Abstract
Tuberculosis (TB) remains the foremost cause of death by an infectious disease globally. Multidrug-resistant or rifampicin-resistant TB (MDR/RR-TB; resistance to rifampicin and isoniazid, or rifampicin alone) is a burgeoning public health challenge in several parts of the world, and especially Eastern Europe, Russia, Asia and sub-Saharan Africa. Pre-extensively drug-resistant TB (pre-XDR-TB) refers to MDR/RR-TB that is also resistant to a fluoroquinolone, and extensively drug-resistant TB (XDR-TB) isolates are additionally resistant to other key drugs such as bedaquiline and/or linezolid. Collectively, these subgroups are referred to as drug-resistant TB (DR-TB). All forms of DR-TB can be as transmissible as rifampicin-susceptible TB; however, it is more difficult to diagnose, is associated with higher mortality and morbidity, and higher rates of post-TB lung damage. The various forms of DR-TB often consume >50% of national TB budgets despite comprising <5–10% of the total TB case-load. The past decade has seen a dramatic change in the DR-TB treatment landscape with the introduction of new diagnostics and therapeutic agents. However, there is limited guidance on understanding and managing various aspects of this complex entity, including the pathogenesis, transmission, diagnosis, management and prevention of MDR-TB and XDR-TB, especially at the primary care physician level.
Similar content being viewed by others
Introduction
Globally, tuberculosis (TB) has once again become the leading cause of death by an infectious disease1. In 2022, TB was the largest drug-resistant airborne epidemic and more than 1 billion people have succumbed to the disease over the past two centuries2,3. Effective drugs against the TB-causing bacterium, Mycobacterium tuberculosis, were first developed in the 1940s. Drug-susceptible TB (DS-TB) can be cured with a 6-month regimen that consists of four drugs: isoniazid, rifampicin, pyrazinamide and ethambutol (HRZE), although there are regimens as short as 4 months4. However, over time, resistance to these first-line drugs, the main ones being rifampicin and isoniazid, has developed (see Mechanisms section below).
Multidrug-resistant tuberculosis (MDR-TB) refers to TB that has resistance to both rifampicin and isoniazid, whereas rifampicin-resistant tuberculosis (RR-TB) refers to rifampicin resistance only (pyrazinamide and ethambutol resistance are not considered) (Box 1). Because both RR-TB and MDR-TB have a similar prognosis and management strategy, they are collectively referred to as MDR/RR-TB5.
In 2019, based on the impact of specific drugs, the WHO released a revised classification of second-line drugs used to treat MDR/RR-TB6. Group A drugs (levofloxacin or moxifloxacin, bedaquiline and linezolid), improved mortality and outcomes; group B drugs (clofazimine and cycloserine or terizidone) improved treatment-related outcomes, and group C drugs were useful treatment adjuncts (ethambutol, delamanid, pyrazinamide, imipenem-cilastin or meropenem (with clavulanic acid), amikacin or streptomycin, ethionamide or prothionamide, and p-aminosalicylic acid).
Pre-extensively drug-resistant TB (pre-XDR-TB) refers to MDR/RR-TB that is also resistant to a fluoroquinolone (levofloxacin or moxifloxacin)5 and extensively drug-resistant TB (XDR-TB) is additionally resistant to the other group A drugs, that is, bedaquiline and/or linezolid5. Collectively, these groups are referred to as drug-resistant TB (DR-TB). In this Primer, we discuss all forms of DR-TB. However, there are instances in which the data available distinguish between the different forms of DR-TB; therefore, we refer to these when appropriate.
In 2020, the COVID-19 pandemic destabilized TB control and, in 2022, it was estimated that ~10.6 million people became newly ill with TB and ~410,000 of these were patients with MDR/RR-TB1. Drug resistance remains a major problem in several parts of the world, especially in Eastern Europe, Russia, Asia and sub-Saharan Africa. Although only ~5% of the total burden of TB strains are rifampicin resistant, mortality is substantially higher — contributing ~15–20% to global TB mortality7. Remarkably, only one in three patients with MDR/RR-TB is ever detected1, and late diagnosis means that morbidity is higher (more substantial post-TB lung disease and chronic pulmonary disability; Supplementary Box 1 for first-hand patient journeys). MDR/RR-TB is also very costly to manage with substantial negative economic consequences for both patients and countries.
It is estimated that DR-TB will cost the global economy about US$16.7 trillion between 2015 and 2050 (ref. 2), and ~20–25% of the total global estimated cost of antimicrobial resistance by the year 2050 will be due to DR-TB8. In countries that include India, Russia, China and South Africa, 2017 gross domestic product losses due to absence from work or early mortality varied from approximately $1 billion to $8 billion excluding catastrophic costs on affected households8. The 2017 cost to Europe alone was approximately US$5 billion. According to national surveys between 2016 and 2022, 82% of patients with DR-TB and their households faced catastrophic costs related to the illness, compared with 47% of those with drug-susceptible disease7. Similar to South Africa (where DR-TB was <5% of total TB burden), almost all the countries in the WHO Europe Region spent more money for medicines to treat DR-TB than they spent for DS-TB9. The costs for the medicines alone of one treatment course for XDR-TB can exceed €300,000 in Western Europe10.
Indeed, of the top 50 interventions for antimicrobial resistance (a global priority almost on the same footing as global warming11), published by the WHO in 2023, approximately ten of the priority areas are dedicated to DR-TB12. Of note, data from 2018 have shed more light on the pathogenesis of DR-TB, including variable drug penetration into TB lesions (for example, cavities)13, demonstrating that resistance amplification is caused by more than patient non-adherence. Although it is gratifying that five new or repurposed TB drugs have revolutionized the treatment of DR-TB after a gap of almost 50 years, there are several remaining controversies around the composition and length of treatment regimens, and management in specific contexts. Drug resistance in TB is a highly complex topic and there is limited up-to-date direction and guidance on managing and understanding the many facets of this disease.
In this Primer, we address current gaps in the understanding of DR-TB by summarizing its epidemiology, pathogenesis, diagnosis, management and prevention, including important updates from the WHO. We also discuss other aspects, including transmission, socio-ethical dilemmas and palliative care, as well as approaches to antibiotic stewardship and public health case-finding strategies.
Epidemiology
Clinical epidemiology
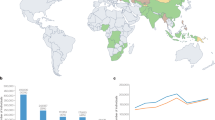
The WHO estimates that ~10.6 million people fell ill with TB in 2022; of these, 3.3% were newly diagnosed with MDR-TB and 17% had been previously treated for TB and were diagnosed with MDR-TB7, amounting to an estimated total of ~410,000 new cases of MDR-TB1 (Fig. 1). Although these numbers are slightly lower than those estimated in 2015, the proportion of all DR-TB cases that are also XDR seems to be rising and is currently estimated at ~18%1.
Global map displaying a range of estimated incidence of cases of multidrug-resistant or rifampicin-resistant tuberculosis (MDR/RR-TB). The seven countries with the highest burden in terms of numbers of MDR/RR-TB cases, which accounted for two-thirds of global MDR/RR-TB cases in 2022, are labelled. Reprinted with permission from ref. 1, WHO.
Geographical variation
Global figures mask marked geographical heterogeneity. The proportion of TB that is found to be drug resistant among new and previously treated patients is ~45% in Turkmenistan compared with 1% in Bangladesh7; notably all countries in which >20% of new TB diagnoses are drug resistant are in Central Asia and Eastern Europe7,14. Despite the small decline in the proportion of cases that are drug resistant7, the number of people diagnosed with DR-TB increased by 6.4% in 2021 (ref. 7) — consistent with the estimated rise in all TB that has been attributed to COVID-19-related disruptions in TB case detection and treatment15. Although multiple studies have identified locally specific risk factors for DR-TB in comparison with DS-TB (incarceration in the Russian Federation16, immigrant status in Europe17, South Korea18 and China19), previous TB treatment is the only host-specific determinant of DR-TB that has been consistently identified across these different locales. Notably, the WHO estimates that only one in three patients with MDR/RR-TB is detected and treated1.
Social determinants
DR-TB remains a disease associated with poverty20, and ecological analyses show that country-specific gross domestic product is strongly correlated with TB incidence21. In the early 1900s, before the development of anti-TB therapy, TB incidence and mortality in the industrialized world declined rapidly22, likely because of improvements in living conditions and nutrition. This highlights the importance of an intersectoral approach to TB in which determinants of health must be addressed in conjunction with strategies aimed at TB control and treatment.
On an individual level, multiple factors associated with TB risk including undernutrition, overcrowding and smoking are also more prevalent in indigent populations23. One study estimated that 24% of TB in the 30 high-burden countries is due to undernutrition24.
Comorbidities
In addition to social determinants, comorbidities have a major role in susceptibility to TB infection, with HIV, diabetes mellitus, malignancies and silicosis all being strong risk factors for TB progression and severity. The WHO estimates that, annually, 0.86 million new TB cases are associated with HIV globally, with many originating from southern Africa7. It has been observed that among people living with HIV, the risk of MDR/RR-TB and the risk of primary multidrug resistance (multidrug resistance associated with transmission) was 1.42-fold and 2.7-fold higher than for those not living with HIV, respectively25. People living with HIV and MDR/RR-TB also experience higher case fatality rates with one study from seven countries reporting a 19.0% case fatality rate compared with 9.4% for patients with MDR/RR-TB not living with HIV26. Diabetes mellitus is also a potent risk factor for TB progression, and as the burden of diabetes mellitus has grown globally over the past three decades, now reaching a global prevalence of 6.1%27, the co-occurrence of TB and diabetes mellitus is increasingly common. The WHO estimates that 0.37 million cases of incident TB were associated with diabetes mellitus7. Diabetes mellitus also increases the risk of MDR/RR-TB by approximately twofold28 and is associated with an increased risk of poor treatment outcomes7,29 (Supplementary Table 1). With global diabetes mellitus prevalence projected to reach 10% in 2050 (ref. 27), diabetes mellitus is expected to have an increasingly important role in the epidemiology of TB in the coming decades.
Molecular epidemiology
Tools that enable the molecular characterization of M. tuberculosis strains have had an increasingly important role in the study of drug resistance in TB7. Over the past three decades, molecular tools have been used to differentiate lineages and study their phenotypic consequences30 (for example, the presence of cavitary lesions, site of TB infection, mutation rate) as well as to track the evolution of drug resistance in individuals and populations over time and resolve long-standing debates about drug resistance in TB. For example, genomic epidemiological studies (matching the genomic fingerprints/barcodes of two strains) show that many patients with DR-TB with previously treated TB have been re-infected with a new drug-resistant strain, suggesting that some cases of purported relapse are due to a new transmission event, likely owing to community-based transmission and sometimes owing to nosocomial spread of DR-TB in the setting of hospitalization for TB treatment31. Molecular tools have also been used to assess the relative fitness of drug-resistant M. tuberculosis strains, both in longitudinal studies of TB contacts and in studies that have inferred the population structure of M. tuberculosis strains from phylogenetic reconstructions of transmission chains. One study from Peru matched genotypes between index patients with TB and their household contacts and observed that drug-resistant M. tuberculosis strains can be equally likely as drug-susceptible strains to be transmitted and cause disease32; a finding that was confirmed in a cough aerosol sampling study33. Similarly, a case-only study in South Africa reported that 212 of 386 patients with XDR-TB diagnosed over 3 years in KwaZulu Natal were part of a single genomically defined transmission cluster, suggesting that this specific XDR M. tuberculosis strain was highly transmissible34.
Whole-genome sequencing has also been used to show that the fitness of drug-resistant M. tuberculosis strains is heterogeneous, dependent not only on the specific mutation that confers resistance, but also on the presence of compensatory mutations that may offset the metabolic consequences of drug-resistance mutations as well as on the specific lineage of the drug-resistant M. tuberculosis strain. One study from Georgia found that drug-resistant M. tuberculosis strains from lineage 4 were less fit than their drug-susceptible counterparts but those from lineage 2 were more fit and that this advantage arose from epistatic interactions between the specific drug resistance and compensatory mutations as well as other pre-existing genetic features specific to the circulating lineage 2 clade35. Notably, other studies have shown that some lineages harbour mutations that confer resistance to bedaquiline, delamanid and pretomanid; phylogenetic reconstructions indicate that some of these variants pre-existed the roll-out of these drugs36,37. These findings emphasize the need for ongoing real-time molecular surveillance to promptly identify transmissible drug-resistant M. tuberculosis strains and implement control measures to interrupt their circulation. Moreover, a phylogenomic approach to reconstruct the acquisition of drug-resistance mutations in a highly prevalent MDR/RR-TB strain from Moldova suggested a temporal association between national TB policies, including the hospitalization of patients with DR-TB, and the evolution of drug-resistant M. tuberculosis in Moldova38.
Transmission and the spectrum of TB infection
The earlier phase of the DR-TB epidemic in the early 1990s was characterized mainly by acquired resistance but by the end of the decade, most newly ill people with DR-TB were infected by already resistant strains (primary resistance)39. This is hypothesized to repeat itself for new and repurposed drugs such as bedaquiline and linezolid. Evidence suggests that individuals with DR-TB are as infectious as those with DS-TB, and transmissions occur to a similar extent32. It is also clear that there is considerable heterogeneity in infectiousness of individuals with DR-TB and the determinants of this are poorly understood but may include lineage and strain type37 and epigenetic factors (as one large study could not identify genomically encoded markers associated with highly infectious strains)35. Thus, a small proportion of individuals are likely to transmit most of the disease (the superspreader phenomenon), which is also observed with other respiratory infections such as measles and COVID-19 (ref. 40). There have been numerous instances of DR-TB outbreaks globally41,42,43,44,45, highlighting the need for prompt diagnosis and transmission-interrupting action.
It is known that certain factors increase the likelihood of infectiousness, such as a high concentration of mycobacteria in sputum, cavitary disease, the presence of cough, advanced HIV co-infection and younger fitter patients who are highly mobile and with significant cough strength33; however, there is no consensus on how to define infectiousness, and the optimum way to measure infectiousness is controversial. A model in guinea pigs has yielded very useful data about transmission but is not practical for routine use46, whereas a cough aerosol sampling system has been validated against human end points, such as tuberculin skin test (TST) conversion (which is a proxy of transmission and latent TB infection)32,47. By contrast, transmission can be measured by TST or interferon-γ release assay (IGRA) conversion in the newly infected host48, prospective follow-up of such individuals and near-identical DNA fingerprinting readouts (whole-genome sequencing analysis). However, these methods all have their drawbacks; for example, IGRA has poor predictive value for TB infection (and progression to active TB disease)49, and other biomarkers (for example, TST or IGRA conversion) are prone to long latency periods before active TB disease emerges. A public health strategy oriented to community-based active case finding (ACF, active searching for cases) facilitates rapid and early diagnosis, thus minimizing transmission within the community and amplification of the epidemic50.
Although individuals exposed to drug-resistant strains of M. tuberculosis may eliminate the infection, with only a small number progressing over the short term (<2 years) to active disease (Fig. 2), infected persons can develop incipient TB (an asymptomatic form of TB disease) and subclinical TB (asymptomatic but with microbiological evidence of TB present), which represents at least 50–60% of the total DR-TB burden in any community51. The precise definition of subclinical TB is contentious (and symptoms may be absent, ascribed to another condition, for example, smoker’s cough, or under-reported owing to disease stigmatization and other factors)52. Incipient TB is likely amenable to TB preventive treatment. These different categories of TB (drug resistant or susceptible) merge into one another and may progress or revert between stages, making up the spectrum of TB infection (Table 1). This is an additional reason why a community-based ACF strategy should be adopted to facilitate early diagnosis and treatment of DR-TB.
Individuals exposed to Mycobacterium tuberculosis aerosol (step 1) may eliminate the infection (step 2) at the site of disease or in the lung or other compartments through innate or adaptive immune mechanisms. ‘Lung and other compartments’ includes the alveolar space, lung interstitium, airways, mediastinal lymph nodes, whole blood and other specific organ systems where M. tuberculosis has seeded. Alternatively, individuals (approximately two-thirds) may rapidly progress from M. tuberculosis infection (step 3) to active disease (step 6) within a few months (~2 to ~18 months), or after many years through intervening asymptomatic disease stages of incipient tuberculosis (TB) (step 4) or subclinical TB (step 5)211. Those with active disease may succumb over several months or years (step 9) or be cured in most cases (step 7). However, such patients are at higher risk of relapse (step 8), leading to further transmission and exposure (step 1). Note that there is no consensus on the term latent TB infection (LTBI); the WHO guidance recommends using the term TB infection, which we have adopted here225, along with an alternative, MET-A-NAD (memory T cell immune response, asymptomatic, no active disease). *2–5% may rapidly progress from infection to active disease (~2 to ~18 months).
Mechanisms/pathophysiology
Mechanisms of drug resistance
The mechanisms of drug resistance in TB are complex (Fig. 3). M. tuberculosis is transmitted via the airborne route to the host, which results in lung granuloma (an aggregation of macrophages) formation, a progressive localized pneumonitis and consequent cavity formation, with resultant re-aerosolization of the organism perpetuating onward transmission (Fig. 2). The immunopathogenesis of human pulmonary TB is incompletely understood, there are no well-established correlates of protection (biomarkers associated with protection against infection). Furthermore, there is little understanding as to why some people (in the absence of obvious risk factors such as HIV or diabetes) get TB and others do not53.
a, Spontaneously occurring drug-resistant Mycobacterium tuberculosis organisms proliferate with inappropriate drug exposure or interruption of therapy. b, Direct extrusion of individual or multiple drugs by efflux pumps contribute to the development of high-level resistance. Efflux pump efficiency may be increased by mutations over time. c, Acquired resistance may also occur through non-DNA-encoding mechanisms (epigenetic mechanisms). d, Selective interruption of drug therapy or suboptimal or no effective drug at the site of disease may occur owing to pharmacokinetic variability. This may be due to variability in metabolism, absorption and/or elimination of drugs, but may also be due to heterogeneity in drug penetration of tuberculosis (TB) lesions and cavities, resulting in pharmacokinetic mismatch (inappropriate concentration of drug relative to minimum inhibitory concentration). e, Psychosocial and programmatic factors may impact drug delivery, adherence, monitoring and detection of low-level resistance, contributing to high-level acquired resistance. f, With time, there is an increasing level of transmission of drug-resistant strains, which results in amplification of the epidemic (primary or transmitted resistance) and this becomes the dominant mechanism of resistance acquisition. There is considerable heterogeneity in the infectiousness of individuals with TB disease, and most transmission occurs within the community (such as households, transport networks, schools and workplaces). A passive case-finding public health strategy means that diagnosis occurs late with most transmission having already occurred. Thus, targeted active case finding strategies will mitigate transmission through earlier diagnosis and reduce further amplification of the epidemic. Other offsets of earlier diagnosis may include reduced lung damage and ameliorated chronic pulmonary disability (post-TB lung disease).
Acquired drug resistance can be established through genomic mutations or epigenetic alterations (Fig. 3b,c,d). In the patient, spontaneous random mutants of M. tuberculosis can be selectively amplified owing to variability in drug pharmacokinetics or drug mismatch (between the desired and the actual concentration of a drug at the disease site), which can be facilitated by factors such as reduced drug exposure that often do not relate to patient non-adherence (Fig. 3d,e). In addition, there is widespread transmission of the resistant M. tuberculosis organisms at population level (primary resistance; Fig. 3f).
Mutational frequency and generation of variants
Spontaneous and random chance mutation is the main source of acquisition of genomic resistance to known antimycobacterial drugs (plasmid transfer does not occur in M. tuberculosis)39,54,55. Thus, there are spontaneously occurring drug-specific mutants, even to drugs not yet used in regimens56,57. Fluctuation assays, which are used to detect induction of mutations in the presence of a specific drug, have shown differences in the rate (per bacterium per generation) of spontaneously occurring in vitro mutants: 2.25 × 10−10 for rifampicin (R), 2.56 × 10−8 for isoniazid, 2 × 10−12 for rifampicin + isoniazid together, 2.56 × 10−7 for ethambutol, 1 × 10−25 for a three-drug first-line regimen, 5 × 10−5 for delamanid and 1 × 10−8 for bedaquiline58,59,60,61,62. Thus, a TB cavity (an excavated lesion in the lung) that contains ~108 organisms can contain two or three isoniazid-resistant organisms. Monotherapy will thus eradicate susceptible organisms but not resistant ones, which will multiply, leading to sequential acquisition of resistance (Fig. 3a). The frequency of mutant generation is a function of the bacillary load of replicating bacteria and exposure to suboptimal concentration of a drug (that is, below the concentration expected to produce a therapeutic effect)63,64,65, which may also be mediated by differential penetration of the drugs into TB lesions13,66,67 (Fig. 3d). Exposure to a low dose of drug favours mutant selection and resistance amplification60. Of note, a mutation encoding drug resistance may be associated with a bacterial fitness cost (discussed below). Prodrugs (such as isoniazid, pyrazinamide, ethionamide, delamanid and pretomanid) require activation inside the cells; if the prodrug-activating enzymes are not essential for mycobacterial growth and survival, spontaneously generated mutants can subvert the mechanism (causing drug resistance) at no cost to the mycobacterial cell.
Genomic mechanisms of resistance
Most of the known drug-resistance mechanisms are linked to mutations in genes known to be involved in drug resistance68, which includes genes encoding transcriptional regulators69. The type of mutation and its position can strongly influence minimal inhibitory concentration (MIC). Thus, MIC values may be useful to guide addition of higher doses of drugs such as fluoroquinolones and isoniazid. M. tuberculosis strain and lineage can also modulate the likelihood of resistance36. Low-level resistance can be overcome by increasing dosage of the drug70 (for example, moxifloxacin, isoniazid) but may be underestimated by phenotypic drug susceptibility testing (pDST) performed at the critical concentration71,72. The WHO catalogue versions 1 and 2 (refs. 73,74) provides a list of mutations based on the confidence of their association with the drug-resistance phenotype71,72. Sometimes, mutations, instead of impacting the function of an enzyme or key protein, can upregulate efflux pumps that excrete the drug from the cytoplasm (Fig. 3b). For example, resistance to bedaquiline and clofazimine is linked to the activation of the pump encoded by the mmpS5–mmpL5 operon owing to mutations in the transcriptional repressor encoded by Rv0678 (refs. 75,76,77).
In addition to genetic mutations, resistance may also be mediated by changes in cellular processes that do not involve alterations in the DNA sequence (Fig. 3c). Examples of these epigenetic mechanisms include DNA methylation or acetylation, histone protein modification and various RNA-based mechanisms (short and long non-coding RNA and RNA methylation, which produce different forms of the protein)60. These mechanisms may also mediate post-translational modification of proteins (and thus are also not mediated by sequence-encoded changes). Although epigenetic changes have been best studied for their effects on immune modulation, there is accumulating evidence that they have an important role in mediating drug resistance78,79,80. Epigenetic mechanisms have been found to mediate or facilitate resistance to several drugs including isoniazid81,82, p‐aminosalicylic acid83, ethambutol84 and streptomycin78,79,80,85.
Pharmacokinetics
Drug disposition (comprising absorption, distribution, metabolism and excretion) of second-line drugs may be influenced by host factors (age, sex, genetics, comorbidities) or by drug interactions with companion drugs (for example, antiretrovirals for treatment of HIV)86. Fortunately, for most second-line drugs, dosing is flat (instead of in milligrams per kilogram), and standard dosing produces target-range concentrations in most patients (unlike isoniazid and rifampicin for DS-TB). Bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) is now a recommended standard-of-care regimen for those with MDR/RR-TB who are not pregnant and are older than 14 years4. Bedaquiline is a cytochrome P450 (CYP) isoenzyme 3A (CYP3A; a liver enzyme) substrate, and its concentrations are thus affected by CYP3A inducers and inhibitors (for example, efavirenz and boosted protease inhibitors, respectively)87,88. It has a long terminal half-life, and higher clearance (and lower exposures) of bedaquiline has been associated with Black race, perhaps explained by population differences in CYP3A5*3 SNP frequencies89. For bedaquiline, there is a strong exposure–response relationship90,91, so high adherence to maintain constant therapeutic exposures is crucial to successful use of this drug.
Delamanid is extensively protein bound (99.5%) and metabolized by albumin92. It is neither a substrate, an inhibitor or an inducer of CYP enzymes, so drug interaction liability for this drug is low. Its maximum concentration is at 4 h post-dose, and it has a long terminal half-life of 30–38 h. Its main metabolite, DM-6705, has a half-life of 121–425 h, and it is responsible for the modest effect of the drug on the QT interval (time from the beginning of the QRS complex to the end of the T wave; see Table 2)93. The drug is largely excreted in the faeces. Absorption is enhanced by food (and even more with a high-fat meal)92,94,95.
Pretomanid, by contrast, has multiple metabolic pathways, with CYP3A being responsible for ~20% of its metabolism. Its concentrations are also reduced by CYP3A inducers, such as efavirenz96. Toxicities (hepatotoxicity specifically) seem to be driven by co-administration with pyrazinamide instead of by high pretomanid exposure97.
Among the drugs in the BPaLM regimen, linezolid has the narrowest therapeutic margin, and doses needed for efficacy typically cause toxicity in a subset of patients98. Toxicity is driven by cumulative exposure, mostly occurring after longer than 8 weeks of treatment99, with trough concentration being the pharmacokinetic (PK) parameter most highly associated with its well-described mitochondrial toxicities. Older age, low weight and renal dysfunction are associated with higher concentrations of linezolid100.
Moxifloxacin, given at a fixed daily dose, is conjugated in the liver and does not have meaningful drug interactions with HIV drugs. Although its pharmacokinetic–pharmacodynamic (PK–PD) relationships are not well-characterized for TB, some evidence suggests that doses greater than the standard dose do not improve outcomes but do produce more musculoskeletal adverse effects72. Important PK characteristics of other second-line anti-TB drugs are described in Table 3 (refs. 72,101). In summary, PK determinants and related factors may result in suboptimal drug concentrations at the site of disease thus driving resistance amplification (Fig. 3).
Heterogeneity in the drug penetration of lesions
Biopsy samples and explanted lungs from individuals failing DR-TB treatment have established that there is considerable heterogeneity in the penetration of drugs into TB lesions13. With specific regard to TB cavities, it has been demonstrated that relative to the blood compartment and the outer wall of the TB cavity, concentrations of moxifloxacin and other drugs were substantially lower in the centre of the cavity and within the liquified caseum (necrotic material) where there were high concentrations of mycobacteria13. This differential drug penetration has been associated with the acquisition of drug-specific resistance13, and the mutational analysis of the sputum sample reflected only part of the resistance profile noted within the TB cavity (likely because only some mutants were expectorated or grown from sputum). These phenomena may in part explain why, despite perfect adherence, ~10–15% of persons taking MDR/RR-TB therapy develop resistance to second-line drugs such as fluoroquinolone102. These findings not only have implications for selection of drugs in regimens (choosing agents based on drug penetration rather than mycobactericidal activity), drug dosing and delivery methods (adjunct inhaled antibiotics) but are also a stark reminder that resistance amplification is often not the fault of the patient when acquired drug resistance occurs.
Heteroresistance and fitness cost
Heteroresistance, defined as the co-existence of susceptible and resistant strains in the same clinical samples, has been linked to either co-infection with genetically different strains (on the same occasion or on different occasions) or co-existence of subclones of the parent wild-type strain with different susceptibility profiles103. The frequency varies between 5% and 10% and is drug dependent (~5% for isoniazid, ~7% for rifampicin and ~10% for fluoroquinolones)104, and detection in terms of sensitivity varies depending on the type of test used (highest with pDST, excellent with the line probe assays, modest with nested assays and targeted sequencing assays, which detect ~50% of heteroresistance)105. However, the mechanisms leading to heteroresistance are still poorly understood.
Bacterial fitness is defined as the capacity of bacteria to survive and grow in a hostile environment60,106. Mutations associated with drug resistance may influence bacterial fitness, and this may be mitigated by compensatory mutations. The fitness cost can be lineage related; the same mutation can impact the bacterial fitness cost differently in strains with different genetic backgrounds106. For example, in the lineage 2, but not the lineage 4 strain, resistance to rifampicin was mitigated by compensatory mutations, confirming the positive effect of the epistatic interaction between the compensatory and resistance-related mutations35.
Initially, and based on laboratory studies106, the concept of fitness cost was assumed to be associated with lower transmissibility of drug-resistant strains. However, molecular epidemiological studies have disproved this hypothesis, demonstrating efficient transmission of drug-resistant strains in various regions worldwide107,108,109, and individuals with DR-TB were shown to be as infectious as those with DS-TB. Realistically, sequence variations may give rise to a range of fitness impact (less fit, unaffected or more fit) depending on whether vital functions, such as replication or protein synthesis, are affected and the nature of compensatory mutations110.
Diagnosis, screening and prevention
Signs and symptoms
Classic symptoms of both DS-TB and DR-TB include fever, loss of weight and appetite, night sweats, cough (sometimes with blood) and chest pain. It is now well recognized that symptoms, although generally chronic, can be of less than 2 weeks duration and can present as an acute lower respiratory tract infection111. TB can be associated with trivial symptoms or may be asymptomatic (subclinical TB). Signs are commensurate with the organ involved and in the case of the lung may include crackles, bronchial breathing over cavities (amphoric breathing) and sometimes wheezing (owing to endobronchial and small airway involvement). The differential diagnosis at clinical presentation for pulmonary TB may include community-acquired pneumonia due to bacterial or viral infections, non-tuberculous mycobacterial disease, Pneumocystis pneumonia (especially in the presence of hypoxia and in immunocompromised persons), malignancy and pulmonary vascular pathologies.
Screening and diagnosis
The current global public health strategy, except for screening high-risk groups such as close contacts and people living with HIV, is predominantly one of passive case finding (the patient self-reports with symptoms to a health-care facility). However, by that time there has been uninterrupted transmission and infection of many persons in the community. As ~50% of the total TB case-load within a community is relatively asymptomatic (subclinical TB)112, two of every five patients with TB remain undetected by community surveys globally (and up to one out of three with MDR-TB)1. Current diagnostic methods are suboptimal, and as a consequence of the slow global decline in TB incidence, there is a crucial need for increased prioritization of community-based ACF113. However, there is debate about the optimal strategy (indiscriminate door-to-door versus targeted screening in the community, or variations of these), the diagnostic tools to be used for ACF (such as X-ray screening, which may used in computer-assisted detection (CAD), sputum-based molecular testing, smear microscopy) and how this should be used in practice (for example, molecular testing alone or using a biomarker (for example, X-ray) to guide molecular testing). Unfortunately, there is no available effective TB screening test and CAD often fails to meet target product profile thresholds especially in those with previous TB, the elderly and people living with HIV112. Strategies for future ACF include the use of a mini-mobile clinic approach that incorporates a low-cost vehicle, point-of-care battery-operated molecular testing and linkage to care114. Multicentric randomized controlled trials are ongoing to address some of the knowledge gaps around ACF (for example, NCT04303104 and NCT05220163)115,116.
Diagnostic testing
Whether by passive or active case finding, the diagnosis of DR-TB involves several steps and challenges, including first obtaining a representative biological sample (patients may be sputum scarce or have extrapulmonary TB), followed by diagnosis of M. tuberculosis infection using existing diagnostic tools (sensitivity is often suboptimal, and research has shown that molecular tests miss up to half the community-based TB case-load compared with culture)114. Diagnosis remains particularly challenging in children and people living with HIV.
Drug susceptibility testing
The WHO recommends (step 3, benchmark 9) that all patients with bacteriologically confirmed TB undergo universal drug susceptibility testing (DST) to determine whether their TB is resistant to commonly used anti-TB drugs50. Testing for isoniazid and fluoroquinolone resistance, in addition to rifampicin, is increasingly important, especially in settings where the prevalence of resistance to these drugs is >5%50. Ideally, testing should also be performed at lower prevalence50 or when there are other features that increase the risk of drug resistance (known contact with DR-TB, poor response to conventional treatment, history of previous TB or exposure to specific drugs and poor adherence, including factors that drive poor adherence)60.
Traditional and sensitive diagnostic methods of pDST use cultured isolates subjected to bacterial growth in the presence of antibiotics117, which is the current reference method for most drugs. However, drawbacks include lengthy time to result, highly complex and labour-intensive process117 and the requirement for specialized infrastructure, which limits its accessibility and impact (Table 4 and Fig. 4). pDST is available using both solid and liquid medium but critical concentrations are interim or not yet established for all new DR-TB drugs118.
Over the past decade, several advances have been made to improve the detection of drug resistance by understanding the molecular mechanism associated with drug resistance at a genomic level54. This has resulted in development of rapid molecular diagnostic assays for genotypic DST. Rapid molecular tests have been recommended119 as an option for smear replacement technology for the primary diagnosis of pulmonary TB in presumptive patients (including children), and they can simultaneously detect rifampicin resistance through detection of specific genetic markers that confirm M. tuberculosis species (for example, IS6110 insertion) and rifampicin resistance (rifampicin resistance determining region (RRDR) rpoB 1–4). Using proper infection control measures, rapid molecular tests can be deployed as a near-patient technology in clinics120 or at point of care in the community setting114. However, these assays are limited to the selected drugs detected by the assay and their sensitivity is restricted to the genetic markers they probe. The WHO has endorsed several platforms5 that provide DST readouts of first-line drugs (rifampicin + isoniazid), and those that detect resistance to fluoroquinolones and second-line injectables (although the latter are no longer recommended in DR-TB regimens). Phenotypic and genotypic test discordance may also occur owing to technical factors, misclassification bias, heteroresistance, and depending on the clinical context, such discordance is often treated as MDR/RR-TB when the discordance is rifampicin specific39,60. A similar consideration would apply to discordant isoniazid resistance readouts.
Addressing the diagnostic gap has been facilitated by the development of methods using next-generation DNA sequencing121 that have leveraged the WHO-endorsed catalogue of mutations122. These methods have been successfully evaluated for drug-resistance detection: from a sputum sample using the targeted approach (targeted next-generation sequencing; tNGS)123,124,125 or from clinical isolates using the whole-genome sequencing approach126,127. The sensitivity of these methods is heavily dependent on the amount of DNA available in a sputum sample (≥95% of sputum DNA is non-TB in origin and thus there is a limited amount of mycobacterial DNA). Research is ongoing to develop strategies to improve concentration or amplification of bacterial genomes directly from a sputum sample. tNGS is rapid and holds the most promise as a tool to provide comprehensive information about resistance for many drugs simultaneously; however, it is highly complex to perform, dependent on skilled personnel, has complex infrastructural requirements and high costs, which are currently prohibitive for wide-scale application. Table 4 outlines the high-confidence genetic markers that confer resistance to anti-TB drugs and the limitations of each sequencing method.
One of the key issues faced by TB programmes is the misalignment of diagnostic tools with the introduction of new or repurposed drugs; to date, WHO-approved or commercial assays for genomic DST are not available for new and repurposed drugs such as pretomanid, delamanid, linezolid and bedaquiline. Furthermore, the genetic basis of resistance to such drugs may not have been fully understood before drug introduction, therefore impeding the development of rapid molecular methods. Newer molecular methods may provide a mutation-based prediction of resistance (ideally within days) with relatively high levels of accuracy when high-confidence gene targets are identified54,128.
Prevention
Prevention of DR-TB disease, in addition to addressing social determinants of health and infection control aspects, can be accomplished by four major strategies: first, by preventing the emergence of isoniazid resistance; second, by preventing acquisition of rifampicin resistance in persons with isoniazid-resistant TB; third, by prompt identification and treatment of persons with DR-TB, so that transmission of drug-resistant M. tuberculosis is minimized; and fourth, by preventing progression from infection (Fig. 3) to disease among persons infected with drug-resistant strains of M. tuberculosis but without disease.
Preventing the emergence of isoniazid monoresistance
Both clinical and molecular evidence supports the conclusion that the first step in the development of MDR-TB is acquisition of isoniazid resistance129,130. Several factors can contribute to the emergence of isoniazid resistance among persons being treated for DS-TB, including failure to adhere to the regimen, rapid isoniazid acetylation, which is known to reduce drug concentration within the patient, and failure of adequate drug penetration to areas of extensive disease131,132,133. Neither assessment of isoniazid acetylator status nor monitoring of isoniazid concentrations is routinely performed, with the result that emergence of drug resistance to isoniazid is observed in ~1% of persons being treated for DS-TB134,135.
Preventing acquisition of rifampicin resistance
Emergence of rifampicin resistance during treatment of persons with isoniazid resistance can occur if isoniazid resistance has not been recognized, which results in treatment with an inadequate regimen, or owing to various factors that may cause PK mismatch136. Unfortunately, although molecular tests for isoniazid resistance are available, they are not widely used, so inadequate treatment of isoniazid-resistant TB occurs far too often. In addition, persons with HIV infection, especially those treated with intermittent regimens, can develop rifamycin resistance without isoniazid resistance; for this population intermittent regimens are to be avoided under any circumstances.
Prompt identification and treatment of persons with MDR-TB
Although acquired resistance was previously the major route by which MDR/RR-TB is contracted, in the past decade primary resistance has become predominant137. MDR/RR strains of M. tuberculosis are effectively transmitted to contacts, and failure to promptly diagnose persons with MDR/RR-TB and start them on effective treatment regimens has led to substantial community transmission. Until all persons with TB are promptly diagnosed and assessed for rifampicin resistance, MDR strains of M. tuberculosis will continue to spread in the community; interrupting such transmission will require ACF and broad availability of genotypic resistance testing. The WHO now recommends a 6-month course of daily levofloxacin be considered as preventive therapy for persons of all ages in contact with MDR/RR-TB138.
Preventing progression from infection to disease among persons infected with drug-resistant strains of M. tuberculosis
For persons infected with isoniazid-monoresistant organisms, rifamycin-based regimens are available for treatment to prevent progression to disease139. For a person infected with MDR/RR strains of M. tuberculosis that are fluoroquinolone susceptible, 6 months of fluoroquinolone preventive therapy is effective and recommended140,141. A trial of delamanid for TB infection has also been initiated, but results will not be available for some time142. A phase II study of a promising TB vaccine, M72/AS01E, suggested that it could prevent progression from infection to disease143, which would be especially useful for treatment of persons infected with MDR-TB.
Preventing emergence of resistance to other antimycobacterial agents used for the treatment of MDR/RR-TB
It will be essential to prevent the emergence of resistance to the agents currently effective in treatment of MDR/RR-TB, especially fluoroquinolones, bedaquiline, linezolid and pretomanid or delamanid. This will require availability and implementation of rapid diagnostic tests for these agents, so that patients do not receive regimens that are inadequate to prevent emergence of such additional resistance. Unfortunately, tests are scarce and not widely implemented144.
Management
Treatment regimens for drug-susceptible TB
The standard treatment regimen for adults with pulmonary TB caused by organisms not known or suspected to be drug resistant involves a 2-month intensive phase using HRZE, followed by a 4-month continuation phase with rifampicin and isoniazid4,145,146. In 2021, a 4-month regimen containing rifapentine, moxifloxacin, isoniazid and pyrazinamide was found to be non-inferior to the standard 6-month regimen in terms of efficacy and safety147, resulting in WHO endorsement of this regimen as an alternative treatment option for nonpregnant patients aged ≥12 years with drug-susceptible pulmonary TB148. In children under 16 years of age with paucibacillary non-severe DS-TB, a 4-month treatment regimen with rifampicin, isoniazid, pyrazinamide, with or without ethambutol was non-inferior to 6 months of standard treatment149 and was conditionally endorsed by the WHO in 2022.
Treatment regimens for resistant forms of TB
Treatment regimen for isoniazid-monoresistant TB
Isoniazid resistance compromises the effectiveness of treatment with the standard HRZE regimen. WHO recommends treatment of rifampicin-susceptible, isoniazid-resistant TB with a combination of rifampicin, ethambutol, pyrazinamide and levofloxacin over a duration of 6 months, although the recommendation is conditional with evidence of low certainty136,150 (Table 5).
Treatment regimens for MDR/RR-TB
Until 2016, the recommended duration of treatment for MDR/RR-TB was at least 18 months with a combination of at least four active drugs, often containing an injectable6,151. Treatment success with this regimen did not exceed 60% globally7 and was associated with a high rate of adverse drug effects152 and high costs10. The landscape has rapidly changed since then, first with the introduction of the 9- to 12-month ‘Bangladesh’ regimen, which includes an injectable153 and subsequently with all-oral regimens of 6–9 months’ duration154,155,156,157,158.
In 2020, the results of the NiX-TB trial became available. NiX-TB is an open-label, single-group phase III study that examined the safety and efficacy of an all-oral three-drug regimen containing bedaquiline, pretomanid and linezolid (also known as BPaL) administered over 6 months in patients with treatment-intolerant or non-responsive MDR-TB or XDR-TB (as per the pre-2021 definition, that is, resistance to rifampicin (with or without resistance to isoniazid) plus resistance to a fluoroquinolone and a second-line injectable drug)155. With this regimen, 90% (95% CI 83–95%) of patients achieved a favourable outcome, albeit, with a high frequency of adverse effects attributed to the daily high-dose of linezolid (81% of patients developed peripheral neuropathy and 48% developed anaemia and/or thrombocytopenia).
Lower daily doses of linezolid were evaluated in ZeNiX-TB and TB-PRACTECAL studies154,158. The TB-PRACTECAL trial158 evaluated the efficacy and safety of three 24-week BPaL-based treatment regimens with linezolid (BPaL, BPaLC (with clofazimine), BPaLM (with moxifloxacin)), in comparison with standard-of-care treatment. Only BPaLM was selected in stage 2 and at the end of 72 weeks post-treatment initiation, 89% of the patients in the BPaLM arm experienced treatment success, compared with 52% of patients in the standard-of-care arm. Thus, BPaLM was both non-inferior and superior to the standard-of-care treatment. Furthermore, more severe adverse effects occurred more often in the standard-of-care arm than in the BPaLM arm (59% compared with 19%). Moreover, BPaLM (with a lower dose of linezolid than in NiX-TB) was much better tolerated than BPaL in the NiX-TB study159.
Based on the results of these two trials and data provided by the Department of Health of the Government of South Africa, the WHO updated the guidelines on DR-TB in 2022, recommending the BPaLM regimen as the preferred regimen for patients with MDR/RR-TB when fluoroquinolone susceptibility is presumed or documented4 (Table 5) and BPaL alone for patients with additional fluoroquinolone resistance (pre-XDR-TB). For patients who are not eligible for the shorter BPaLM regimens (Table 5; for example, unavailability of pretomanid or TB with other specific characteristics) but have isolates susceptible to fluoroquinolones, an all-oral regimen of 9–11 months is recommended. Thus, injectables (for example, amikacin) should no longer be used to treat MDR/RR-TB but they may still be considered in rescue regimens for XDR-TB, or resistance beyond XDR-TB, in which there are no other treatment options. The management of DR-TB in children is addressed in Box 2. Although those under 14 years of age are not eligible for pretomanid-based regimens, it is recommended they receive the short 9-month all-oral regimen containing new drugs. It is important to note that the cost of implementing BPaLM and BPaL regimens (excluding patient-incurred costs) is potentially 40–90% lower than current regimens, despite containing two innovative new drugs (bedaquiline and pretomanid)160.
Adverse effects
It is important to promptly address adverse effects of treatment and provide effective patient support to optimize treatment adherence and effectiveness. Adverse effects undermine treatment adherence and can result in treatment interruption, life-threatening complications and death161 (Supplementary Box 1). The use of multiple drugs in the TB regimen and their possible additive contribution to adverse effects (in addition to the use of concomitant antiretroviral therapy) makes it challenging to identify causality. Table 2 shows major treatment-related adverse effects and possible drug causes. Close observation and experience are required to promptly detect an adverse event and manage drug intolerance.
Management of treatment of DR-TB in special patient populations
The treatment of patients affected by DR-TB is complicated by the management of comorbidities, such as HIV co-infection, diabetes mellitus, liver disease, renal failure, undernutrition, and in special situations such as pregnancy and breastfeeding161. Comorbidities are best addressed by early detection and timely management, including of adverse effects, dosage adjustments and recognition of drug–drug interactions and malabsorption (Table 6). Special situations often require additional counselling or mobilization of social resources to enhance treatment adherence. Pregnant or breastfeeding women should have access to all-oral, shorter regimens that do not contain pretomanid, as safety data are limited4.
Role of therapeutic drug monitoring
There is overwhelming evidence that MDR-TB is augmented by way of isoniazid resistance (mediated specifically by the katG mutation), which preceded subsequent resistance to rifampicin across lineage and geographical regions129. As PK variability (with some patients naturally having too-low drug concentrations)162 is thought to be a major contributor to the risk of acquired drug resistance (and/or poor treatment response), therapeutic drug monitoring (TDM; measuring drug levels within patient blood) is commonly practised, where available39,60. The goal is to ensure that patients have drug exposures in the target range, typically defined by population averages (given that concentrations that prevent clinical resistance have not been convincingly established)163. Whether or not TDM improves treatment outcomes and reduces risk of resistance is unclear, even if the rationale for using it is well founded164. However, given the high PK variability in rifampicin and the fact that exposures produced by currently recommended doses are on the steep part of the dose–response curve165, ensuring adequate drug concentrations, either by TDM or higher dosing overall, represents an important component of DR-TB prevention strategies164. Few data currently exist to support TDM for drugs used to treat MDR/RR-TB. However, given the crucial role that bedaquiline has in curing patients with MDR/RR-TB (coupled with the rising threat of bedaquiline resistance) and the narrow therapeutic margin of linezolid166,167, a case can be made for developing TDM strategies for these drugs, recognizing that barriers to access would need to be overcome for TDM to have a population-level effect on treatment efficacy and/or emergence of resistance. Measuring linezolid before dose and 2 h after dose will capture minimum concentration (Cmin, relevant for toxicity) and maximum concentration (Cmax, relevant for efficacy)168. To our knowledge, there is not a TDM sampling strategy for bedaquiline. Use of dried blood spots (DBSs) can reduce practical challenges of TDM in that DBS samples typically do not require a cold chain and can be shipped to a pharmacology lab for testing via standard post. DBSs have been developed for linezolid but are not, to our knowledge, in clinical use. No DBS exists for bedaquiline169.
Personalized medicine versus the pan-TB approach
With new, highly effective and well-tolerated compounds from new drug classes becoming available, there is potential for drugs to be combined in one treatment regimen irrespective of the patient’s M. tuberculosis drug-resistance profile136. Such a ‘pan-TB’ regimen could potentially turn back the clock, creating a situation like the 1960s when the four drugs of the standard anti-TB regimen were highly effective. The advantages of such a regimen, without the need for DST, may include reduction in the complexity of management, rapid reduction in mortality and curtailment of transmission on a wide scale. However, the major problem in implementation would be monitoring of emerging drug resistance, which will occur following the natural laws of evolution70,103. Indeed, even with good adherence, ~10–15% of isolates developed resistance to fluoroquinolones102, which is now widespread70,103. A similar situation may also occur with the roll-out of the BPaLM regimen in the absence of sufficient DST capacities in most affected countries170. Thus, any potential advantages would be neutralized within a decade or two and replaced by transmission of highly resistant strains.
An alternative, with several advantages171,172, is to design accurate treatment regimens based on pathogen-specific genotypic data and the likelihood of adverse effects, including patient preferences128, thus implementing a personalized medicine and patient-centred approach. Although much more difficult to implement in low-resource settings, the advantages would include preservation of a meagre pipeline of new drugs, thus prolonging the utility of existing regimens, minimizing the development of resistance, ongoing promotion of new drug and diagnostic development, and prevention of the emergence of highly resistant strains. The personalized approach could also impact treatment in other ways such as improving outcomes. Biomarkers based on host RNA signatures are being developed to guide individualization of the duration of anti-TB treatments173 and human gene-expression profiles are being explored to provide endotype-specific host-directed therapies174. However, until newer and improved diagnostics are more widely implemented core regimens with limited flexibility seem to be more applicable in TB-endemic countries.
Treatment outcomes and surgical aspects
Following an expert consultation meeting the WHO revised the treatment outcome definitions for all forms of TB in 2021 (refs. 5,175). These revised definitions aim for programmatic use and rely on the assignment of an outcome at the end of the treatment course. They acknowledge that more prolonged follow-up (because of lengthy regimens) is challenging given the limited capacity of the health-care systems in countries where TB is endemic. The new definitions add the new component of ‘sustained treatment success’, which is suggested for use under operational research only9,176. The new definitions also recognize that discontinuation of a single key group A drug may have a more profound impact on outcomes than two or more alternative drugs (the requirement for discontinuation of two drugs for treatment failure is no longer specified in the WHO definition). The definition of failure and the embedded definition of bacteriological response remain central to the outcome definitions to account for the changing potency and mycobactericidal activity of the drug combinations used in individual regimens. Culture negativity at the 6-month time-point and beyond has been shown to be a good marker to indicate relapse-free cure in low- and high-burden settings of TB9,177,178,179. Future treatment outcome definitions could be guided by results of long-term post-treatment follow-up to find markers that best indicate relapse-free cure180,181. Such an approach will likely provide a useful perspective that can be considered in future outcome definitions.
The inherent nature of medical treatment failure (severe illness; limited longevity) and considerable selection bias (limited disease and the need to be fit for surgery), means that data from controlled clinical trials evaluating the impact of surgical interventions are still unavailable. However, in selected circumstances, results from an individual patient data meta-analysis suggest that partial lung resection, but not pneumonectomy, is associated with improved treatment success, although this result may have been due to selection bias inherent in the studies performed182. Data suggest that PET–CT activity and positivity in the contralateral lung (the lung or part of it not planned to be resected) is poorly predictive of surgical outcomes, and that a suitable group A-based rescue regimen is just as important for success as the surgical resection itself183.
Person-centred care
The WHO declared TB a public health emergency in 1993; directly observed therapy short course (DOTS) was promoted as the predominant approach to combat it39,60. Although widely implemented, DOTS was limited in its success22. Criticism of DOTS included its potential ability to undermine the dignity of individuals, contribution to the catastrophic costs incurred by poor individuals who may be required to forgo employment and shoulder travel expenses to attend daily therapy and lack of impact on reduction of the development of drug resistance132. DOTS has since been surpassed by the more collaborative endTB goals and an increasing recognition of the need for a person-centred public health approach in which the communities and persons affected by TB are active participants. Much of this has been successfully modelled by the global HIV response.
In addition to the biomedical strategies outlined thus far, DS-TB and DR-TB programmes should address the psychosocial factors that underpin transmission and disease and work to remove health system barriers to care (Supplementary Box 1). This requires decentralized and family-friendly TB services, which provide necessary adherence such as food support, transport vouchers and access to income replacement184. Care for comorbidities (including HIV, diabetes, mental health and substance use disorders) should be integrated into TB services. Treatment literacy, age-appropriate counselling and differentiated adherence support should be offered (examples include video-observed therapy, peer support models and adolescent-specific adherence support; much space remains for innovation in this field).
Palliative care
Interventions to improve treatment outcomes in patients thought to be affected by ‘incurable’ TB include implementations of personalized medicine; however, despite the application of innovative methods some patients affected by DR-TB may remain incurable. Palliative care aims to improve the life of patients facing terminal illness, through the prevention and relief of suffering185,186,187. Access to palliative care is a human right, and TB programmes have an ethical obligation to provide it, including for persons with incurable forms of DR-TB185. In these cases, in addition to extensive counselling and support for individuals and their loved ones, care providers and patients should seek collaborative solutions that aim to uphold the patient’s right to self-determination and access to socially inclusive palliative care, while also preventing disease transmission to others (including health-care workers and children).
Quality of life
International efforts have been made to define minimum standards of care for the DS-TB and DR-TB care cascade175,188,189. However, the gap between theory and reality remains vast190,191.
In addition to increased DR-TB services coverage, quality of care must considered190. Improving DR-TB services starts with inviting the individuals and communities for whom these services are being provided, together with the health workers at grassroots level, as key partners in design, improvement, innovation and implementation of DR-TB care190,192,193. This includes providing feedback on existing DR-TB services, identifying quality care gaps and impactful improvement projects, and actively participating in the implementation of DR-TB tools and services190. Helpful examples of how this has been done successfully are available in the HIV domain194. Without this participation, evidence-based interventions in DR-TB care are unlikely to have the desired effect195. Similarly, top-down imposed quality improvement projects — often with targets and incentives set by external funders — rarely translate into sustainable change196. Sustainable improvement in quality of DR-TB services requires that all levels of health services work together — with strong national tuberculosis programme leadership — and a focus on overall health system strengthening190. DR-TB care should be decentralized with facility level ownership and accountability of quality of services190.
Post-TB lung disease
Unfortunately, TB is a disease in which symptoms and complications such as pulmonary disability may continue after microbiological cure197. Various structural forms of lung disease may persist after TB treatment and these may include obstructive airways disease (TB is amongst the most common causes of chronic obstructive pulmonary disease in many TB-endemic countries197), bronchiectasis and fibrocavitary disease198. A combination of these entities can occur in the same individual and often there is severe fibrocavitary destructive disease. Residual disability after successful treatment of TB is common; in one meta-analysis, almost 60% of patients after treatment had abnormal spirometry, ~25% had an Medical Research Council (MRC) dyspnoea score of 3–5 (effort tolerance was restricted to only 100 m or less), and lung cancer was four times more common in patients after TB treatment198. However, there are hardly any data about the frequency of post-TB lung disease specifically in patients with DR-TB. Limited data indicate that post-DR-TB treatment obstructive disease together with poor physical health scores are common199, and in two small studies (each with fewer than 50 participants with MDR/RR-TB), almost all had pulmonary function test abnormalities after treatment, and lung damage was extensive200. In an analysis of more than 14,000 persons, lung function impairment in those with MDR/RR-TB was worse than in those with DS-TB, and impairment was severe in 10–15% of survivors201. This may be related to later diagnosis and treatment initiation in patients with DR-TB compared with DS-TB202. Remarkably, there are no reliable data about the natural history of post-DR-TB lung disease, the incidence of secondary respiratory infections (viral, bacterial or mycobacterial), and nothing is known about the effectiveness of interventions to ameliorate the severity of the disability or symptoms.
Socio-ethical dilemmas
The treatment journey may be particularly challenging for individuals who are also facing poverty, mental illness or a substance use disorder, which are often associated with poorer TB treatment outcomes and can result in additional stigma and discrimination (including by health staff)203,204. The interwoven complexities of TB, mental health, social determinants of health and rigid health services are complex205 and can result in socio-ethical dilemmas in which the tension between the individual’s right to autonomy and the public’s right to a safe environment must be carefully navigated206. One example of this is when a person with TB repeatedly struggles to take their treatment or does not wish to receive treatment yet continues to travel and work to provide for their family. Another socio-ethical dilemma may present itself when managing patients who are functionally incurable (therapeutically destitute); often caused by M. tuberculosis strains with resistance to all or almost all existing anti-TB agents. A suboptimal treatment regimen may improve the individual’s clinical condition, but at the risk of increasing resistance in the TB strain, resulting in a greater public health threat by a TB ‘superbug’206.
The WHO released a guide that outlines ethical principles to be used in facing these dilemmas185. In all these scenarios the individual and family affected must be approached without judgement, and with compassion and kindness185. Human dignity and individual rights (carefully balanced against the rights of the public) remain paramount. It is important to acknowledge that most socio-ethical dilemmas have been born as the result of system failures; solutions can often be found when health services are willing to be flexible and partner with the individual and their community to find creative solutions. Adherence may be achieved when treatment is provided in a person-centred way, there is aggressive management of adverse effects and comorbid conditions (including mental Illness or substance use disorder) and with adequate patient support (including counselling, treatment enablers and family engagement).
Outlook
Ending DS-TB and DR-TB is not just a public health problem, but a development challenge and opportunity. One of the targets of the Sustainable Development Goals (SDGs)207 for the period 2015–2030 is to end the global TB epidemic. In line with this target, the WHO end TB strategy208, approved by the World Health Assembly in 2014, calls for a 90% reduction in TB deaths and an 80% decrease in TB incidence by 2030. The resolution calls on governments to adapt and implement the strategy with high-level commitment and financing.
One of the three pillars of the end TB strategy is intensified research and innovation. The key components of this pillar of the strategy are discovery, development and rapid uptake of new tools, interventions and strategies; and research to optimize implementation, and impact and promote innovation.
The development of policies to tackle the problem of DR-TB is challenged by lack of high-quality evidence. To stimulate and guide additional research and innovation in areas with insufficient evidence, the WHO regularly convenes several guideline development groups to develop new policies and also to identify the research gaps that hinder the development of policies (TB Knowledge Sharing Platform). The research gaps that are particularly evident are those around the care and management of DR-TB. Another neglected aspect in general, and highly relevant to DR-TB, is screening for TB and community-based ACF of TB given the realization that 36.1–79.7% (median 50.4%) of the TB and DR-TB burden is subclinical51. Supplementary Table 2 provides a high-level summary of the research goals and activities to fill the knowledge gaps with anticipated deliverables that relate to the management of DR-TB in several research areas.
Although MDR/RR-TB forms only ~5% of the global TB burden (~410,000 newly ill patients per year)1, more than 20% of M. tuberculosis isolates are now resistant to at least one major first-line or second-line TB drug39. Besides the considerable morbidity and mortality associated with DR-TB, including to health-care workers, drug resistance remains a major threat to TB control in many countries because of the extremely high costs associated with the management of the condition, often eclipsing the cost of managing DS-TB. It is estimated that DR-TB will contribute to ~30% of the total costs to the global economy due to antimicrobial resistance by 2050 (ref. 8).
Encouragingly, new automated point-of-care diagnostic technologies and targeted genomic sequencing approaches hold promise to optimize diagnosis and facilitate a personalized medicine approach to DR-TB; thus helping to minimize resistance amplification. There needs to be a paradigm shift in global health strategy with a much bigger focus on community-based ACF, thus circumventing transmission and preventing amplification of the epidemic. To aid this, newer tools and biomarkers are urgently required to identify the most infectious patients so that they may be targeted for transmission-interrupting interventions and early treatment initiation. Shorter all-oral regimens using newer drugs have been developed4; however, antibiotic stewardship measures (rational use of antibiotics to prevent resistance) will have to be implemented to protect these new drugs. Thus, development of rapid DST tools for newer drugs remains a major priority. Nevertheless, despite these advances, resistance to the newer drugs has rapidly emerged, leading to substantial numbers of therapeutically destitute patients in TB-endemic countries209. This raises major socio-ethical dilemmas, and more thought should be given towards creation of palliative care facilities and long-term community-based facilities where such patients can live a meaningful existence210. The goals and priorities outlined in Supplementary Table 2 should occur in tandem with improved global investment in TB, strengthening of health-care systems, change in global economic policies to reduce poverty and overcrowding, and a strong change in political will, to control TB, both globally and in TB-endemic countries.
The COVID-19 pandemic illustrated what could be done within a short period of time to create new diagnostics, drugs and vaccines15. The challenges with DR-TB are complex, and a reinvigorated global approach is required to tackle this scourge of DR-TB, as it is far from eradicated210.
References
WHO. Global Tuberculosis Report 2023. World Health Organization https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (2023).
The Economist Intelligence Unit. It’s time to end drug-resistant tuberculosis. The Economist https://www.eiu.com/graphics/marketing/pdf/its-time-to-end-drug-resistant-tuberculosis-full-report.pdf (2019).
Paulson, T. Epidemiology: a mortal foe. Nature 502, S2–S3 (2013).
WHO. WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment - Drug-Resistant Tuberculosis Treatment, 2022 Update. World Health Organization https://www.who.int/publications/i/item/9789240063129 (2022).
WHO. Meeting Report of the WHO Expert Consultation on Drug-Resistant Tuberculosis Treatment Outcome Definitions. World Health Organization https://www.who.int/publications/i/item/9789240022195 (2021).
WHO Consolidated Guidelines on Drug-resistant Tuberculosis Treatment (WHO, 2019).
WHO. Global Tuberculosis Report 2022. World Health Organization https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (2022).
O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. AMR https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (2016).
Gunther, G. et al. Treatment outcomes in multidrug-resistant tuberculosis. N. Engl. J. Med. 375, 1103–1105 (2016).
Gunther, G. et al. Availability and costs of medicines for the treatment of tuberculosis in Europe. Clin. Microbiol. Infect. 29, 77–84 (2023).
WHO. Ten Threats to Global Health in 2019. World Health Organization https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (2019).
WHO. Global Research Agenda for Antimicrobial Resistance in Human Health. World Health Organization https://www.who.int/publications/m/item/global-research-agenda-for-antimicrobial-resistance-in-human-health#:~:Text=It%20aims%20to%20guide%20policy,and%2Dmiddle%2Dincome%20countries (2023).
Dheda, K. et al. Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am. J. Respir. Crit. Care Med. 198, 1208–1219 (2018). Comprehensive study outlining the differential penetration of drugs into TB cavities and its association with resistance amplification.
WHO. WHO TB country, regional and global profiles. World Health Organization https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&lan=%22EN%22&entity_type=%22country%22&iso2=%22AF%22 (2022).
Dheda, K. et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 10, 603–622 (2022).
Bykov, I. et al. Factors contributing to the high prevalence of multidrug-resistance/rifampicin-resistance in patients with tuberculosis: an epidemiological cross sectional and qualitative study from Khabarovsk Krai region of Russia. BMC Infect. Dis. 22, 612 (2022).
Faustini, A., Hall, A. J. & Perucci, C. A. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax 61, 158–163 (2006).
Jeong, H. E. et al. Socioeconomic disparities and multidrug-resistant tuberculosis in South Korea: focus on immigrants and income levels. J. Microbiol. Immunol. Infect. 56, 424–428 (2023).
Feng, M. et al. Risk factors of multidrug-resistant tuberculosis in China: a meta-analysis. Public Health Nurs. 36, 257–269 (2019).
Wingfield, T., Tovar, M. A., Datta, S., Saunders, M. J. & Evans, C. A. Addressing social determinants to end tuberculosis. Lancet 391, 1129–1132 (2018).
Janssens, J. P. & Rieder, H. L. An ecological analysis of incidence of tuberculosis and ‘per capita′ gross domestic product. Eur. Respir. J. 32, 1415 (2008).
Lienhardt, C. et al. Global tuberculosis control: lessons learnt and future prospects. Nat. Rev. Microbiol. 10, 407–416 (2012).
Oxlade, O. & Murray, M. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS ONE 7, e47533 (2012).
Bhargava, A., Bhargava, M., Beneditti, A. & Kurpad, A. Attributable is preventable: corrected and revised estimates of population attributable fraction of TB related to undernutrition in 30 high TB burden countries. J. Clin. Tuberc. Other Mycobact. Dis. 27, 100309 (2022).
Sultana, Z. Z. et al. HIV infection and multidrug resistant tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis. 21, 51 (2021).
Bastard, M. et al. Outcomes of HIV-infected versus HIV-non-infected patients treated for drug-resistance tuberculosis: multicenter cohort study. PLoS ONE 13, e0193491 (2018).
Ong, K. L. et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402, 203–234 (2023).
Tegegne, B. S., Mengesha, M. M., Teferra, A. A., Awoke, M. A. & Habtewold, T. D. Association between diabetes mellitus and multi-drug-resistant tuberculosis: evidence from a systematic review and meta-analysis. Syst. Rev. 7, 161 (2018).
Xu, G., Hu, X., Lian, Y. & Li, X. Diabetes mellitus affects the treatment outcomes of drug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis. 23, 813 (2023).
Du, D. H. et al. The effect of M. tuberculosis lineage on clinical phenotype. Preprint at medRxiv https://doi.org/10.1101/2023.03.14.23287284 (2023).
Marx, F. M. et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin. Infect. Dis. 58, 1676–1683 (2014).
Becerra, M. C. et al. Transmissibility and potential for disease progression of drug resistant Mycobacterium tuberculosis: prospective cohort study. BMJ 367, l5894 (2019).
Theron, G. et al. Bacterial and host determinants of cough aerosol culture positivity in patients with drug-resistant versus drug-susceptible tuberculosis. Nat. Med. 26, 1435–1443 (2020). Work indicating that DR-TB strains were as infectious as DS-TB strains, and that infectiousness was not associated with M. tuberculosis genetic variants (suggesting that epigenetic factors or host–pathogen interactions are likely important in the pathogenesis of resistance amplification).
Shah, N. S. et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N. Engl. J. Med. 376, 243–253 (2017).
Loiseau, C. et al. The relative transmission fitness of multidrug-resistant Mycobacterium tuberculosis in a drug resistance hotspot. Nat. Commun. 14, 1988 (2023).
Bateson, A. et al. Ancient and recent differences in the intrinsic susceptibility of Mycobacterium tuberculosis complex to pretomanid. J. Antimicrob. Chemother. 77, 1685–1693 (2022).
Gómez-González, P. J. et al. Genetic diversity of candidate loci linked to Mycobacterium tuberculosis resistance to bedaquiline, delamanid and pretomanid. Sci. Rep. 11, 19431 (2021).
Brown, T. S. et al. Evolution and emergence of multidrug-resistant Mycobacterium tuberculosis in Chisinau, Moldova. Microb. Genom. 7, 000620 (2021).
Dheda, K. et al. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir. Med. 7, 820–826 (2019).
Adam, D. C. et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat. Med. 26, 1714–1719 (2020).
Perdigão, J. et al. Using genomics to understand the origin and dispersion of multidrug and extensively drug resistant tuberculosis in Portugal. Sci. Rep. 10, 2600 (2020).
Salvato, R. S. et al. Genomic-based surveillance reveals high ongoing transmission of multi-drug-resistant Mycobacterium tuberculosis in Southern Brazil. Int. J. Antimicrob. Agents 58, 106401 (2021).
Sara, C. A. et al. Extensively drug-resistant tuberculosis in South Africa: genomic evidence supporting transmission in communities. ERJ 52, 1800246 (2018).
Srilohasin, P. et al. Genomic evidence supporting the clonal expansion of extensively drug-resistant tuberculosis bacteria belonging to a rare proto-Beijing genotype. Emerg. Microbes Infect. 9, 2632–2641 (2020).
Vaziri, F. et al. Genetic diversity of multi- and extensively drug-resistant Mycobacterium tuberculosis isolates in the capital of iran, revealed by whole-genome sequencing. J. Clin. Microbiol. 57, e01477–e01518 (2019).
McMurray, D. N. in Tuberculosis: Pathogenesis, Protection, and Control (ed. Bloom B. R.) 135–147 (ASM, 1994).
Jones-López, E. C. et al. Cough aerosols of Mycobacterium tuberculosis predict new infection. A household contact study. Am. J. Respir. Crit. Care Med. 187, 1007–1015 (2013).
Cattamanchi, A. et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 56, 230–238 (2011).
Rangaka, M. X. et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 12, 45–55 (2012).
WHO. WHO standard: universal access to rapid tuberculosis diagnostics. World Health Organization https://www.who.int/publications/i/item/9789240071315 (2023).
Frascella, B. et al. Subclinical tuberculosis disease-a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin. Infect. Dis. 73, e830–e841 (2021).
Kendall, E. A., Shrestha, S. & Dowdy, D. W. The epidemiological importance of subclinical tuberculosis. A critical reappraisal. Am. J. Respir. Crit. Care Med. 203, 168–174 (2021).
Flynn, J. L. & Chan, J. Immune cell interactions in tuberculosis. Cell 185, 4682–4702 (2022). Comprehensive summary of the immunopathogenesis of TB.
Domínguez, J. et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a 2023 TBnet/RESIST-TB consensus statement. Lancet Infect. Dis. 23, e122–e137 (2023).
Mokrousov, I. et al. Molecular insight into Mycobacterium tuberculosis resistance to nitrofuranyl amides gained through metagenomics-like analysis of spontaneous mutants. Pharmaceuticals 15, 1136 (2022).
Ghosh, A., N, S. & Saha, S. Survey of drug resistance associated gene mutations in Mycobacterium tuberculosis, ESKAPE and other bacterial species. Sci. Rep. 10, 8957 (2020).
Green, A. G. et al. Analysis of genome-wide mutational dependence in naturally evolving Mycobacterium tuberculosis populations. Mol. Biol. Evol. https://doi.org/10.1093/molbev/msad131 (2023).
Bergval, I. et al. Pre-existing isoniazid resistance, but not the genotype of Mycobacterium tuberculosis drives rifampicin resistance codon preference in vitro. PLoS ONE 7, e29108 (2012).
David, H. L. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl. Microbiol. 20, 810–814 (1970).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 5, 291–360 (2017).
Ismail, N., Omar, S. V., Ismail, N. A. & Peters, R. P. H. Collated data of mutation frequencies and associated genetic variants of bedaquiline, clofazimine and linezolid resistance in Mycobacterium tuberculosis. Data Brief. 20, 1975–1983 (2018).
Schena, E. et al. Delamanid susceptibility testing of Mycobacterium tuberculosis using the resazurin microtitre assay and the BACTEC™ MGIT™ 960 system. J. Antimicrob. Chemother. 71, 1532–1539 (2016).
Davies Forsman, L. et al. Suboptimal moxifloxacin and levofloxacin drug exposure during treatment of patients with multidrug-resistant tuberculosis: results from a prospective study in China. Eur. Respir. J. 57, 2003463 (2021).
Nguyen, Q. H., Contamin, L., Nguyen, T. V. A. & Bañuls, A. L. Insights into the processes that drive the evolution of drug resistance in Mycobacterium tuberculosis. Evol. Appl. 11, 1498–1511 (2018).
Said, B. N. et al. Pharmacodynamic biomarkers for quantifying the mycobacterial effect of high doses of rifampin in patients with rifampin-susceptible pulmonary tuberculosis. Int. J. Mycobacteriol. 10, 457–462 (2021).
Kokesch-Himmelreich, J. et al. Do anti-tuberculosis drugs reach their target? — high-resolution matrix-assisted laser desorption/ionization mass spectrometry imaging provides information on drug penetration into necrotic granulomas. Anal. Chem. 94, 5483–5492 (2022).
Strydom, N. et al. Tuberculosis drugs’ distribution and emergence of resistance in patient’s lung lesions: a mechanistic model and tool for regimen and dose optimization. PLoS Med. 16, e1002773 (2019).
The CRyPTIC Consortium. A data compendium associating the genomes of 12,289 Mycobacterium tuberculosis isolates with quantitative resistance phenotypes to 13 antibiotics. PLoS Biol. 20, e3001721 (2022).
Miotto, P. et al. Transcriptional regulation and drug resistance in Mycobacterium tuberculosis. Front. Cell Infect. Microbiol. 12, 990312 (2022).
Koehler, N. et al. Pretomanid-resistant tuberculosis. J. Infect. 86, 520–524 (2023).
Heyckendorf, J. et al. What is resistance? Impact of phenotypic versus molecular drug resistance testing on therapy for multi- and extensively drug-resistant tuberculosis. Antimicrob. Agents Chemother. 62, e01550-17 (2018).
Tornheim, J. A. et al. Increased moxifloxacin dosing among patients with multidrug-resistant tuberculosis with low-level resistance to moxifloxacin did not improve treatment outcomes in a tertiary care center in Mumbai, India. Open. Forum Infect. Dis. 9, ofab615 (2022).
WHO Global Tuberculosis Programme. Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. World Health Organization https://www.who.int/publications/i/item/9789240028173 (2021).
WHO Global Tuberculosis Programme. Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. 2nd edn. World Health Organization https://www.who.int/publications/i/item/9789240082410#:~:Text=The%20catalogue%20provides%20a%20reference,countries%20for%20the%2013%20medicines (2023).
Maslov, D. A., Shur, K. V., Vatlin, A. A. & Danilenko, V. N. MmpS5–MmpL5 transporters provide Mycobacterium smegmatis resistance to imidazo[1,2-b][1,2,4,5]tetrazines. Pathogens 9, 166 (2020).
Radhakrishnan, A. et al. Crystal structure of the transcriptional regulator Rv0678 of Mycobacterium tuberculosis. J. Biol. Chem. 289, 16526–16540 (2014).
Vatlin, A. A. et al. Transcriptomic profile of Mycobacterium smegmatis in response to an Imidazo[1,2-b][1,2,4,5]tetrazine reveals its possible impact on iron metabolism. Front. Microbiol. 12, 724042 (2021).
Asaad, M. et al. Methylation in Mycobacterium–host interaction and implications for novel control measures. Infect. Genet. Evol. 83, 104350 (2020).
Fatima, S. et al. Epigenetic code during mycobacterial infections: therapeutic implications for tuberculosis. FEBS J. 289, 4172–4191 (2022).
Peters, J. S. et al. Genetic diversity in Mycobacterium tuberculosis clinical isolates and resulting outcomes of tuberculosis infection and disease. Annu. Rev. Genet. 54, 511–537 (2020).
Chu, H., Hu, Y., Zhang, B., Sun, Z. & Zhu, B. DNA methyltransferase HsdM induce drug resistance on Mycobacterium tuberculosis via multiple effects. Antibiotics 10, 1544 (2021).
Hu, X. et al. The mycobacterial DNA methyltransferase HsdM decreases intrinsic isoniazid susceptibility. Antibiotics 10, 1323 (2021).
Li, H. C. et al. Genome-wide DNA methylation and transcriptome and proteome changes in Mycobacterium tuberculosis with para-aminosalicylic acid resistance. Chem. Biol. Drug Des. 95, 104–112 (2020).
Wu, Z. et al. mbtD and celA1 association with ethambutol resistance in Mycobacterium tuberculosis: a multiomics analysis. Front. Cell. Infect. Microbiol. 12, 959911 (2022).
Wong, S. Y. et al. Functional role of methylation of G518 of the 16S rRNA 530 loop by GidB in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 57, 6311–6318 (2013).
Manosuthi, W., Wiboonchutikul, S. & Sungkanuparph, S. Integrated therapy for HIV and tuberculosis. AIDS Res. Ther. 13, 22 (2016).
Svensson, E. M. et al. Model-based estimates of the effects of efavirenz on bedaquiline pharmacokinetics and suggested dose adjustments for patients coinfected with HIV and tuberculosis. Antimicrob. Agents Chemother. 57, 2780–2787 (2013).
Svensson, E. M., Dooley, K. E. & Karlsson, M. O. Impact of lopinavir–ritonavir or nevirapine on bedaquiline exposures and potential implications for patients with tuberculosis–HIV coinfection. Antimicrob. Agents Chemother. 58, 6406–6412 (2014).
Haas, D. W. et al. Pharmacogenetics of between-individual variability in plasma clearance of bedaquiline and clofazimine in South Africa. J. Infect. Dis. 226, 147–156 (2022).
Svensson, E. M. & Karlsson, M. O. Modelling of mycobacterial load reveals bedaquiline’s exposure–response relationship in patients with drug-resistant TB. J. Antimicrob. Chemother. 72, 3398–3405 (2017).
Tanneau, L., Karlsson, M. O. & Svensson, E. M. Understanding the drug exposure-response relationship of bedaquiline to predict efficacy for novel dosing regimens in the treatment of multidrug-resistant tuberculosis. Br. J. Clin. Pharmacol. 86, 913–922 (2020).
Tanneau, L. et al. Population pharmacokinetics of delamanid and its main metabolite DM-6705 in drug-resistant tuberculosis patients receiving delamanid alone or coadministered with bedaquiline. Clin. Pharmacokinet. 61, 1177–1185 (2022).
Guglielmetti, L. et al. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: a Tuberculosis Network European Trialsgroup (TBnet) study. Eur. Respir. J. 52, 1800537 (2018).
Shimokawa, Y., Sasahara, K., Yoda, N., Mizuno, K. & Umehara, K. Delamanid does not inhibit or induce cytochrome P450 enzymes in vitro. Biol. Pharm. Bull. 37, 1727–1735 (2014).
Tanneau, L. et al. Assessing prolongation of the corrected QT interval with bedaquiline and delamanid coadministration to predict the cardiac safety of simplified dosing regimens. Clin. Pharmacol. Ther. 112, 873–881 (2022).
Dooley, K. E. et al. Phase I safety, pharmacokinetics, and pharmacogenetics study of the antituberculosis drug PA-824 with concomitant lopinavir-ritonavir, efavirenz, or rifampin. Antimicrob. Agents Chemother. 58, 5245–5252 (2014).
TB Alliance. SimpliciTB results and heapatic safety of pretomanid regimens +/− pyrazinamide. TB Alliance https://www.croiconference.org/abstract/simplicitb-results-and-hepatic-safety-of-pretomanid-regimens-pyrazinamide/#:~:Text=Conclusions%3A,6%2D7%25%20of%20patients (2023).
Wasserman, S. et al. Linezolid pharmacokinetics in South African patients with drug-resistant tuberculosis and a high prevalence of HIV coinfection. Antimicrob. Agents Chemother. 63, e02164-18 (2019).
Imperial, M. Z., Nedelman, J. R., Conradie, F. & Savic, R. M. Proposed linezolid dosing strategies to minimize adverse events for treatment of extensively drug-resistant tuberculosis. Clin. Infect. Dis. 74, 1736–1747 (2022).
Wasserman, S., Meintjes, G. & Maartens, G. Linezolid in the treatment of drug-resistant tuberculosis: the challenge of its narrow therapeutic index. Expert Rev. Anti Infect. Ther. 14, 901–915 (2016).
Wilby, K. J. & Hussain, F. N. A review of clinical pharmacokinetic and pharmacodynamic relationships and clinical implications for drugs used to treat multi-drug resistant tuberculosis. Eur. J. Drug Metab. Pharmacokinet. 45, 305–313 (2020).
Cegielski, J. P. et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin. Infect. Dis. 59, 1049–1063 (2014).
Hoffmann, H. et al. Delamanid and bedaquiline resistance in Mycobacterium tuberculosis ancestral beijing genotype causing extensively drug-resistant tuberculosis in a tibetan refugee. Am. J. Respir. Crit. Care Med. 193, 337–340 (2016).
Ye, M. et al. Antibiotic heteroresistance in Mycobacterium tuberculosis isolates: a systematic review and meta-analysis. Ann. Clin. Microb. Antimicrob. 20, 73 (2021).
Ng, K. C. S. et al. How well do routine molecular diagnostics detect rifampin heteroresistance in Mycobacterium tuberculosis? J. Clin. Microbiol. 57, e00717–e00719 (2019).
Gagneux, S. Fitness cost of drug resistance in Mycobacterium tuberculosis. Clin. Microbiol. Infect. 15 (Suppl. 1), 66–68 (2009).
Casali, N. et al. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat. Genet. 46, 279–286 (2014).
Merker, M. et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat. Genet. 47, 242–249 (2015).
Zhao, Y. et al. National survey of drug-resistant tuberculosis in China. N. Engl. J. Med. 366, 2161–2170 (2012).
Gagneux, S. et al. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. 2, e61 (2006).
Dheda, K., Makambwa, E. & Esmail, A. The great masquerader: tuberculosis presenting as community-acquired pneumonia. Semin. Respir. Crit. Care Med. 41, 592–604 (2020).
Sossen, B. et al. The natural history of untreated pulmonary tuberculosis in adults: a systematic review and meta-analysis. Lancet Respir. Med. 11, 367–379 (2023).
WHO. Optimizing active case-finding for tuberculosis: implementation lessons from South-East Asia. World Health Organization https://www.who.int/publications/i/item/9789290228486 (2021).
Esmail, A. et al. Comparison of two diagnostic intervention packages for community-based active case finding for tuberculosis: an open-label randomized controlled trial. Nat. Med. 29, 1009–1016 (2023). Validation of an ACF model using a mobile van shows that portable PCR technology can be used to effectively diagnose DR-TB in the community.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04303104?term=NCT04303104&rank=1 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT01990274?term=NCT01990274&rank=1 (2015).
Gilpin, C., Korobitsyn, A. & Weyer, K. Current tools available for the diagnosis of drug-resistant tuberculosis. Ther. Adv. Infect. Dis. 3, 145–151 (2016).
Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. Report NO. 9789241514842 (WHO, 2018).
WHO. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. World Health Organization https://www.ncbi.nlm.nih.gov/books/NBK258608/ (2013).
Theron, G. et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 383, 424–435 (2014).
Köser, C. U. et al. Whole-genome sequencing for rapid susceptibility testing of M. tuberculosis. N. Engl. J. Med. 369, 290–292 (2013).
Walker, T. M. et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe 3, e265–e273 (2022).
Feuerriegel, S. et al. Rapid genomic first- and second-line drug resistance prediction from clinical Mycobacterium tuberculosis specimens using Deeplex-MycTB. Eur. Respir. J. 57, 2001796 (2021).
Kambli, P. et al. Targeted next generation sequencing directly from sputum for comprehensive genetic information on drug resistant Mycobacterium tuberculosis. Tuberculosis 127, 102051 (2021).
Wu, S. H., Xiao, Y. X., Hsiao, H. C. & Jou, R. Development and assessment of a novel whole-gene-based targeted next-generation sequencing assay for detecting the susceptibility of Mycobacterium tuberculosis to 14 drugs. Microbiol. Spectr. 10, e0260522 (2022).
Allix-Béguec, C. et al. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N. Engl. J. Med. 379, 1403–1415 (2018).
Walker, T. M. et al. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect. Dis. 15, 1193–1202 (2015).
Grobbel, H. P. et al. Design of multidrug-resistant tuberculosis treatment regimens based on DNA sequencing. Clin. Infect. Dis. 73, 1194–1202 (2021).
Manson, A. L. et al. Genomic analysis of globally diverse Mycobacterium tuberculosis strains provides insights into the emergence and spread of multidrug resistance. Nat. Genet. 49, 395–402 (2017).
O’Donnell, M. Isoniazid monoresistance: a precursor to multidrug-resistant tuberculosis. Ann. Am. Thorac. Soc. 15, 306–307 (2018).
Donald, P. R. et al. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur. J. Clin. Pharmacol. 63, 633–639 (2007).
Pasipanodya, J. G. & Gumbo, T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin. Infect. Dis. 57, 21–31 (2013).
Sarathy, J. P. et al. Extreme drug tolerance of Mycobacterium tuberculosis in caseum. Antimicrob. Agents Chemother. 62, e02266–e02317 (2018).
Rockwood, N. et al. Low frequency of acquired isoniazid and rifampicin resistance in rifampicin-susceptible pulmonary tuberculosis in a setting of high HIV-1 infection and tuberculosis coprevalence. J. Infect. Dis. 216, 632–640 (2017).
Vree, M. et al. Survival and relapse rate of tuberculosis patients who successfully completed treatment in Vietnam. Int. J. Tuberc. Lung Dis. 11, 392–397 (2007).
Fregonese, F. et al. Comparison of different treatments for isoniazid-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir. Med. 6, 265–275 (2018).
Ragonnet, R., Trauer, J. M., Denholm, J. T., Marais, B. J. & McBryde, E. S. High rates of multidrug-resistant and rifampicin-resistant tuberculosis among re-treatment cases: where do they come from. BMC Infect. Dis. 17, 36 (2017).
WHO. Tuberculosis preventive treatment: rapid communication. World Health Organization https://www.who.int/publications/i/item/9789240089723 (2024).
Polesky, A. et al. Rifampin preventive therapy for tuberculosis in Boston’s homeless. Am. J. Respir. Crit. Care Med. 154, 1473–1477 (1996).
Australian New Zealand Clinical Trials Registry. The V-QUIN MDR TRIAL: A Randomized Controlled Trial of Six Months of Daily Levofloxacin for the Prevention of Tuberculosis Among Household Contacts of Patients with Multi-Drug Resistant Tuberculosis. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369817 (2019).
ISRCTN registry. Tuberculosis Child Multidrug-Resistant Preventive Therapy: TB CHAMP trial. https://www.isrctn.com/ISRCTN92634082 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03568383?term=NCT03568383&rank=1 (2023).
Tait, D. R. et al. Final analysis of a trial of M72/AS01(E) vaccine to prevent tuberculosis. N. Engl. J. Med. 381, 2429–2439 (2019). Phase II(b) study showing that the M72 vaccine had a 50% efficacy in preventing the development of active TB. This will have implications for reducing both DS-TB and DR-TB burden.
Lazarchik, A., Nyaruhirira, A. U., Chiang, C. Y., Wares, F. & Horsburgh, C. R. Global availability of susceptibility testing for second-line anti-tuberculosis agents. Int. J. Tuberc. Lung Dis. 26, 524–528 (2022).
Nahid, P. et al. Treatment of drug-resistant tuberculosis. an official ATS/CDC/ERS/IDSA clinical practice guideline. Am. J. Respir. Crit. Care Med. 200, e93–e142 (2019).
NICE. Tuberculosis: NICE guideline 33. National Institute for Health and Care Excellence https://www.nice.org.uk/guidance/ng33 (2016).
Dorman, S. E. et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N. Engl. J. Med. 384, 1705–1718 (2021).
WHO. WHO operational handbook on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. World Health Organization https://www.who.int/publications/i/item/9789240065116 (2022).
Turkova, A. et al. Shorter treatment for nonsevere tuberculosis in African and Indian children. N. Engl. J. Med. 386, 911–922 (2022).
WHO. WHO consolidated guidelines on tuberculosis: Module 4: treatment: drug-susceptible tuberculosis treatment. World Health Organization https://www.who.int/publications/i/item/9789240048126 (2022).
Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis (WHO, 2008).
Lan, Z. et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir. Med. 8, 383–394 (2020).
Nunn, A. J. et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 380, 1201–1213 (2019). Randomized controlled trial showing that a shorter 9- to 11-month injection-based regimen had similar outcomes to the longer 18- to 20-month injection-based regimen. This established the foundational basis for treatment shortening.
Conradie, F. et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N. Engl. J. Med. 387, 810–823 (2022). Demonstrates that the BPaL regimen performed well in patients with pre-XDR-TB, XDR-TB and MDR-TB treatment failures.
Conradie, F. et al. Treatment of highly drug-resistant pulmonary tuberculosis. N. Engl. J. Med. 382, 893–902 (2020).
Esmail, A. et al. An all-oral 6-month regimen for multidrug-resistant tuberculosis: a multicenter, randomized controlled clinical trial (the NExT Study). Am. J. Respir. Crit. Care Med. 205, 1214–1227 (2022). RCT of 6-month all-oral regimen for MDR-TB demonstrating the ability of such a regimen to improve outcomes within the context of a controlled trial.
Ndjeka, N. et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study. Lancet Infect. Dis. 22, 1042–1051 (2022).
Nyang’wa, B. T. et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 387, 2331–2343 (2022). Demonstration that an all-oral 6-month regimen (BPaLM) produced equivalent outcomes, and with reduced adverse effects, to a longer injectable-containing control regimen.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02333799?term=NCT02333799&rank=1 (2020).
Gupta, A. et al. Lifesaving, cost-saving: innovative simplified regimens for drug-resistant tuberculosis. PLoS Glob. Public Health 2, e0001287 (2022).
Esmail, A., Sabur, N. F., Okpechi, I. & Dheda, K. Management of drug-resistant tuberculosis in special sub-populations including those with HIV co-infection, pregnancy, diabetes, organ-specific dysfunction, and in the critically ill. J. Thorac. Dis. 10, 3102–3118 (2018).
Pasipanodya, J. G. et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 208, 1464–1473 (2013).
Sekaggya-Wiltshire, C. et al. The utility of pharmacokinetic studies for the evaluation of exposure-response relationships for standard dose anti-tuberculosis drugs. Tuberculosis 108, 77–82 (2018).
Mota, L. et al. Therapeutic drug monitoring in anti-tuberculosis treatment: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 20, 819–826 (2016).
Boeree, M. J. et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 191, 1058–1065 (2015).
Mallick, J. S., Nair, P., Abbew, E. T., Van Deun, A. & Decroo, T. Acquired bedaquiline resistance during the treatment of drug-resistant tuberculosis: a systematic review. JAC Antimicrob. Resist. 4, dlac029 (2022).
Pai, H. et al. Bedaquiline safety, efficacy, utilization and emergence of resistance following treatment of multidrug-resistant tuberculosis patients in South Africa: a retrospective cohort analysis. BMC Infect. Dis. 22, 870 (2022).
Kamp, J. et al. Simple strategy to assess linezolid exposure in patients with multi-drug-resistant and extensively-drug-resistant tuberculosis. Int. J. Antimicrob. Agents 49, 688–694 (2017).
Rao, P. S. et al. Alternative methods for therapeutic drug monitoring and dose adjustment of tuberculosis treatment in clinical settings: a systematic review. Clin. Pharmacokinet. 62, 375–398 (2023).
Lange, C., Kohler, N. & Gunther, G. Regimens for drug-resistant tuberculosis. N. Engl. J. Med. 388, 190 (2023).
Dheda, K., Gumbo, T., Lange, C., Horsburgh, C. R. Jr. & Furin, J. Pan-tuberculosis regimens: an argument against. Lancet Respir. Med. 6, 240–242 (2018).
Lange, C. et al. Perspective for precision medicine for tuberculosis. Front. Immunol. 11, 566608 (2020).
Heyckendorf, J. et al. Prediction of anti-tuberculosis treatment duration based on a 22-gene transcriptomic model. Eur. Respir. J. 58, 2003492 (2021).
DiNardo, A. R. et al. Gene expression signatures identify biologically and clinically distinct tuberculosis endotypes. Eur. Respir. J. 60, 2102263 (2022).
International Standards for Tubcerculosis Care. 3rd edn (TB CARE I, 2014).
Chesov, D. et al. Failing treatment of multidrug-resistant tuberculosis: a matter of definition. Int. J. Tuberc. Lung Dis. 23, 522–524 (2019).
Butov, D. et al. Multidrug-resistant tuberculosis in the Kharkiv region, Ukraine. Int. J. Tuberc. Lung Dis. 24, 485–491 (2020).
Chesov, D. et al. Impact of bedaquiline on treatment outcomes of multidrug-resistant tuberculosis in a high-burden country. Eur. Respir. J. 57, 2002544 (2021).
Heyckendorf, J. et al. Relapse-free cure from multidrug-resistant tuberculosis in Germany. Eur. Respir. J. 51, 1702122 (2018).
Maier, C. et al. Long-term treatment outcomes in patients with multidrug-resistant tuberculosis. Clin. Microbiol. Infect. 29, 751–757 (2023).
Otto-Knapp, R. et al. Long-term multidrug- and rifampicin-resistant tuberculosis treatment outcome by new WHO definitions in Germany. Eur. Respir. J. 60, 2200765 (2022).
Fox, G. J. et al. Surgery as an adjunctive treatment for multidrug-resistant tuberculosis: an individual patient data metaanalysis. Clin. Infect. Dis. 62, 887–895 (2016).
Calligaro, G. L. et al. Outcomes of patients undergoing lung resection for drug-resistant TB and the prognostic significance of pre-operative positron emission tomography/computed tomography (PET/CT) in predicting treatment failure. EClinicalMedicine 55, 101728 (2023).
Myburgh, H. et al. A scoping review of patient-centred tuberculosis care interventions: gaps and opportunities. PLoS Glob. Public Health 3, e0001357 (2023).
Ethics Guidance for the Implementation of the End TB Strategy (WHO, 2017).
Connor, S. R. Palliative care for tuberculosis. J. Pain. Symptom Manag. 55, S178–S180 (2018).
WHO. Palliative care fact sheet. World Health Organization https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/palliative-care/palliative-care-essential-facts.pdf?sfvrsn=c5fed6dc_1 (2020).
ECDC. European Union standards for tuberculosis care - 2017 update. European Centre for Disease Prevention and Control https://www.ecdc.europa.eu/en/publications-data/european-union-standards-tuberculosis-care-2017-update (2018).
Migliori, G. B., Sotgiu, G., Rosales-Klintz, S. & van der Werf, M. J. European Union standard for tuberculosis care on treatment of multidrug-resistant tuberculosis following new World Health Organization recommendations. Eur. Respir. J. 52, 1801617 (2018).
Agins, B. D. et al. Improving the cascade of global tuberculosis care: moving from the “what” to the “how” of quality improvement. Lancet Infect. Dis. 19, e437–e443 (2019).
Cobelens, F., van Kampen, S., Ochodo, E., Atun, R. & Lienhardt, C. Research on implementation of interventions in tuberculosis control in low- and middle-income countries: a systematic review. PLoS Med. 9, e1001358 (2012).
Batalden, M. et al. Coproduction of healthcare service. BMJ Qual. Saf. 25, 509–517 (2016).
Pai, M., Yadav, P. & Anupindi, R. Tuberculosis control needs a complete and patient-centric solution. Lancet Glob. Health 2, e189–e190 (2014).
Grimsrud, A. et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J. Int. AIDS Soc. 19, 21484 (2016).
Pai, M., Schumacher, S. G. & Abimbola, S. Surrogate endpoints in global health research: still searching for killer apps and silver bullets? BMJ Glob. Health 3, e000755 (2018).
Bouchet, B., Francisco, M. & Ovretveit, J. The Zambia quality assurance program: successes and challenges. Int. J. Qual. Health Care 14 (Suppl. 1), 89–95 (2002).
Fan, H. et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann. Transl. Med. 9, 390 (2021).
Taylor, J. et al. Residual respiratory disability after successful treatment of pulmonary tuberculosis: a systematic review and meta-analysis. EClinicalMedicine 59, 101979 (2023). Demonstrates that there was significant pulmonary disability after successful treatment of pulmonary tuberculosis highlighting this condition as a non-communicable disease of global epidemic proportions.
Byrne, A. L. et al. Chronic airflow obstruction after successful treatment of multidrug-resistant tuberculosis. ERJ Open Res. 3, 00026-2017 (2017).
de Vallière, S. & Barker, R. D. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 8, 767–771 (2004).
Ivanova, O., Hoffmann, V. S., Lange, C., Hoelscher, M. & Rachow, A. Post-tuberculosis lung impairment: systematic review and meta-analysis of spirometry data from 14 621 people. Eur. Respir. Rev. 32, 220221 (2023).
Sharma, N. et al. A comparison of patient treatment pathways among multidrug-resistant and drug-sensitive TB cases in Delhi, India: a cross-sectional study. Indian J. Tuberculosis 67, 502–508 (2020).
Lee, G. et al. Impact of mental disorders on active TB treatment outcomes: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 24, 1279–1284 (2020).
Ragan, E. J. et al. The impact of alcohol use on tuberculosis treatment outcomes: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 24, 73–82 (2020).
Pietersen, E. et al. Variation in missed doses and reasons for discontinuation of anti-tuberculosis drugs during hospital treatment for drug-resistant tuberculosis in South Africa. PLoS ONE 18, e0281097 (2023).
Ashley-Norman, P. Balancing tuberculosis therapy and substance use. Lancet Infect. Dis. 23, 542 (2023).
UN. Resolution adopted by the General Assembly on 25 September 2015. 70/1. Transforming our world: the 2030 Agenda for Sustainable Development. United Nations https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (2015).
WHO. The end TB strategy (WHO, 2015).
Dheda, K. et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir. Med. 5, 269–281 (2017).
Dheda, K. & Migliori, G. B. The global rise of extensively drug-resistant tuberculosis: is the time to bring back sanatoria now overdue. Lancet 379, 773–775 (2012).
Behr, M. A., Edelstein, P. H. & Ramakrishnan, L. Is Mycobacterium tuberculosis infection life long? BMJ 367, l5770 (2019).
Menzies, D., Gardiner, G., Farhat, M., Greenaway, C. & Pai, M. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. Int. J. Tuberc. Lung Dis. 12, 498–505 (2008).
Curry International Tuberculosis Center & State of California Department of Public Health Tuberculosis Control Branch. Drug-resistant Tuberculosis: A Survival Guide for Clinicians. 3rd edn (2016).
Diacon, A. H. et al. Phase II dose-ranging trial of the early bactericidal activity of PA-824. Antimicrob. Agents Chemother. 56, 3027–3031 (2012).
Lange, C. et al. Management of patients with multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 23, 645–662 (2019).
Li, M. et al. Phase 1 study of the effects of the tuberculosis treatment pretomanid, alone and in combination with moxifloxacin, on the QTc interval in healthy volunteers. Clin. Pharmacol. Drug Dev. 10, 634–646 (2021).
Liu, Y. et al. Safety and pharmacokinetic profile of pretomanid in healthy Chinese adults: results of a phase I single dose escalation study. Pulm. Pharmacol. Ther. 73–74, 102132 (2022).
WHO. Use of targeted next-generation sequencing to detect drug-resistant tuberculosis: rapid communication, July 2023. World Health Organization https://www.who.int/publications/i/item/9789240076372 (2023).
Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, 27–29 October 2020. Report No. 9789240018662 (electronic version) 9789240018679 (print version) (WHO, 2021).
Campbell, J. R. et al. Low body mass index at treatment initiation and rifampicin-resistant tuberculosis treatment outcomes: an individual participant data meta-analysis. Clin. Infect. Dis. 75, 2201–2210 (2022).
Golightly, L. K. et al. Renal Pharmacotherapy. Dosage Adjustment of Medications Eliminated by the Kidneys (Springer, 2013).
Huynh, D. & Nguyen, N. Q. in Diet and Nutrition in Critical Care (eds Rajendram, R., Preedy, V. R. & Patel V. B.) (Springer, 2015).
Maugans, C. et al. Best practices for the care of pregnant people living with TB. Int. J. Tuberc. Lung Dis. 27, 357–366 (2023).
Winter, M. A., Guhr, K. N. & Berg, G. M. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft–Gault equation. Pharmacotherapy 32, 604–612 (2012).
WHO. Consensus meeting report: development of a target product profile (TPP) and a framework for evaluation for a test for predicting progression from tuberculosis infection to active disease. World Health Organization https://www.who.int/publications/i/item/WHO-HTM-TB-2017.18 (2017).
WHO. Definitions. World Health Organization https://tbksp.org/en/node/1882 (2023).
Musonda, H. K., Rose, P. C., Switala, J. & Schaaf, H. S. Paediatric admissions to a TB hospital: reasons for admission, clinical profile and outcomes. Int. J. Tuberc. Lung Dis. 26, 217–223 (2022).
Global Fund. List of tuberculosis pharmaceutical products classified according to the global fund quality assurance policy. The Global Fund https://www.theglobalfund.org/media/4757/psm_productstb_list_en.pdf (2023).
WHO. Use of bedaquiline in children and adolescents with multidrug- and rifampicin-resistant tuberculosis: information note. World Health Organization https://www.who.int/publications/i/item/9789240074286 (2023).
WHO. Use of delamanid in children and adolescents with multidrug- and rifampicin-resistant tuberculosis: information note. World Health Organization https://www.who.int/publications/i/item/9789240074309 (2023).
WHO. WHO consolidated guidelines on tuberculosis: module 5: management of tuberculosis in children and adolescents. World Health Organization https://www.who.int/publications/i/item/9789240046764 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04062201?term=NCT04062201&rank=1 (2022).
Patankar, S. et al. Making the case for all-oral, shorter regimens for children with drug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 208, 130–131 (2023).
The Sentinel Project for Pediatric Drug-Resistant Tuberculosis. Management of Drug-Resistant Tuberculosis In Children: A Field Guide (Boston, 2021).
endTB Consortium. endTB clinical trial results. endTB https://endtb.org/endtb-clinical-trial-results (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02754765 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02589782?term=NCT02589782&rank=1 (2021).
Mvelase, N. R. & Mlisana, K. P. Xpert MTB/XDR for rapid detection of drug-resistant tuberculosis beyond rifampicin. Lancet Infect. Dis. 22, 156–157 (2022).
Chesov, E. et al. Emergence of bedaquiline resistance in a high tuberculosis burden country. Eur. Respir. J. 59, 2100621 (2022).
Ghodousi, A. et al. Acquisition of cross-resistance to bedaquiline and clofazimine following treatment for tuberculosis in Pakistan. Antimicrob. Agents Chemother. 63, e00915-19 (2019).
Lange, C., Vasiliu, A. & Mandalakas, A. M. Emerging bedaquiline-resistant tuberculosis. Lancet Microbe 4, e964–e965 (2023).
Holt, E. Phase 2 trial of a novel tuberculosis drug launched. Lancet Microbe https://doi.org/10.1016/S2666-5247(23)00401-9 (2024).
Mok, J. et al. 9 months of delamanid, linezolid, levofloxacin, and pyrazinamide versus conventional therapy for treatment of fluoroquinolone-sensitive multidrug-resistant tuberculosis (MDR-END): a multicentre, randomised, open-label phase 2/3 non-inferiority trial in South Korea. Lancet 400, 1522–1530 (2022).
Motta, I. et al. Recent advances in the treatment of tuberculosis. Clin. Microbiol. Infect. https://doi.org/10.1016/j.cmi.2023.07.013 (2023).
Goodall, R. L. et al. Evaluation of two short standardised regimens for the treatment of rifampicin-resistant tuberculosis (STREAM stage 2): an open-label, multicentre, randomised, non-inferiority trial. Lancet 400, 1858–1868 (2022).
Padmapriyadarsini, C. et al. Bedaquiline, delamanid, linezolid, and clofazimine for treatment of pre-extensively drug-resistant tuberculosis. Clin. Infect. Dis. 76, e938–e946 (2022).
Boeree, M. J. et al. UNITE4TB: a new consortium for clinical drug and regimen development for TB. Int. J. Tuberc. Lung Dis. 25, 886–889 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT05941052?term=NCT05941052&rank=1 (2023).
PAN-TB collaboration. Project to accelerate new treatments for tuberculosis (PAN-TB). PAN-TB https://www.pan-tb.org/newsroom/ (2021).
Acknowledgements
The authors acknowledge the patients who have contributed the patient journeys. K.D. is supported by the UK MRC, Wellcome Trust, European Union Horizon 2020 (EDCTP) and the South African MRC. C.L. is supported by the German Center of Infection Research (DZIF).
Author information
Authors and Affiliations
Contributions
Introduction (K.D. and C.L.); Epidemiology (K.D., M.M. and T.P.); Mechanisms/pathophysiology (K.D., K.E.D., D.M.C., A.R. and T.P.); Diagnosis, screening and prevention (K.D., D.M.C., S.V.O., F.M., A.R. and T.P.); Management (K.D., Z.U., K.E.D., K.-C.C., C.R.H., C.L. and A.R.); Quality of life (K.D., M.M. and A.R.); Outlook (K.D., Z.U., K.-C.C., C.R.H., C.L. and F.M.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks S. Hoffner, H. S. Schaaf, J.-J. Yim and M. Viveiros for their contribution to the peer review of this work.
Additional information
Informed consent
The authors affirm that human research participants provided informed consent for publication of their experiences and accompanying images in Supplementary Box 1.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dheda, K., Mirzayev, F., Cirillo, D.M. et al. Multidrug-resistant tuberculosis. Nat Rev Dis Primers 10, 22 (2024). https://doi.org/10.1038/s41572-024-00504-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-024-00504-2