The LXR inverse agonist SR9238 suppresses fibrosis in a model of non-alcoholic steatohepatitis. Mol. Metab. 4, 353–357 (2015).
Cave, M. C. et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim. Biophys. Acta 1859, 1083–1099 (2016).
Tanaka, N., Aoyama, T., Kimura, S. & Gonzalez, F. J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 179, 142–157 (2017).
Meex, R. C. R. & Watt, M. J. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 13, 509–520 (2017).
Lai, K. K., Kolippakkam, D. & Beretta, L. Comprehensive and quantitative proteome profiling of the mouse liver and plasma. Hepatology 47, 1043–1051 (2008).
Joshi-Barve, S. et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 46, 823–830 (2007).
Meex, R. C. et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab. 22, 1078–1089 (2015).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Jung, T. W., Yoo, H. J. & Choi, K. M. Implication of hepatokines in metabolic disorders and cardiovascular diseases. BBA Clin. 5, 108–113 (2016).
Lebensztejn, D. M., Flisiak-Jackiewicz, M., Bialokoz-Kalinowska, I., Bobrus-Chociej, A. & Kowalska, I. Hepatokines and non-alcoholic fatty liver disease. Acta Biochim. Pol. 63, 459–467 (2016).
Lotze, M. T. et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 220, 60–81 (2007).
Arrese, M., Cabrera, D., Kalergis, A. M. & Feldstein, A. E. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 61, 1294–1303 (2016).
Kesar, V. & Odin, J. A. Toll-like receptors and liver disease. Liver Int. 34, 184–196 (2014).
Bieghs, V. & Trautwein, C. Innate immune signaling and gut-liver interactions in non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 3, 377–385 (2014).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Miura, K. et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 57, 577–589 (2013).
Cai, C. et al. NLRP3 deletion inhibits the non-alcoholic steatohepatitis development and inflammation in kupffer cells induced by palmitic acid. Inflammation 40, 1875–1883 (2017).
Watanabe, A. et al. Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1248–G1257 (2009).
Krenkel, O. & Tacke, F. Macrophages in nonalcoholic fatty liver disease: a role model of pathogenic immunometabolism. Semin. Liver Dis. 37, 189–197 (2017).
Baeck, C. et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61, 416–426 (2012).
Reid, D. T. et al. Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation. PLOS ONE 11, e0159524 (2016).
Tosello-Trampont, A. C., Landes, S. G., Nguyen, V., Novobrantseva, T. I. & Hahn, Y. S. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J. Biol. Chem. 287, 40161–40172 (2012).
Soehnlein, O., Steffens, S., Hidalgo, A. & Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 17, 248–261 (2017).
Xu, R., Huang, H., Zhang, Z. & Wang, F. S. The role of neutrophils in the development of liver diseases. Cell. Mol. Immunol. 11, 224–231 (2014).
Rensen, S. S. et al. Neutrophil-derived myeloperoxidase aggravates non-alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLOS ONE 7, e52411 (2012).
Ibusuki, R. et al. Transgenic expression of human neutrophil peptide-1 enhances hepatic fibrosis in mice fed a choline-deficient, L-amino acid-defined diet. Liver Int. 33, 1549–1556 (2013).
Alkhouri, N. et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 32, 297–302 (2012).
Anstee, Q. M., Seth, D. & Day, C. P. Genetic factors that affect risk of alcoholic and nonalcoholic fatty liver disease. Gastroenterology 150, 1728–1744.e7 (2016).
Dongiovanni, P. et al. PNPLA3 I148M polymorphism and progressive liver disease. World J. Gastroenterol. 19, 6969–6978 (2013).
Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40, 1461–1465 (2008).
Li, J. Z. et al. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Invest. 122, 4130–4144 (2012).
BasuRay, S., Smagris, E., Cohen, J. C. & Hobbs, H. H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 66, 1111–1124 (2017).
Bruschi, F. V. et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology 65, 1875–1890 (2017).
Mancina, R. M. et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 150, 1219–1230.e6 (2016).
Sookoian, S. et al. Mitochondrial genome architecture in non-alcoholic fatty liver disease. J. Pathol. 240, 437–449 (2016).
Lee, J., Kim, Y., Friso, S. & Choi, S. W. Epigenetics in non-alcoholic fatty liver disease. Mol. Aspects Med. 54, 78–88 (2017).
Pirola, C. J. et al. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut 62, 1356–1363 (2013).
Murphy, S. K. et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology 145, 1076–1087 (2013).
Celikbilek, M. et al. Circulating microRNAs in patients with non-alcoholic fatty liver disease. World J. Hepatol. 6, 613–620 (2014).
Fernandez-Hernando, C., Suarez, Y., Rayner, K. J. & Moore, K. J. MicroRNAs in lipid metabolism. Curr. Opin. Lipidol. 22, 86–92 (2011).
Gori, M., Arciello, M. & Balsano, C. MicroRNAs in nonalcoholic fatty liver disease: novel biomarkers and prognostic tools during the transition from steatosis to hepatocarcinoma. Biomed. Res. Int. 2014, 741465 (2014).
Tryndyak, V. P. Plasma microRNAs are sensitive indicators of inter-strain differences in the severity of liver injury induced in mice by a choline- and folate-deficient diet. Toxicol Appl Pharmacol. 262, 52–59 (2012).
Cortez, M. A. et al. MicroRNAs in body fluids — the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 8, 467–477 (2011).
Fullerton, J. N. & Gilroy, D. W. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 (2016).
Rius, B. et al. The specialized pro-resolving lipid mediator maresin 1 protects hepatocytes from lipotoxic and hypoxia-induced endoplasmic reticulum stress. FASEB J 31, 5384–5398 (2017).
Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 66, 1300–1312 (2017).
Li, P., He, K., Li, J., Liu, Z. & Gong, J. The role of Kupffer cells in hepatic diseases. Mol. Immunol. 85, 222–229 (2017).
Alisi, A. et al. The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediators Inflamm. 2017, 8162421 (2017).
Serhan, C. N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 31, 1273–1288 (2017).
Lopez-Vicario, C. et al. Molecular interplay between delta5/delta6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 63, 344–355 (2014).
Araya, J. et al. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obes 18, 1460–1463 (2010).
Rius, B. et al. Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 28, 836–848 (2014).
Borgeson, E. et al. Lipoxin A4 attenuates adipose inflammation. FASEB J. 26, 4287–4294 (2012).
Martinez-Fernandez, L. et al. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 31, 2135–2145 (2017).
Hotta, K. et al. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med. Genet. 11, 172 (2010).
Petta, S. et al. IL28B and PNPLA3 polymorphisms affect histological liver damage in patients with non-alcoholic fatty liver disease. J. Hepatol. 56, 1356–1362 (2012).
Rotman, Y., Koh, C., Zmuda, J. M., Kleiner, D. E. & Liang, T. J. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 52, 894–903 (2010).
Dongiovanni, P. et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 61, 506–514 (2015).
Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356 (2014).
Liu, Y. L. et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 5, 4309 (2014).
Petta, S. et al. Glucokinase regulatory protein gene polymorphism affects liver fibrosis in non-alcoholic fatty liver disease. PLOS ONE 9, e87523 (2014).
Santoro, N. et al. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology 55, 781–789 (2012).
Cefalu, A. B. et al. A novel APOB mutation identified by exome sequencing cosegregates with steatosis, liver cancer, and hypocholesterolemia. Arterioscler Thromb. Vasc. Biol. 33, 2021–2025 (2013).
Di Filippo, M. et al. Homozygous MTTP and APOB mutations may lead to hepatic steatosis and fibrosis despite metabolic differences in congenital hypocholesterolemia. J. Hepatol. 61, 891–902 (2014).
Nobili, V. et al. A 4-polymorphism risk score predicts steatohepatitis in children with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 58, 632–636 (2014).
Valenti, L. et al. LPIN1 rs13412852 polymorphism in pediatric nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 54, 588–593 (2012).
Fares, R. et al. The UCP2 -866 G>A promoter region polymorphism is associated with nonalcoholic steatohepatitis. Liver Int. 35, 1574–1580 (2015).
Chalasani, N. et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology 139, 1567–1576 (2010).
Bernard, S. et al. Association between microsomal triglyceride transfer protein gene polymorphism and the biological features of liver steatosis in patients with type II diabetes. Diabetologia 43, 995–999 (2000).
Oliveira, C. P. et al. Association of polymorphisms of glutamate-cystein ligase and microsomal triglyceride transfer protein genes in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 25, 357–361 (2010).
Chen, Y. et al. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology 45, 1118–1128 (2007).
Petta, S. et al. Interferon lambda 4 rs368234815 TT> δG variant is associated with liver damage in patients with nonalcoholic fatty liver disease. Hepatology 66, 1885–1893 (2017).
Acknowledgements
The work of the authors was supported by the US National Institutes of Health (R01 DK113592-01,1U01AA024206-01 TO A.E.F.), the German Research Foundation (SCHU3146/1-2 to S.S.) and the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1150327 to M.A. and 11171001 to D.C.) and the Comisión Nacional de Investigación Científica y Tecnológica (grant CONICYT PIA/Basal PFB12, Basal Centre for Excellence in Science and Technology to M.A.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. S.S., M.A. and A.E.F. contributed substantially to the discussion of content. S.S., D.C. and M.A. wrote the manuscript, and all authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hepatocyte ballooning
-
A histomorphological change observed in hepatocytes during cell death, usually defined on tissue stained with haematoxylin and eosin as a cellular enlargement in normal hepatocyte diameter of 1.5–2 times with rarefied cytoplasm.
- Lipotoxicity
-
The toxic effects of excessive free fatty acids (FFAs) leading to cellular injury and death caused by FFAs themselves or by their metabolites.
- Sterile cell death
-
Cell death induced by activation of a sterile inflammatory response via interactions with immune cells that can result in a full inflammatory response in the absence of an infection.
- Gut–liver axis
-
Physiological crosstalk between the liver and gut that consists of a myriad of signals with both immunological and metabolic effects in each organ.
- Leaky gut
-
Leakage that occurs as a result of damage to the intestinal lining, which makes the internal environment of the gut leak out of the intestines into the bloodstream, triggering an immune response.
- Fenton reaction
-
A reaction in which iron acts as a catalyst and reacts with hydrogen peroxide to generate highly reactive hydroxyl radicals.
- Unfolded protein response
-
(UPR). A response that is activated by an accumulation of misfolded and/or unfolded proteins in the endoplasmic reticulum. The unfolded protein response aims to restore normal cellular function by inhibiting translation, degrading misfolded proteins and activating signalling pathways that increase the production of molecular chaperones that participate in protein folding.
- Free cholesterol
-
A measure of cholesterol that has not been esterified.
- Intestinal dysbiosis
-
A state in which there are perturbations of the composition of gut commensal communities.
- Autacoids
-
Physiologically active factors that are produced by the body and typically act near the site of synthesis for a brief duration.
Rights and permissions
About this article
Cite this article
Schuster, S., Cabrera, D., Arrese, M. et al. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol 15, 349–364 (2018). https://doi.org/10.1038/s41575-018-0009-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0009-6
This article is cited by
-
Development of non-alcoholic steatohepatitis is associated with gut microbiota but not with oxysterol enzymes CH25H, EBI2, or CYP7B1 in mice
BMC Microbiology (2024)
-
Integrated traditional Chinese and Western medicine in the prevention and treatment of non-alcoholic fatty liver disease: future directions and strategies
Chinese Medicine (2024)
-
Machine learning-based integration identifies the ferroptosis hub genes in nonalcoholic steatohepatitis
Lipids in Health and Disease (2024)
-
Sonic hedgehog-heat shock protein 90β axis promotes the development of nonalcoholic steatohepatitis in mice
Nature Communications (2024)
-
Identification of pyroptosis-related gene signature in nonalcoholic steatohepatitis
Scientific Reports (2024)