Abstract
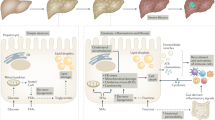
Chronic liver diseases such as nonalcoholic fatty liver disease (NAFLD) or viral hepatitis are characterized by persistent inflammation and subsequent liver fibrosis. Liver fibrosis critically determines long-term morbidity (for example, cirrhosis or liver cancer) and mortality in NAFLD and nonalcoholic steatohepatitis (NASH). Inflammation represents the concerted response of various hepatic cell types to hepatocellular death and inflammatory signals, which are related to intrahepatic injury pathways or extrahepatic mediators from the gut–liver axis and the circulation. Single-cell technologies have revealed the heterogeneity of immune cell activation concerning disease states and the spatial organization within the liver, including resident and recruited macrophages, neutrophils as mediators of tissue repair, auto-aggressive features of T cells as well as various innate lymphoid cell and unconventional T cell populations. Inflammatory responses drive the activation of hepatic stellate cells (HSCs), and HSC subsets, in turn, modulate immune mechanisms via chemokines and cytokines or transdifferentiate into matrix-producing myofibroblasts. Current advances in understanding the pathogenesis of inflammation and fibrosis in the liver, mainly focused on NAFLD or NASH owing to the high unmet medical need, have led to the identification of several therapeutic targets. In this Review, we summarize the inflammatory mediators and cells in the diseased liver, fibrogenic pathways and their therapeutic implications.
Key points
-
The liver harbours a dense network of phagocytes that maintain tolerance under non-inflammatory conditions and quickly sense hepatocyte stress and injury signals leading to the activation of pro-inflammatory cascades.
-
Upon injury, leukocytes rapidly infiltrate the liver parenchyma, contributing to inflammation and fibrogenesis by producing soluble mediators that activate other immune cells and non-parenchymal cell populations.
-
Inflammatory mediators can activate hepatic stellate cells (HSCs), the main effector cells during hepatic fibrogenesis, resulting in excessive extracellular matrix deposition as a wound-healing or scarring response.
-
Production of pro-inflammatory mediators by activated HSCs, in turn, perpetuates hepatic inflammation, resulting in a chronic cycle of inflammation and formation of scar tissue, ultimately leading to organ failure.
-
Current technological advances have led to an unprecedented comprehensive understanding of the hepatic inflammatory processes underlying fibrogenesis, resulting in the identification of novel potential anti-inflammatory and antifibrotic targets.
-
Treatment of the underlying liver disease seems the most effective antifibrotic strategy, but developing efficient therapies remains challenging as preclinical results rarely translate to human disease in clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Henderson, N. C., Rieder, F. & Wynn, T. A. Fibrosis: from mechanisms to medicines. Nature 587, 555–566 (2020).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 385, 1559–1569 (2021).
Simon, T. G., Roelstraete, B., Khalili, H., Hagstrom, H. & Ludvigsson, J. F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 70, 1375–1382 (2021).
Lin, M. H. et al. Liver fibrosis in the natural course of chronic hepatitis B viral infection: a systematic review with meta-analysis. Dig. Dis. Sci. 67, 2608–2626 (2022).
Calvaruso, V. & Craxi, A. Hepatic benefits of HCV cure. J. Hepatol. 73, 1548–1556 (2020).
Marcellin, P. et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381, 468–475 (2013).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378.e5 (2015).
Verrastro, O. et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial. Lancet 401, 1786–1797 (2023).
Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18, 151–166 (2021).
Elkington, P. T., O’Kane, C. M. & Friedland, J. S. The paradox of matrix metalloproteinases in infectious disease. Clin. Exp. Immunol. 142, 12–20 (2005).
Wen, Y., Lambrecht, J., Ju, C. & Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol. Immunol. 18, 45–56 (2021).
Mederacke, I. et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 4, 2823 (2013).
Lei, L. et al. Portal fibroblasts with mesenchymal stem cell features form a reservoir of proliferative myofibroblasts in liver fibrosis. Hepatology 76, 1360–1375 (2022).
Marra, F. Hepatic stellate cells and the regulation of liver inflammation. J. Hepatol. 31, 1120–1130 (1999).
Iredale, J. P. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 117, 539–548 (2007).
Friedman, S. L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 328, 1828–1835 (1993).
Mederacke, I. et al. The purinergic P2Y14 receptor links hepatocyte death to hepatic stellate cell activation and fibrogenesis in the liver. Sci. Transl Med. 14, eabe5795 (2022).
Kao, Y. H. et al. High-mobility group box 1 protein activates hepatic stellate cells in vitro. Transpl. Proc. 40, 2704–2705 (2008).
Wiesner, R. J., Ruegg, J. C. & Morano, I. Counting target molecules by exponential polymerase chain reaction: copy number of mitochondrial DNA in rat tissues. Biochem. Biophys. Res. Commun. 183, 553–559 (1992).
An, P. et al. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 11, 2362 (2020).
Knorr, J., Wree, A., Tacke, F. & Feldstein, A. E. The NLRP3 inflammasome in alcoholic and nonalcoholic steatohepatitis. Semin. Liver Dis. 40, 298–306 (2020).
McHedlidze, T. et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 39, 357–371 (2013).
Tan, Z. et al. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell Mol. Immunol. 15, 388–398 (2018).
Allen, K., Jaeschke, H. & Copple, B. L. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 178, 175–186 (2011).
Keitel, V. & Haussinger, D. Role of TGR5 (GPBAR1) in liver disease. Semin. Liver Dis. 38, 333–339 (2018).
Guillot, A. et al. Bile acid-activated macrophages promote biliary epithelial cell proliferation through integrin αvβ6 upregulation following liver injury. J. Clin. Invest. 131, e132305 (2021).
Tacke, F., Puengel, T., Loomba, R. & Friedman, S. L. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J. Hepatol. https://doi.org/10.1016/j.jhep.2023.03.038 (2023).
Tilg, H., Adolph, T. E. & Trauner, M. Gut–liver axis: pathophysiological concepts and clinical implications. Cell Metab. 34, 1700–1718 (2022).
Bruneau, A., Hundertmark, J., Guillot, A. & Tacke, F. Molecular and cellular mediators of the gut–liver axis in the progression of liver diseases. Front. Med. 8, 725390 (2021).
Chopyk, D. M. & Grakoui, A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology 159, 849–863 (2020).
Yuan, J. et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 30, 675–688.e7 (2019).
Begley, M., Gahan, C. G. & Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 (2005).
Islam, K. B. et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141, 1773–1781 (2011).
Wang, Y. D. et al. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 48, 1632–1643 (2008).
Azzu, V., Vacca, M., Virtue, S., Allison, M. & Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology 158, 1899–1912 (2020).
Mandard, S. et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281, 934–944 (2006).
Imajo, K. et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 16, 44–54 (2012).
Chatterjee, S. et al. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J. Hepatol. 58, 778–784 (2013).
Wang, J. et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 137, 713–723 (2009).
Marra, F. & Tacke, F. Roles for chemokines in liver disease. Gastroenterology 147, 577–594.e1 (2014).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Seki, E. et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 50, 185–197 (2009).
Krenkel, O. et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 67, 1270–1283 (2018).
Heinrichs, D. et al. The chemokine CCL3 promotes experimental liver fibrosis in mice. PLoS ONE 8, e66106 (2013).
Berres, M. L. et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J. Clin. Invest. 120, 4129–4140 (2010).
Aoyama, T., Inokuchi, S., Brenner, D. A. & Seki, E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52, 1390–1400 (2010).
Karlmark, K. R. et al. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 52, 1769–1782 (2010).
Tacke, F., Zimmermann, H. W., Berres, M. L., Trautwein, C. & Wasmuth, H. E. Serum chemokine receptor CXCR3 ligands are associated with progression, organ dysfunction and complications of chronic liver diseases. Liver Int. 31, 840–849 (2011).
Zeremski, M. et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 48, 1440–1450 (2008).
Wasmuth, H. E. et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology 137, 309–319.e3 (2009).
Zhang, X. et al. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J. Hepatol. 64, 160–170 (2016).
Schrage, A. et al. Enhanced T cell transmigration across the murine liver sinusoidal endothelium is mediated by transcytosis and surface presentation of chemokines. Hepatology 48, 1262–1272 (2008).
Eksteen, B. et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J. Immunol. 177, 593–603 (2006).
Wehr, A. et al. Chemokine receptor CXCR6-dependent hepatic NK T cell accumulation promotes inflammation and liver fibrosis. J. Immunol. 190, 5226–5236 (2013).
Wehr, A. et al. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS ONE 9, e112327 (2014).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Mossanen, J. C. et al. CXCR6 inhibits hepatocarcinogenesis by promoting natural killer T- and CD4+ T-cell-dependent control of senescence. Gastroenterology 156, 1877–1889.e4 (2019).
Dudek, M. et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature 592, 444–449 (2021).
Pfister, D. et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592, 450–456 (2021).
Affo, S. et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut 63, 1782–1792 (2014).
Hammerich, L. et al. Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 59, 630–642 (2014).
Wang, S. et al. Emerging importance of chemokine receptor CXCR4 and its ligand in liver disease. Front. Cell Dev. Biol. 9, 716842 (2021).
Kim, H. M. et al. iNKT cells prevent obesity-induced hepatic steatosis in mice in a C-C chemokine receptor 7-dependent manner. Int. J. Obes. 42, 270–279 (2018).
Bonacchi, A. et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology 125, 1060–1076 (2003).
Guilliams, M. et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 185, 379–396.e38 (2022).
Bonnardel, J. et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity 51, 638–654.e9 (2019).
Gola, A. et al. Commensal-driven immune zonation of the liver promotes host defence. Nature 589, 131–136 (2021).
Duan, Y. et al. CRIg on liver macrophages clears pathobionts and protects against alcoholic liver disease. Nat. Commun. 12, 7172 (2021).
Heymann, F. et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 62, 279–291 (2015).
Heymann, F. & Tacke, F. Immunology in the liver – from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 (2016).
Scott, C. L. et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 7, 10321 (2016).
Bleriot, C. et al. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity 54, 2101–2116.e6 (2021).
Krenkel, O. et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut 69, 551–563 (2020).
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
Krenkel, O. & Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 17, 306–321 (2017).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Daemen, S. et al. Dynamic shifts in the composition of resident and recruited macrophages influence tissue remodeling in NASH. Cell Rep. 34, 108626 (2021).
Remmerie, A. et al. Osteopontin expression identifies a subset of recruited macrophages distinct from Kupffer cells in the fatty liver. Immunity 53, 641–657.e14 (2020).
Seidman, J. S. et al. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity 52, 1057–1074.e7 (2020).
Tran, S. et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity 53, 627–640.e5 (2020).
Guillot, A. et al. Mapping the hepatic immune landscape identifies monocytic macrophages as key drivers of steatohepatitis and cholangiopathy progression. Hepatology 78, 150–166 (2023).
Connolly, M. K. et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J. Clin. Invest. 119, 3213–3225 (2009).
Sutti, S. et al. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin. Sci. 129, 797–808 (2015).
Sutti, S. et al. CX3CR1 mediates the development of monocyte-derived dendritic cells during hepatic inflammation. Cells 8, 1099 (2019).
Pradere, J. P. et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 58, 1461–1473 (2013).
Blois, S. M. et al. Dendritic cells regulate angiogenesis associated with liver fibrogenesis. Angiogenesis 17, 119–128 (2014).
Henning, J. R. et al. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology 58, 589–602 (2013).
Heier, E. C. et al. Murine CD103+ dendritic cells protect against steatosis progression towards steatohepatitis. J. Hepatol. 66, 1241–1250 (2017).
Deczkowska, A. et al. XCR1+ type 1 conventional dendritic cells drive liver pathology in non-alcoholic steatohepatitis. Nat. Med. 27, 1043–1054 (2021).
Xu, R., Huang, H., Zhang, Z. & Wang, F. S. The role of neutrophils in the development of liver diseases. Cell Mol. Immunol. 11, 224–231 (2014).
Liang, W. et al. Metabolically induced liver inflammation leads to NASH and differs from LPS- or IL-1β-induced chronic inflammation. Lab. Invest. 94, 491–502 (2014).
O’Brien, K. M. et al. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am. J. Pathol. 183, 1498–1507 (2013).
Ma, J. et al. Distinct histopathological phenotypes of severe alcoholic hepatitis suggest different mechanisms driving liver injury and failure. J. Clin. Invest. 132, e157780 (2022).
Moles, A. et al. A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J. Hepatol. 60, 782–791 (2014).
Calvente, C. J. et al. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J. Clin. Invest. 129, 4091–4109 (2019).
Weiskirchen, R., Meurer, S. K., Liedtke, C. & Huber, M. Mast cells in liver fibrogenesis. Cells 8, 1429 (2019).
Huang, S. et al. Exploring the role of mast cells in the progression of liver disease. Front. Physiol. 13, 964887 (2022).
Kennedy, L. et al. Mast cells promote nonalcoholic fatty liver disease phenotypes and microvesicular steatosis in mice fed a western diet. Hepatology 74, 164–182 (2021).
Hargrove, L. et al. Bile duct ligation-induced biliary hyperplasia, hepatic injury, and fibrosis are reduced in mast cell-deficient Kit(W-sh) mice. Hepatology 65, 1991–2004 (2017).
Meadows, V. et al. Downregulation of hepatic stem cell factor by Vivo-Morpholino treatment inhibits mast cell migration and decreases biliary damage/senescence and liver fibrosis in Mdr2−/− mice. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 165557 (2019).
Matsunaga, Y. & Terada, T. Mast cell subpopulations in chronic inflammatory hepatobiliary diseases. Liver 20, 152–156 (2000).
Koruk, S. T., Ozardali, I., Dincoglu, D. & Bitiren, M. Increased liver mast cells in patients with chronic hepatitis C. Indian J. Pathol. Microbiol. 54, 736–740 (2011).
Jones, H. et al. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2−/− mice. Hepatology 64, 1202–1216 (2016).
Kennedy, L. et al. Blocking H1/H2 histamine receptors inhibits damage/fibrosis in Mdr2−/− mice and human cholangiocarcinoma tumorigenesis. Hepatology 68, 1042–1056 (2018).
Ficht, X. & Iannacone, M. Immune surveillance of the liver by T cells. Sci. Immunol. 5, eaba2351 (2020).
Benechet, A. P. et al. Dynamics and genomic landscape of CD8+ T cells undergoing hepatic priming. Nature 574, 200–205 (2019).
De Simone, G. et al. Identification of a Kupffer cell subset capable of reverting the T cell dysfunction induced by hepatocellular priming. Immunity 54, 2089–2100.e8 (2021).
Sutti, S. & Albano, E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 17, 81–92 (2020).
Freitas-Lopes, M. A., Mafra, K., David, B. A., Carvalho-Gontijo, R. & Menezes, G. B. Differential location and distribution of hepatic immune cells. Cells 6, 48 (2017).
Thapa, M. et al. Liver fibrosis occurs through dysregulation of MyD88-dependent innate B-cell activity. Hepatology 61, 2067–2079 (2015).
Barrow, F. et al. Microbiota-driven activation of intrahepatic B cells aggravates NASH through innate and adaptive signaling. Hepatology 74, 704–722 (2021).
Karl, M. et al. Dual roles of B lymphocytes in mouse models of diet-induced nonalcoholic fatty liver disease. Hepatology 76, 1135–1149 (2022).
Kotsiliti, E. et al. Intestinal B-cells license metabolic T-cell activation in NASH microbiota/antigen-independently and contribute to fibrosis by IgA-FcR signalling. J. Hepatol. https://doi.org/10.1016/j.jhep.2023.04.037 (2023).
Cheever, A. W. et al. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J. Immunol. 153, 753–759 (1994).
Chiaramonte, M. G., Donaldson, D. D., Cheever, A. W. & Wynn, T. A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Invest. 104, 777–785 (1999).
Lee, C. G. et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 194, 809–821 (2001).
Wynn, T. A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 4, 583–594 (2004).
Gieseck, R. L. III et al. Interleukin-13 activates distinct cellular pathways leading to ductular reaction, steatosis, and fibrosis. Immunity 45, 145–158 (2016).
Wynn, T. A. et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 376, 594–596 (1995).
Fabre, T. et al. Type 3 cytokines IL-17A and IL-22 drive TGF-β-dependent liver fibrosis. Sci. Immunol. 3, eaar7754 (2018).
Meng, F. et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776.e3 (2012).
Lemmers, A. et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology 49, 646–657 (2009).
Li, J. et al. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS ONE 7, e39307 (2012).
Xiang, X. et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J. Hepatol. 72, 736–745 (2020).
Zenewicz, L. A. et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659 (2007).
Kong, X. et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 56, 1150–1159 (2012).
Arab, J. P. et al. An open-label, dose-escalation study to assess the safety and efficacy of IL-22 agonist F-652 in patients with alcohol-associated hepatitis. Hepatology 72, 441–453 (2020).
Wangoo, A., Laban, C., Cook, H. T., Glenville, B. & Shaw, R. J. Interleukin-10- and corticosteroid-induced reduction in type I procollagen in a human ex vivo scar culture. Int. J. Exp. Pathol. 78, 33–41 (1997).
Breous, E., Somanathan, S., Vandenberghe, L. H. & Wilson, J. M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 50, 612–621 (2009).
Louis, H. et al. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology 28, 1607–1615 (1998).
Zhang, X. et al. Persistence of cirrhosis is maintained by intrahepatic regulatory T cells that inhibit fibrosis resolution by regulating the balance of tissue inhibitors of metalloproteinases and matrix metalloproteinases. Transl. Res. 169, 67–79.e2 (2016).
Novobrantseva, T. I. et al. Attenuated liver fibrosis in the absence of B cells. J. Clin. Invest. 115, 3072–3082 (2005).
Safadi, R. et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology 127, 870–882 (2004).
Breuer, D. A. et al. CD8+ T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G211–G224 (2020).
Koda, Y. et al. CD8+ tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat. Commun. 12, 4474 (2021).
Pellicci, D. G., Koay, H. F. & Berzins, S. P. Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat. Rev. Immunol. 20, 756–770 (2020).
de Lalla, C. et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J. Immunol. 173, 1417–1425 (2004).
Park, O. et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology 49, 1683–1694 (2009).
Li, Y. et al. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Front. Immunol. 9, 1994 (2018).
Hegde, P. et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 9, 2146 (2018).
Bottcher, K. et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 68, 172–186 (2018).
Mabire, M. et al. MAIT cell inhibition promotes liver fibrosis regression via macrophage phenotype reprogramming. Nat. Commun. 14, 1830 (2023).
Kurioka, A., Walker, L. J., Klenerman, P. & Willberg, C. B. MAIT cells: new guardians of the liver. Clin. Transl. Immunol. 5, e98 (2016).
Toubal, A., Nel, I., Lotersztajn, S. & Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 19, 643–657 (2019).
Seo, W. et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology 64, 616–631 (2016).
Liu, M., Hu, Y., Yuan, Y., Tian, Z. & Zhang, C. γδT cells suppress liver fibrosis via strong cytolysis and enhanced NK cell-mediated cytotoxicity against hepatic stellate cells. Front. Immunol. 10, 477 (2019).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Curio, S. & Belz, G. T. The unique role of innate lymphoid cells in cancer and the hepatic microenvironment. Cell. Mol. Immunol. 19, 1012–1029 (2022).
Muhanna, N. et al. Amelioration of hepatic fibrosis by NK cell activation. Gut 60, 90–98 (2011).
Gur, C. et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut 61, 885–893 (2012).
Tosello-Trampont, A. C. et al. NKp46+ natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology 63, 799–812 (2016).
Wijaya, R. S. et al. KLRG1+ natural killer cells exert a novel antifibrotic function in chronic hepatitis B. J. Hepatol. 71, 252–264 (2019).
Eisenhardt, M. et al. The CXCR3(+)CD56Bright phenotype characterizes a distinct NK cell subset with anti-fibrotic potential that shows dys-regulated activity in hepatitis C. PLoS ONE 7, e38846 (2012).
Jeong, W. I. et al. Suppression of innate immunity (natural killer cell/interferon-γ) in the advanced stages of liver fibrosis in mice. Hepatology 53, 1342–1351 (2011).
Trivedi, P., Wang, S. & Friedman, S. L. The power of plasticity-metabolic regulation of hepatic stellate cells. Cell Metab. 33, 242–257 (2021).
Gilgenkrantz, H., Mallat, A., Moreau, R. & Lotersztajn, S. Targeting cell-intrinsic metabolism for antifibrotic therapy. J. Hepatol. 74, 1442–1454 (2021).
Rosenthal, S. B. et al. Heterogeneity of HSCs in a mouse model of NASH. Hepatology 74, 667–685 (2021).
Krenkel, O., Hundertmark, J., Ritz, T. P., Weiskirchen, R. & Tacke, F. Single cell RNA sequencing identifies subsets of hepatic stellate cells and myofibroblasts in liver fibrosis. Cells 8, 503 (2019).
Filliol, A. et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature 610, 356–365 (2022).
Dobie, R. et al. Single-cell transcriptomics uncovers zonation of function in the mesenchyme during liver fibrosis. Cell Rep. 29, 1832–1847.e8 (2019).
Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl Acad. Sci. USA 109, 9448–9453 (2012).
Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14, 397–411 (2017).
Wang, S. et al. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis. Sci. Transl Med. 15, eadd3949 (2023).
Schwabe, R. F., Tabas, I. & Pajvani, U. B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology 158, 1913–1928 (2020).
Kourtzelis, I., Hajishengallis, G. & Chavakis, T. Phagocytosis of apoptotic cells in resolution of inflammation. Front. Immunol. 11, 553 (2020).
Tacke, F. et al. Bone morphogenetic protein 7 is elevated in patients with chronic liver disease and exerts fibrogenic effects on human hepatic stellate cells. Dig. Dis. Sci. 52, 3404–3415 (2007).
Dewidar, B., Meyer, C., Dooley, S. & Meindl-Beinker, A. N. TGF-β in hepatic stellate cell activation and liver fibrogenesis — updated 2019. Cells 8, 1419 (2019).
Fabre, T., Kared, H., Friedman, S. L. & Shoukry, N. H. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 193, 3925–3933 (2014).
Wipff, P. J., Rifkin, D. B., Meister, J. J. & Hinz, B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323 (2007).
Henderson, N. C. et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Zhang, J., Jiang, N., Ping, J. & Xu, L. TGF-β1-induced autophagy activates hepatic stellate cells via the ERK and JNK signaling pathways. Int. J. Mol. Med. 47, 256–266 (2021).
Hernandez-Gea, V. et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142, 938–946 (2012).
Ying, H. Z. et al. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics. Mol. Med. Rep. 16, 7879–7889 (2017).
Wong, L., Yamasaki, G., Johnson, R. J. & Friedman, S. L. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J. Clin. Invest. 94, 1563–1569 (1994).
Huang, Y. et al. Bevacizumab attenuates hepatic fibrosis in rats by inhibiting activation of hepatic stellate cells. PLoS ONE 8, e73492 (2013).
Rangwala, F. et al. Increased production of sonic hedgehog by ballooned hepatocytes. J. Pathol. 224, 401–410 (2011).
Povero, D. et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell Mol. Gastroenterol. Hepatol. 1, 646–663.e4 (2015).
Ioannou, G. N. et al. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J. Lipid Res. 58, 1067–1079 (2017).
Ioannou, G. N., Haigh, W. G., Thorning, D. & Savard, C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J. Lipid Res. 54, 1326–1334 (2013).
Kaffe, E. et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology 65, 1369–1383 (2017).
Li, C. et al. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J. Hepatol. 54, 1205–1213 (2011).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Arteel, G. E. & Naba, A. The liver matrisome – looking beyond collagens. JHEP Rep. 2, 100115 (2020).
Lu, P., Takai, K., Weaver, V. M. & Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3, a005058 (2011).
Beier, J. I. et al. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology 49, 1545–1553 (2009).
Gillis, S. E. & Nagy, L. E. Deposition of cellular fibronectin increases before stellate cell activation in rat liver during ethanol feeding. Alcohol. Clin. Exp. Res. 21, 857–861 (1997).
Massey, V. L. et al. The hepatic “matrisome” responds dynamically to injury: characterization of transitional changes to the extracellular matrix in mice. Hepatology 65, 969–982 (2017).
Klaas, M. et al. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 6, 27398 (2016).
Mazza, G. et al. Cirrhotic human liver extracellular matrix 3D scaffolds promote Smad-dependent TGF-β1 epithelial mesenchymal transition. Cells 9, 83 (2019).
Hinz, B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 47, 54–65 (2015).
Manicardi, N. et al. Transcriptomic profiling of the liver sinusoidal endothelium during cirrhosis reveals stage-specific secretory signature. Cancers 13, 2688 (2021).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Heydtmann, M. et al. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 174, 1055–1062 (2005).
Monneau, Y., Arenzana-Seisdedos, F. & Lortat-Jacob, H. The sweet spot: how GAGs help chemokines guide migrating cells. J. Leukoc. Biol. 99, 935–953 (2016).
Lassailly, G. et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology 159, 1290–1301.e5 (2020).
Tacke, F. & Trautwein, C. Mechanisms of liver fibrosis resolution. J. Hepatol. 63, 1038–1039 (2015).
Troeger, J. S. et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143, 1073–1083.e22 (2012).
Liu, X. et al. Identification of lineage-specific transcription factors that prevent activation of hepatic stellate cells and promote fibrosis resolution. Gastroenterology 158, 1728–1744.e14 (2020).
Lefere, S. et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J. Hepatol. 73, 757–770 (2020).
Boettcher, E., Csako, G., Pucino, F., Wesley, R. & Loomba, R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 35, 66–75 (2012).
Francque, S. M. et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N. Engl. J. Med. 385, 1547–1558 (2021).
Nakano, Y. et al. A deactivation factor of fibrogenic hepatic stellate cells induces regression of liver fibrosis in mice. Hepatology 71, 1437–1452 (2020).
Arroyo, N. et al. GATA4 induces liver fibrosis regression by deactivating hepatic stellate cells. JCI Insight 6, e150059 (2021).
Radaeva, S. et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 130, 435–452 (2006).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012).
Popov, Y. et al. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G323–G334 (2010).
Uchinami, H., Seki, E., Brenner, D. A. & D’Armiento, J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology 44, 420–429 (2006).
Fallowfield, J. A. et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 178, 5288–5295 (2007).
Dal-Secco, D. et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med. 212, 447–456 (2015).
Wan, J. et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 59, 130–142 (2014).
Saijou, E. et al. Neutrophils alleviate fibrosis in the CCl4-induced mouse chronic liver injury model. Hepatol. Commun. 2, 703–717 (2018).
Wang, J. et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116 (2017).
He, Y. et al. MicroRNA-223 ameliorates nonalcoholic steatohepatitis and cancer by targeting multiple inflammatory and oncogenic genes in hepatocytes. Hepatology 70, 1150–1167 (2019).
He, Y. et al. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. J. Clin. Invest. 131, e141513 (2021).
Ratziu, V., Francque, S. & Sanyal, A. Breakthroughs in therapies for NASH and remaining challenges. J. Hepatol. 76, 1263–1278 (2022).
Rinella, M. E., Tacke, F., Sanyal, A. J. & Anstee, Q. M. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J. Hepatol. 71, 823–833 (2019).
Intercept Pharmaceuticals. Intercept announces outcome of FDA advisory committee meeting for obeticholic acid as a treatment for pre-cirrhotic fibrosis due to NASH. Intercept https://ir.interceptpharma.com/news-releases/news-release-details/intercept-announces-outcome-fda-advisory-committee-meeting (2023).
Wiering, L. & Tacke, F. Treating inflammation to combat non-alcoholic fatty liver disease. J. Endocrinol. 256, e220194 (2022).
Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 66, 1300–1312 (2017).
Ratziu, V. et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology 72, 892–905 (2020).
Anstee, Q. M. et al. Cenicriviroc lacked efficacy to treat liver fibrosis in nonalcoholic steatohepatitis: AURORA phase III randomized study. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2023.04.003 (2023).
Liu, S. et al. TAK-242 ameliorates hepatic fibrosis by regulating the liver–gut axis. Biomed. Res. Int. 2022, 4949148 (2022).
Puengel, T. et al. The medium-chain fatty acid receptor GPR84 mediates myeloid cell infiltration promoting steatohepatitis and fibrosis. J. Clin. Med. 9, 1140 (2020).
Harrison, S. A. et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J. Hepatol. 73, 26–39 (2020).
Boyer-Diaz, Z. et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J. Hepatol. 74, 1188–1199 (2021).
Francque, S. et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat. Rev. Gastroenterol. Hepatol. 18, 24–39 (2021).
Ratziu, V. & Charlton, M. Rational combination therapy for NASH: insights from clinical trials and error. J. Hepatol. 78, 1073–1079 (2023).
Puengel, T. et al. Combined therapy with a CCR2/CCR5 antagonist and FGF21 analogue synergizes in ameliorating steatohepatitis and fibrosis. Int. J. Mol. Sci. 23, 6696 (2022).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347 (2023).
Fumagalli, V. et al. Group 1 ILCs regulate T cell-mediated liver immunopathology by controlling local IL-2 availability. Sci. Immunol. 7, eabi6112 (2022).
Iannacone, M., Andreata, F. & Guidotti, L. G. Immunological insights in the treatment of chronic hepatitis B. Curr. Opin. Immunol. 77, 102207 (2022).
Vuerich, M., Wang, N., Kalbasi, A., Graham, J. J. & Longhi, M. S. Dysfunctional immune regulation in autoimmune hepatitis: from pathogenesis to novel therapies. Front. Immunol. 12, 746436 (2021).
Liberal, R., de Boer, Y. S. & Heneghan, M. A. Established and novel therapeutic options for autoimmune hepatitis. Lancet Gastroenterol. Hepatol. 6, 315–326 (2021).
Gao, B. & Bataller, R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585 (2011).
Kelly, A. et al. Human monocytes and macrophages regulate immune tolerance via integrin αvβ8-mediated TGFβ activation. J. Exp. Med. 215, 2725–2736 (2018).
Rahman, S. R. et al. Integrins as a drug target in liver fibrosis. Liver Int. 42, 507–521 (2022).
Puengel, T. et al. Nuclear receptors linking metabolism, inflammation, and fibrosis in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 23, 2668 (2022).
Schwabl, P. et al. The non-steroidal FXR agonist cilofexor improves portal hypertension and reduces hepatic fibrosis in a rat NASH model. Biomedicines 9, 60 (2021).
Zhou, J. et al. SUMOylation inhibitors synergize with FXR agonists in combating liver fibrosis. Nat. Commun. 11, 240 (2020).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Wirth, E. K., Puengel, T., Spranger, J. & Tacke, F. Thyroid hormones as a disease modifier and therapeutic target in nonalcoholic steatohepatitis. Expert Rev. Endocrinol. Metab. 17, 425–434 (2022).
Kaufmann, B., Reca, A., Kim, A. D. & Feldstein, A. E. Novel mechanisms for resolution of liver inflammation: therapeutic implications. Semin. Liver Dis. 41, 150–162 (2021).
Baeck, C. et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C+ macrophage infiltration in mice. Hepatology 59, 1060–1072 (2014).
Thomas, J. A. et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology 53, 2003–2015 (2011).
Ma, P. F. et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J. Hepatol. 67, 770–779 (2017).
Moroni, F. et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat. Med. 25, 1560–1565 (2019).
Dwyer, B. J., Macmillan, M. T., Brennan, P. N. & Forbes, S. J. Cell therapy for advanced liver diseases: repair or rebuild. J. Hepatol. 74, 185–199 (2021).
Psaraki, A., Ntari, L., Karakostas, C., Korrou-Karava, D. & Roubelakis, M. G. Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology 75, 1590–1603 (2022).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Kaur, S. et al. In vitro models for the study of liver biology and diseases: advances and limitations. Cell Mol. Gastroenterol. Hepatol. 15, 559–571 (2022).
Nevzorova, Y. A., Boyer-Diaz, Z., Cubero, F. J. & Gracia-Sancho, J. Animal models for liver disease – a practical approach for translational research. J. Hepatol. 73, 423–440 (2020).
Saviano, A., Henderson, N. C. & Baumert, T. F. Single-cell genomics and spatial transcriptomics: discovery of novel cell states and cellular interactions in liver physiology and disease biology. J. Hepatol. 73, 1219–1230 (2020).
Wallace, S. J., Tacke, F., Schwabe, R. F. & Henderson, N. C. Understanding the cellular interactome of non-alcoholic fatty liver disease. JHEP Rep. 4, 100524 (2022).
Lambrecht, J., van Grunsven, L. A. & Tacke, F. Current and emerging pharmacotherapeutic interventions for the treatment of liver fibrosis. Expert Opin. Pharmacother. 21, 1637–1650 (2020).
Acknowledgements
L.H. is supported by the German Research Foundation (DFG SPP2306) and the Else-Kroener-Fresenius-Stiftung (grant ID 2021_EKEA.145). F.T. is supported by the German Research Foundation (DFG SFB/TRR 296 and CRC1382, project ID 403224013) and the German Ministry of Education and Research (BMBF DEEP-HCC consortium, BMBF Immun Avatar consortium).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
F.T.’s laboratory has received research funding from Allergan, Bristol-Myers Squibb, Gilead and Inventiva (funding to the institution). F.T. has received honoraria for consulting or lectures from Astra Zeneca, Gilead, AbbVie, BMS, Boehringer, Madrigal, Intercept, Falk, Ionis, Inventiva, Merz, Pfizer, Alnylam, NGM, CSL Behring, Novo Nordisk and Novartis. L.H. declares no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Fabio Marra and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NCT02548351: https://clinicaltrials.gov/ct2/show/NCT02548351
NCT03900429: https://clinicaltrials.gov/ct2/show/NCT03900429
NCT04849728: https://clinicaltrials.gov/ct2/show/NCT04849728
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hammerich, L., Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol 20, 633–646 (2023). https://doi.org/10.1038/s41575-023-00807-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00807-x
This article is cited by
-
DCDC2 inhibits hepatic stellate cell activation and ameliorates CCl4-induced liver fibrosis by suppressing Wnt/β-catenin signaling
Scientific Reports (2024)
-
The m6A reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis
Cell Death & Disease (2024)
-
Development of a high throughput system to screen compounds that revert the activated hepatic stellate cells to a quiescent-like state
Scientific Reports (2024)
-
Protein neddylation and its role in health and diseases
Signal Transduction and Targeted Therapy (2024)
-
Phillygenin Inhibits TGF-β1-induced Hepatic Stellate Cell Activation and Inflammation: Regulation of the Bax/Bcl-2 and Wnt/β-catenin Pathways
Inflammation (2024)