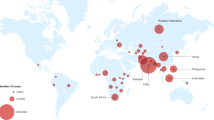
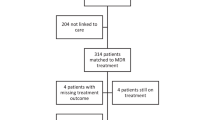
Abstract
Drug-resistant tuberculosis (TB) is estimated to cause 13% of all antimicrobial resistance-attributable deaths worldwide and is driven by both ongoing resistance acquisition and person-to-person transmission. Poor outcomes are exacerbated by late diagnosis and inadequate access to effective treatment. Advances in rapid molecular testing have recently improved the diagnosis of TB and drug resistance. Next-generation sequencing of Mycobacterium tuberculosis has increased our understanding of genetic resistance mechanisms and can now detect mutations associated with resistance phenotypes. All-oral, shorter drug regimens that can achieve high cure rates of drug-resistant TB within 6–9 months are now available and recommended but have yet to be scaled to global clinical use. Promising regimens for the prevention of drug-resistant TB among high-risk contacts are supported by early clinical trial data but final results are pending. A person-centred approach is crucial in managing drug-resistant TB to reduce the risk of poor treatment outcomes, side effects, stigma and mental health burden associated with the diagnosis. In this Review, we describe current surveillance of drug-resistant TB and the causes, risk factors and determinants of drug resistance as well as the stigma and mental health considerations associated with it. We discuss recent advances in diagnostics and drug-susceptibility testing and outline the progress in developing better treatment and preventive therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Peto, H. M., Pratt, R. H., Harrington, T. A., LoBue, P. A. & Armstrong, L. R. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin. Infect. Dis. 49, 1350–1357 (2009).
WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment — Drug-resistant Tuberculosis Treatment, 2022 Update. World Health Organization https://www.who.int/publications/i/item/9789240063129 (2022).
WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment — Drug-susceptible Tuberculosis Treatment. World Health Organization https://www.who.int/publications/i/item/9789240048126 (2022).
WHO Consolidated Guidelines on Tuberculosis. Module 3: Diagnosis — Rapid Diagnostics For Tuberculosis Detection, 2021 Update. World Health Organization https://www.who.int/publications/i/item/9789240029415 (2021).
Cohen, K. A., Manson, A. L., Desjardins, C. A., Abeel, T. & Earl, A. M. Deciphering drug resistance in Mycobacterium tuberculosis using whole-genome sequencing: progress, promise, and challenges. Genome Med. 11, 45 (2019).
Cohen, K. A. et al. Extensive global movement of multidrug-resistant Mycobacterium tuberculosis strains revealed by whole-genome analysis. Thorax 74, 882–889 (2019).
Short-course chemotherapy in pulmonary tuberculosis: a controlled trial by the British Thoracic and Tuberculosis Association. Lancet 305, 119–124 (1975).
Zhang, Y. The magic bullets and tuberculosis drug targets. Annu. Rev. Pharmacol. Toxicol. 45, 529–564 (2005).
Global Tuberculosis Report 2022. World Health Organization https://www.who.int/publications/i/item/9789240061729 (2022).
Global Tuberculosis Report 2021. World Health Organization https://www.who.int/publications/i/item/9789240037021 (2021).
Boehme, C. C. et al. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363, 1005–1015 (2010).
The Use of Molecular Line Probe Assay for the Detection of Resistance to Isoniazid and Rifampicin: Policy Update. World Health Organization https://www.who.int/publications/i/item/9789241511261 (2016).
Dixit, A. et al. Estimation of country-specific tuberculosis antibiograms using genomic data. Preprint at medRxiv https://doi.org/10.1101/2021.09.23.21263991 (2021).
O’Connor, C. & Brady, M. F. Isoniazid. StatPearls [Internet] https://pubmed.ncbi.nlm.nih.gov/32491549/ (updated 8 April 2022).
Yee, D. et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am. J. Respir. Crit. Care Med. 167, 1472–1477 (2003).
Murray, J. F., Schraufnagel, D. E. & Hopewell, P. C. Treatment of tuberculosis: a historical perspective. Ann. Am. Thorac. Soc. 12, 1749–1759 (2015).
Jacobson, K. R. et al. Treatment outcomes of isoniazid-resistant tuberculosis patients, Western Cape Province, South Africa. Clin. Infect. Dis. 53, 369–372 (2011).
Ahmad, N., Ahuja, S. & Akkerman, O. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 392, 821–834 (2018).
Stagg, H. R. et al. Fluoroquinolones and isoniazid-resistant tuberculosis: implications for the 2018 WHO guidance. Eur. Respir. J. 54, 1900982 (2019).
WHO Treatment Guidelines for Isoniazid-resistant Tuberculosis: Supplement to the WHO Treatment Guidelines for Drug-resistant Tuberculosis. World Health Organization https://www.who.int/publications/i/item/9789241550079 (2018).
Meeting Report of the WHO Expert Consultation on the Definition of extensively Drug-Resistant Tuberculosis, 27-29 October 2020. World Health Organization https://www.who.int/publications/i/item/9789240018662 (2021).
Global Tuberculosis Report 2023. World Health Organization https://www.who.int/publications/i/item/9789240083851 (2023).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Knight, G. M., McQuaid, C. F., Dodd, P. J. & Houben, R. Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect. Dis. 19, 903–912 (2019).
WHO Global Task Force on TB Impact Measurement: Report of a Subgroup Meeting on Methods Used by WHO to Estimate TB Disease Burden, 11-12 May 2022, Geneva, Switzerland. World Health Organization https://www.who.int/publications/i/item/9789240057647 (2022).
Global Tuberculosis Report 2015. World Health Organization https://www.who.int/publications/i/item/9789241565059 (2015).
Global Tuberculosis Report 2020. World Health Organization https://www.who.int/publications/i/item/9789240013131 (2020).
Villegas, L. et al. Prevalence, risk factors, and treatment outcomes of isoniazid- and rifampicin-mono-resistant pulmonary tuberculosis in Lima, Peru. PLoS ONE 11, e0152933 (2016).
Sharling, L., Marks, S. M., Goodman, M., Chorba, T. & Mase, S. Rifampin-resistant tuberculosis in the United States, 1998-2014. Clin. Infect. Dis. 70, 1596–1605 (2019).
Ismail, N. A. et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect. Dis. 18, 779–787 (2018).
Dean, A. S. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: a multicountry analysis of cross-sectional data. PLoS Med. 17, e1003008 (2020).
Subbaraman, R., Jhaveri, T. & Nathavitharana, R. R. Closing gaps in the tuberculosis care cascade: an action-oriented research agenda. J. Clin. Tuberc. Mycobact. Dis. 19, 100144 (2020).
Subbaraman, R. et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 16, e1002754 (2019).
Naidoo, P. et al. The South African tuberculosis care cascade: estimated losses and methodological challenges. J. Infect. Dis. 216, S702–S713 (2017).
Subbaraman, R. et al. The tuberculosis cascade of care in India’s public sector: a systematic review and meta-analysis. PLoS Med. 13, e1002149 (2016).
Migliori, G. B. et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur. Respir. J. 58, 2101786 (2021).
Daniels, B. et al. Use of standardised patients to assess quality of healthcare in Nairobi, Kenya: a pilot, cross-sectional study with international comparisons. BMJ Glob. Health 2, e000333 (2017).
Daniels, B., Kwan, A. & Pai, M. Lessons on the quality of tuberculosis diagnosis from standardized patients in China, India, Kenya, and South. Afr. J. Clin. Tuberc. Mycobact. Dis. 16, 100109 (2019).
Boffa, J. et al. Quality of care for tuberculosis and HIV in the private health sector: a cross-sectional, standardised patient study in South Africa. BMJ Glob. Health 6, e005250 (2021).
Kwan, A. et al. Variations in the quality of tuberculosis care in urban India: a cross-sectional, standardized patient study in two cities. PLoS Med. 15, e1002653 (2018).
Daniels, B. et al. Tuberculosis diagnosis and management in the public versus private sector: a standardised patients study in Mumbai, India. BMJ Glob. Health 7, 009657 (2022).
Demers, A. M. et al. Drug susceptibility patterns of Mycobacterium tuberculosis from adults with multidrug-resistant tuberculosis and implications for a household contact preventive therapy trial. BMC Infect. Dis. 21, 205 (2021).
Step up for TB 2020 report: Tuberculosis Policies in 37 Countries. Médecins Sans Frontières & Stop TB Partnership https://msfaccess.org/step-tb-tb-policies-37-countries-4th-ed (2020).
Omar, S. V., Ismail, F., Ndjeka, N., Kaniga, K. & Ismail, N. A. Bedaquiline-resistant tuberculosis associated with Rv0678 mutations. N. Engl. J. Med. 386, 93–94 (2022).
Ismail, N. A. et al. Assessment of epidemiological and genetic characteristics and clinical outcomes of resistance to bedaquiline in patients treated for rifampicin-resistant tuberculosis: a cross-sectional and longitudinal study. Lancet Infect. Dis. 22, 496–506 (2022).
Azimi, T. et al. Linezolid resistance in multidrug-resistant Mycobacterium tuberculosis: a systematic review and meta-analysis. Front. Pharmacol. 13, 955050 (2022).
Chesov, E. et al. Emergence of bedaquiline resistance in a high tuberculosis burden country. Eur. Respir. J. 59, 2100621 (2022).
Mallick, J. S., Nair, P., Abbew, E. T., Van Deun, A. & Decroo, T. Acquired bedaquiline resistance during the treatment of drug-resistant tuberculosis: a systematic review. JAC Antimicrob. Resist. 4, dlac029 (2022).
Jenkins, H. E. & Yuen, C. M. The burden of multidrug-resistant tuberculosis in children. Int. J. Tuberc. Lung Dis. 22, 3–6 (2018).
WHO Consolidated Guidelines on Tuberculosis, Module 5: Management of Tuberculosis in children and Adolescents. World Health Organization https://www.who.int/publications/i/item/9789240046764 (2022).
Dodd, P. J., Mafirakureva, N., Seddon, J. A. & McQuaid, C. F. The global impact of household contact management for children on multidrug-resistant and rifampicin-resistant tuberculosis cases, deaths, and health-system costs in 2019: a modelling study. Lancet Glob. Health 10, 1034–1044 (2022).
Dookie, N., Rambaran, S., Padayatchi, N., Mahomed, S. & Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: a review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 73, 1138–1151 (2018).
Casali, N. et al. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat. Genet. 46, 279–286 (2014).
Cohen, K. A. et al. Evolution of extensively drug-resistant tuberculosis over four decades: whole genome sequencing and dating analysis of Mycobacterium tuberculosis isolates from KwaZulu-Natal. PLoS Med. 12, e1001880 (2015).
Jiang, Q. et al. The evolution and transmission dynamics of multidrug-resistant tuberculosis in an isolated high-plateau population of Tibet, China. Microbiol. Spectr. 11, e03991-22 (2023).
Ektefaie, Y., Dixit, A., Freschi, L. & Farhat, M. R. Globally diverse Mycobacterium tuberculosis resistance acquisition: a retrospective geographical and temporal analysis of whole genome sequences. Lancet Microbe 2, e96–e104 (2021).
Auld, S. C. et al. Extensively drug-resistant tuberculosis in South Africa: genomic evidence supporting transmission in communities. Eur. Respir. J. 52, 1800246 (2018).
Kendall, E. A., Fofana, M. O. & Dowdy, D. W. Burden of transmitted multidrug resistance in epidemics of tuberculosis: a transmission modelling analysis. Lancet Respir. Med. 3, 963–972 (2015).
Yang, C. et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect. Dis. 17, 275–284 (2017).
Becerra, M. C. et al. Transmissibility and potential for disease progression of drug resistant Mycobacterium tuberculosis: prospective cohort study. BMJ 367, l5894 (2019).
Atre, S. R. et al. Tuberculosis pathways to care and transmission of multidrug resistance in India. Am. J. Respir. Crit. Care Med. 205, 233–241 (2022).
El Halabi, J. et al. Measuring health-care delays among privately insured patients with tuberculosis in the USA: an observational cohort study. Lancet Infect. Dis. 21, 1175–1183 (2021).
Odone, A. et al. Acquired and transmitted multidrug resistant tuberculosis: the role of social determinants. PLoS ONE 11, e0146642 (2016).
Bayer, R. & Wilkinson, D. Directly observed therapy for tuberculosis: history of an idea. Lancet 345, 1545–1548 (1995).
Pasipanodya, J. G., Srivastava, S. & Gumbo, T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin. Infect. Dis. 55, 169–177 (2012).
Srivastava, S., Pasipanodya, J. G., Meek, C., Leff, R. & Gumbo, T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 204, 1951–1959 (2011).
McKay, B., Castellanos, M., Ebell, M., Whalen, C. C. & Handel, A. An attempt to reproduce a previous meta-analysis and a new analysis regarding the impact of directly observed therapy on tuberculosis treatment outcomes. PLoS ONE 14, e0217219 (2019).
Manson, A. L. et al. Genomic analysis of globally diverse Mycobacterium tuberculosis strains provides insights into the emergence and spread of multidrug resistance. Nat. Genet. 49, 395–402 (2017).
Dreyer, V. et al. High fluoroquinolone resistance proportions among multidrug-resistant tuberculosis driven by dominant L2 Mycobacterium tuberculosis clones in the Mumbai Metropolitan Region. Genome Med. 14, 95 (2022).
Cox, H. et al. Potential contribution of HIV during first-line tuberculosis treatment to subsequent rifampicin-monoresistant tuberculosis and acquired tuberculosis drug resistance in South Africa: a retrospective molecular epidemiology study. Lancet Microbe 2, e584–e593 (2021).
Wang, Z. et al. Epidemiological characteristics and risk factors of multidrug-resistant tuberculosis in Luoyang, China. Front. Public Health 11, 1117101 (2023).
Hang, N. T. L. et al. Primary drug-resistant tuberculosis in Hanoi, Viet Nam: present status and risk factors. PLoS ONE 8, e71867 (2013).
Vashakidze, L. et al. Prevalence and risk factors for drug resistance among hospitalized tuberculosis patients in Georgia. Int. J. Tuberc. Lung Dis. 13, 1148–1153 (2009).
Andrews, J. R. et al. Predictors of multidrug-and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS ONE 5, e15735 (2010).
Mesfin, E. A. et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS ONE 13, e0197737 (2018).
Mbuh, T. P. et al. Predictors of drug-resistant tuberculosis among high-risk population diagnosed under national program conditions in the Littoral region, Cameroon. BioMed. Res. Int. 2021, 8817442 (2021).
Skrahina, A. et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull. World Health Organ. 91, 36–45 (2013).
Urrego, J. et al. The impact of ventilation and early diagnosis on tuberculosis transmission in Brazilian prisons. Am. J. Trop. Med. Hyg. 93, 739–746 (2015).
Kerubo, G., Amukoye, E., Niemann, S. & Kariuki, S. Drug susceptibility profiles of pulmonary Mycobacterium tuberculosis isolates from patients in informal urban settlements in Nairobi, Kenya. BMC Infect. Dis. 16, 583 (2016).
Oliveira, O. et al. Using Bayesian spatial models to map and to identify geographical hotspots of multidrug-resistant tuberculosis in Portugal between 2000 and 2016. Sci. Rep. 10, 16646 (2020).
Jenkins, H. E. et al. Assessing spatial heterogeneity of multidrug-resistant tuberculosis in a high-burden country. Eur. Respir. J. 42, 1291–1301 (2013).
Alene, K. A., Viney, K., McBryde, E. S. & Clements, A. C. A. Spatial patterns of multidrug resistant tuberculosis and relationships to socio-economic, demographic and household factors in northwest Ethiopia. PLoS ONE 12, e0171800 (2017).
Paleckyte, A., Dissanayake, O., Mpagama, S., Lipman, M. C. & McHugh, T. D. Reducing the risk of tuberculosis transmission for HCWs in high incidence settings. Antimicrob. Resist. Infect. Control 10, 106 (2021).
Escombe, A. R. et al. Tuberculosis transmission risk and infection control in a hospital emergency department in Lima, Peru. Int. J. Tuberc. Lung Dis. 14, 1120–1126 (2010).
Telisinghe, L. et al. High tuberculosis prevalence in a South African prison: the need for routine tuberculosis screening. PLoS ONE 9, e87262 (2014).
Dheda, K. et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 5, 291–360 (2017).
Houben, R. M. G. J. & Glynn, J. R. A systematic review and meta-analysis of molecular epidemiological studies of tuberculosis: development of a new tool to aid interpretation. Trop. Med. Int. Health 14, 892–909 (2009).
Chen, S. et al. Risk factors for multidrug resistance among previously treated patients with tuberculosis in eastern China: a case-control study. Int. J. Infect. Dis. 17, e1116-20 (2013).
Pradipta, I. S., Forsman, L. D., Bruchfeld, J., Hak, E. & Alffenaar, J. W. Risk factors of multidrug-resistant tuberculosis: a global systematic review and meta-analysis. J. Infect. 77, 469–478 (2018).
Lomtadze, N. et al. Prevalence and risk factors for multidrug-resistant tuberculosis in the Republic of Georgia: a population-based study. Int. J. Tuberc. Lung Dis. 13, 68–73 (2009).
Lee, E. G. et al. Age-stratified anti-tuberculosis drug resistance profiles in South Korea: a multicenter retrospective study. BMC Infect. Dis. 20, 446 (2020).
Behr, M. A., Edelstein, P. H. & Ramakrishnan, L. Revisiting the timetable of tuberculosis. BMJ 362, k2738 (2018).
Oladimeji, O. et al. Gender and drug-resistant tuberculosis in Nigeria. Trop. Med. Infect. Dis. 8, 104 (2023).
McQuaid, C. F., Horton, K. C., Dean, A. S., Knight, G. M. & White, R. G. The risk of multidrug-or rifampicin-resistance in males versus females with tuberculosis. Eur. Respir. J. 56, 2000626 (2020).
O’Donnell, M. R. et al. Extensively drug-resistant tuberculosis in women, KwaZulu-Natal, South Africa. Emerg. Infect. Dis. 17, 1942–1945 (2011).
Gandhi, N. R. et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368, 1575–1580 (2006).
Kolla, B. P., Oesterle, T., Gold, M., Southwick, F. & Rummans, T. Infectious diseases occurring in the context of substance use disorders: a concise review. J. Neurol. Sci. 411, 116719 (2020).
Soboka, M. et al. Substance use disorders and adherence to antituberculosis medications in Southwest Ethiopia: a prospective cohort study. BMJ Open 11, e043050 (2021).
Mekonnen, H. S. & Azagew, A. W. Non-adherence to anti-tuberculosis treatment, reasons and associated factors among TB patients attending at Gondar town health centers, Northwest Ethiopia. BMC Res. Notes 11, 691 (2018).
Chaves Torres, N. M., Quijano Rodríguez, J. J., Porras Andrade, P. S., Arriaga, M. B. & Netto, E. M. Factors predictive of the success of tuberculosis treatment: a systematic review with meta-analysis. PLoS ONE 14, e0226507 (2019).
Dixit, A. et al. Modern lineages of Mycobacterium tuberculosis were recently introduced in Western India and demonstrate increased transmissibility. Preprint at medRxiv https://doi.org/10.1101/2022.01.04.22268645 (2022).
Casali, N. et al. Microevolution of extensively drug-resistant tuberculosis in Russia. Genome Res. 22, 735–745 (2012).
Gygli, S. M. et al. Publisher Correction: prisons as ecological drivers of fitness-compensated multidrug-resistant Mycobacterium tuberculosis. Nat. Med. 27, 1308–1308 (2021).
Loiseau, C. et al. The relative transmission fitness of multidrug-resistant Mycobacterium tuberculosis in a drug resistance hotspot. Nat. Commun. 14, 1988 (2023).
Gygli, S. M., Borrell, S., Trauner, A. & Gagneux, S. Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives. FEMS Microbiol. Rev. 41, 354–373 (2017).
Li, S. et al. CRISPRi chemical genetics and comparative genomics identify genes mediating drug potency in Mycobacterium tuberculosis. Nat. Microbiol. 7, 766–779 (2022).
Nguyen, L. & Pieters, J. Mycobacterial subversion of chemotherapeutic reagents and host defense tactics: challenges in tuberculosis drug development. Annu. Rev. Pharmacol. Toxicol. 49, 427–453 (2009).
Morris, R. P. et al. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 102, 12200–12205 (2005).
Mailaender, C. et al. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 150, 853–864 (2004).
Rodriguez-Rivera, F. P., Zhou, X., Theriot, J. A. & Bertozzi, C. R. Visualization of mycobacterial membrane dynamics in live cells. J. Am. Chem. Soc. 139, 3488–3495 (2017).
Madsen, C. T. et al. Methyltransferase Erm(37) slips on rRNA to confer atypical resistance in Mycobacterium tuberculosis. J. Biol. Chem. 280, 38942–38947 (2005).
Wang, F., Cassidy, C. & Sacchettini, J. C. Crystal structure and activity studies of the Mycobacterium tuberculosis beta-lactamase reveal its critical role in resistance to β-lactam antibiotics. Antimicrob. Agents Chemother. 50, 2762–2771 (2006).
Chambers, H. F., Kocagoz, T., Sipit, T., Turner, J. & Hopewell, P. C. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin. Infect. Dis. 26, 874–877 (1998).
Donald, P. R. & Sirge, F. A. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand. J. Infect. Dis. 33, 466–469 (2001).
Hugonnet, J.-E., Tremblay, L. W., Boshoff, H. I., Barry, C. E. & Blanchard, J. S. Meropenem-clavulanate is effective against extensively drug-desistant Mycobacterium tuberculosis. Science 323, 1215–1218 (2009).
Vargas, R. Jr et al. Phase variation as a major mechanism of adaptation in Mycobacterium tuberculosis complex. Proc. Natl Acad. Sci. USA 120, e2301394120 (2023).
Vargas, R. Jr Role of epistasis in amikacin, kanamycin, bedaquiline, and clofazimine resistance in Mycobacterium tuberculosis complex. Antimicrob. Agents Chemother. 65, e0116421 (2021).
Farhat, M. R. et al. Genetic determinants of drug resistance in Mycobacterium tuberculosis and their diagnostic value. Am. J. Respir. Crit. Care Med. 194, 621–630 (2016).
Nebenzahl-Guimaraes, H., Jacobson, K. R., Farhat, M. R. & Murray, M. B. Systematic review of allelic exchange experiments aimed at identifying mutations that confer drug resistance in Mycobacterium tuberculosis. J. Antimicrob. Chemother. 69, 331–342 (2013).
Kadura, S. et al. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J. Antimicrob. Chemother. 75, 2031–2043 (2020).
Zhang, Y. & Yew, W. W. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 13, 1320–1330 (2009).
Green, A. G. et al. Analysis of genome-wide mutational dependence in naturally evolving Mycobacterium tuberculosis populations. Mol. Biol. Evol. 40, msad131 (2023).
Barilar, I. et al. Quantitative measurement of antibiotic resistance in Mycobacterium tuberculosis reveals genetic determinants of resistance and susceptibility in a target gene approach. Nat. Commun. 15, 488 (2024).
Farhat, M. R. et al. GWAS for quantitative resistance phenotypes in Mycobacterium tuberculosis reveals resistance genes and regulatory regions. Nat. Commun. 10, 2128 (2019).
Walker, T. M. et al. The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe 3, e265–e273 (2022).
Ghodousi, A. et al. Isoniazid resistance in Mycobacterium tuberculosis is a heterogeneous phenotype composed of overlapping MIC distributions with different underlying resistance mechanisms. Antimicrob. Agents Chemother. 63, e00092-19 (2019).
Spitaleri, A., Ghodousi, A., Miotto, P. & Cirillo, D. M. Whole genome sequencing in Mycobacterium tuberculosis. Ann. Transl. Med. 7, S197 (2019).
Farhat, M. R. et al. Gyrase mutations are associated with variable levels of fluoroquinolone resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 54, 727–733 (2016).
Chen, M. L. et al. Beyond multidrug resistance: leveraging rare variants with machine and statistical learning models in Mycobacterium tuberculosis resistance prediction. EBioMedicine 43, 356–369 (2019).
Green, A. G. et al. A convolutional neural network highlights mutations relevant to antimicrobial resistance in Mycobacterium tuberculosis. Nat. Commun. 13, 3817 (2022).
Yang, Y. et al. Machine learning for classifying tuberculosis drug-resistance from DNA sequencing data. Bioinformatics 34, 1666–1671 (2018).
Gröeschel, M. I. et al. GenTB: a user-friendly genome-based predictor for tuberculosis resistance powered by machine learning. Genome Med. 13, 138 (2021).
Safi, H. et al. Phase variation in Mycobacterium tuberculosis glpK produces transiently heritable drug tolerance. Proc. Natl Acad. Sci. USA 116, 19665–19674 (2019).
Hicks, N. D. et al. Clinically prevalent mutations in Mycobacterium tuberculosis alter propionate metabolism and mediate multidrug tolerance. Nat. Microbiol. 3, 1032–1042 (2018).
Liu, Q. et al. Tuberculosis treatment failure associated with evolution of antibiotic resilience. Science 378, 1111–1118 (2022).
Kreutzfeldt, K. M. et al. CinA mediates multidrug tolerance in Mycobacterium tuberculosis. Nat. Commun. 13, 2203 (2022).
Martini, M. C. et al. Loss of RNase J leads to multi-drug tolerance and accumulation of highly structured mRNA fragments in Mycobacterium tuberculosis. PLoS Pathog. 18, e1010705 (2022).
Andersson, D. I., Nicoloff, H. & Hjort, K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat. Rev. Microbiol. 17, 479–496 (2019).
Vargas, R. et al. In-host population dynamics of Mycobacterium tuberculosis complex during active disease. eLife 10, e61805 (2021).
Nimmo, C. et al. Dynamics of within-host Mycobacterium tuberculosis diversity and heteroresistance during treatment. EBioMedicine 55, 102747 (2020).
Engelthaler, D. M. et al. Minority Mycobacterium tuberculosis genotypic populations as an indicator of subsequent phenotypic resistance. Am. J. Respir. Cell Mol. Biol. 61, 789–791 (2019).
WHO Standard: Universal Access to Rapid Tuberculosis Diagnostics. World Health Organization https://www.who.int/publications/i/item/9789240071315 (2023).
Report of the 16th Meeting of the Strategic and Technical Advisory Group for Tuberculosis 2016. World Health Organization https://www.who.int/publications/m/item/report-of-the-16th-meeting-of-the-strategic-and-technical-advisory-group-for-tb (2016).
Jacobson, K. R. et al. Implications of failure to routinely diagnose resistance to second-line drugs in patients with rifampicin-resistant tuberculosis on Xpert MTB/RIF: a multisite observational study. Clin. Infect. Dis. 64, 1502–1508 (2017).
Oga-Omenka, C. et al. Factors influencing diagnosis and treatment initiation for multidrug-resistant/rifampicin-resistant tuberculosis in six sub-Saharan African countries: a mixed-methods systematic review. BMJ Glob. Health 5, e002280 (2020).
Svadzian, A., Sulis, G., Gore, G., Pai, M. & Denkinger, C. M. Differential yield of universal versus selective drug susceptibility testing of patients with tuberculosis in high-burden countries: a systematic review and meta-analysis. BMJ Glob. Health 5, e003438 (2020).
Kim, S. J. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur. Respir. J. 25, 564–569 (2005).
Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. World Health Organization https://www.who.int/publications/i/item/9789241514842 (2018).
Yu, H.-J. et al. Performance evaluation of the BACTEC MGIT 960 system for rifampin drug-susceptibility testing of Mycobacterium tuberculosis using the current WHO critical concentration. J. Clin. Microbiol. 61, e01086-22 (2023).
Shea, J. et al. Low-level rifampin resistance and rpoB mutations in Mycobacterium tuberculosis: an analysis of whole-genome sequencing and drug susceptibility test data in New York.J. Clin. Microbiol. 59, e01885-20 (2021).
Torrea, G. et al. Variable ability of rapid tests to detect Mycobacterium tuberculosis rpoB mutations conferring phenotypically occult rifampicin resistance. Sci. Rep. 9, 11826 (2019).
Automated Real-time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System: Policy Statement. World Health Organization https://www.who.int/publications/i/item/9789241501545 (2011).
Rapid Implementation of the Xpert MTB/RIF Diagnostic Test: Technical and Operational “How-to”; Practical Considerations. World Health Organization https://www.who.int/publications/i/item/9789241501569 (2011).
Dorman, S. E. et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 18, 76–84 (2018).
Albert, H. et al. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur. Respir. J. 48, 516–525 (2016).
Zifodya, J. S. et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst. Rev. 2021, CD009593 (2021).
Penn-Nicholson, A. et al. A prospective multicentre diagnostic accuracy study for the Truenat tuberculosis assays. Eur. Respir. J. 58, 2100526 (2021).
Gomathi, N. S. et al. Validation of an indigenous assay for rapid molecular detection of rifampicin resistance in presumptive multidrug-resistant pulmonary tuberculosis patients. Indian J. Med. Res. 152, 482–489 (2020).
Molbio Diagnostics: Molbio launches Truenat MTB-INH test for drug resistance in TB patients. Health News, ET HealthWorld https://health.economictimes.indiatimes.com/news/diagnostics/molbio-launches-truenat-mtb-inh-test-for-drug-resistance-in-tb-patients/96963161 (2023).
Theron, G. et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 383, 424–435 (2014).
Yoon, C. et al. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLoS ONE 7, e48599 (2012).
Di Tanna, G. L. et al. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Glob. Health 7, e191–e199 (2019).
Churchyard, G. J. et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob. Health 3, 450–457 (2015).
Cattamanchi, A. et al. Multicomponent strategy with decentralized molecular testing for tuberculosis. N. Engl. J. Med. 385, 2441–2450 (2021).
Penn-Nicholson, A. et al. Detection of isoniazid, fluoroquinolone, ethionamide, amikacin, kanamycin, and capreomycin resistance by the Xpert MTB/XDR assay: a cross-sectional multicentre diagnostic accuracy study. Lancet Infect. Dis. 22, 242–249 (2022).
Cao, Y. et al. Xpert MTB/XDR: a 10-color reflex assay suitable for point-of-care settings to detect isoniazid, fluoroquinolone, and second-line-injectable-drug resistance directly from Mycobacterium tuberculosis-positive sputum. J. Clin. Microbiol. 59, e02314-20 (2021).
De Vos, M. et al. Comparative analytical evaluation of four centralized platforms for the detection of Mycobacterium tuberculosis complex and resistance to rifampicin and isoniazid. J. Clin. Microbiol. 59, e02168-20 (2021).
Meaza, A. et al. Evaluation of genotype MTBDRplus VER 2.0 line probe assay for the detection of MDR-TB in smear positive and negative sputum samples. BMC Infect. Dis. 17, 280 (2017).
Nathavitharana, R. R. et al. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 49, 1601075 (2017).
Driesen, M. et al. Evaluation of a novel line probe assay to detect resistance to pyrazinamide, a key drug used for tuberculosis treatment. Clin. Microbiol. Infect. 24, 60–64 (2018).
Willby, M. J. et al. Detection of Mycobacterium tuberculosis pncA mutations by the Nipro Genoscholar PZA-TB II assay compared to conventional sequencing. Antimicrob. Agents Chemother. 62, e01871-17 (2018).
Catalogue of Mutations in Mycobacterium tuberculosis Complex and their Association with Drug Resistance, 2nd edn. World Health Organization https://www.who.int/publications/i/item/9789240082410 (2023).
Ismail, N. et al. Genetic variants and their association with phenotypic resistance to bedaquiline in Mycobacterium tuberculosis: a systematic review and individual isolate data analysis. Lancet Microbe 2, e604–e616 (2021).
An, Q., Lin, R., Yang, Q., Wang, C. & Wang, D. Evaluation of genetic mutations associated with phenotypic resistance to fluoroquinolones, bedaquiline, and linezolid in clinical Mycobacterium tuberculosis: a systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 34, 214–226 (2023).
Gan, W. C., Ng, H. F. & Ngeow, Y. F. Mechanisms of linezolid resistance in mycobacteria. Pharmaceuticals 16, 784 (2023).
CRyPTIC Consortium & The 100,000 Genomes Project. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N. Engl. J. Med. 379, 1403–1415 (2018).
Pankhurst, L. J. Rapid, comprehensive, and affordable mycobacterial diagnosis with whole-genome sequencing: a prospective study. Lancet Respir. Med. 4, 49–58 (2016).
Use of Targeted Next-generation Sequencing to Detect Drug-resistant Tuberculosis: Rapid Communication. World Health Organization https://www.who.int/publications/i/item/9789240076372 (2023).
The Use of Next-Generation Sequencing for the Surveillance of Drug-Resistant Tuberculosis: An Implementation Manual. World Health Organization https://www.who.int/publications/i/item/9789240078079 (2023).
Dippenaar, A. et al. Nanopore sequencing for Mycobacterium tuberculosis: a critical review of the literature, new developments, and future opportunities. J. Clin. Microbiol. 60, e00646-21 (2022).
Sanchez-Padilla, E. et al. Detection of drug-resistant tuberculosis by Xpert MTB/RIF in Swaziland. N. Engl. J. Med. 372, 1181–1182 (2015).
Ng, K. C. et al. Xpert Ultra can unambiguously identify specific rifampin resistance-conferring mutations. J. Clin. Microbiol. 56, 10–1128 (2018).
Daum, L. T. et al. Next-generation sequencing for characterizing drug resistance-conferring Mycobacterium tuberculosis genes from clinical isolates in the Ukraine. J. Clin. Microbiol. 56, e00009-18 (2018).
Tagliani, E. et al. Culture and next-generation sequencing-based drug susceptibility testing unveil high levels of drug-resistant-TB in Djibouti: results from the first national survey. Sci. Rep. 7, 17672 (2017).
Walker, T. M. et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect. Dis. 13, 137–146 (2013).
Mahomed, S., Mlisana, K., Cele, L. & Naidoo, K. Discordant line probe genotypic testing vs culture-based drug susceptibility phenotypic testing in TB endemic KwaZulu-Natal: impact on bedside clinical decision making. J. Clin. Tuberc. Mycobact. Dis. 20, 100176 (2020).
Milimo, D. et al. Diagnosis of rifampicin-resistant tuberculosis: discordant results by diagnostic methods. Afr. J. Lab. Med. 7, 1–4 (2018).
Votintseva, A. A. et al. Same-day diagnostic and surveillance data for ttuberculosis via whole-genome sequencing of direct respiratory samples. J. Clin. Microbiol. 55, 1285–1298 (2017).
Brown, A. C. et al. Rapid whole-genome sequencing of Mycobacterium tuberculosis isolates directly from clinical samples. J. Clin. Microbiol. 53, 2230–2237 (2015).
Doyle, R. M. et al. Direct whole-genome sequencing of sputum accurately identifies drug-resistant Mycobacterium tuberculosis faster than MGIT culture sequencing. J. Clin. Microbiol. 56, e00666–18 (2018).
Goig, G. A. et al. Whole-genome sequencing of Mycobacterium tuberculosis directly from clinical samples for high-resolution genomic epidemiology and drug resistance surveillance: an observational study. Lancet Microbe 1, e175–e183 (2020).
Van Rie, A. et al. Sequencing mycobacteria and algorithm-determined resistant tuberculosis treatment (SMARTT): a study protocol for a phase-IV pragmatic randomized controlled patient management strategy trial. Trials 23, 864 (2022).
Sibandze, D. B. et al. Rapid molecular diagnostics of tuberculosis resistance by targeted stool sequencing. Genome Med. 14, 52 (2022).
Iyer, A. et al. Operationalising targeted next-generation sequencing for routine diagnosis of drug-resistant TB. Public Health Action. 13, 43–49 (2023).
Cox, H. et al. Whole-genome sequencing has the potential to improve treatment for rifampicin-resistant tuberculosis in high-burden settings: a retrospective cohort study. J. Clin. Microbiol. 60, e02362–21 (2022).
Kwong, J. C., McCallum, N., Sintchenko, V. & Howden, B. P. Whole genome sequencing in clinical and public health microbiology. Pathology 47, 199–210 (2015).
Conradie, F. et al. Treatment of highly drug-resistant pulmonary tuberculosis. N. Engl. J. Med. 382, 893–902 (2020).
Nyang’wa, B.-T. et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 387, 2331–2343 (2022).
Padmapriyadarsini, C. et al. Bedaquiline, delamanid, linezolid and clofazimine for treatment of pre-extensively drug-resistant tuberculosis. Clin. Infect. Dis. 76, e938–e946 (2022).
Pym, A. S. et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur. Respir. J. 47, 564–574 (2016).
Schnippel, K. et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir. Med. 6, 699–706 (2018).
Ndjeka, N. et al. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur. Respir. J. 52, 1801528 (2018).
Ndjeka, N. et al. Treatment outcomes 24 months after initiating short bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens: a retrospective cohort study in South Africa.Lancet Infect. Dis. 22, 1042–1051 (2022).
Zhao, Y. et al. Improved treatment outcomes with bedaquiline when substituted for second-line injectable agents in multidrug-resistant tuberculosis: a retrospective cohort study. Clin. Infect. Dis. 68, 1522–1529 (2019).
Conradie, F. et al. Bedaquiline–pretomanid–linezolid regimens for drug-resistant tuberculosis. N. Engl. J. Med. 387, 810–823 (2022).
Esmail, A. et al. An all-oral 6-month regimen for multidrug-resistant tuberculosis: a multicenter, randomized controlled clinical trial (the NExT study). Am. J. Respir. Crit. Care Med. 205, 1214–1227 (2022).
Goodall, R. L. et al. Evaluation of two short standardised regimens for the treatment of rifampicin-resistant tuberculosis (STREAM stage 2): an open-label, multicentre, randomised, non-inferiority trial. Lancet 400, 1858–1868 (2022).
WHO Operational Handbook on Tuberculosis. Module 4: Treatment of Drug-resistant Tuberculosis. World Health Organization https://www.who.int/publications/i/item/9789240006997 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02589782 (2021).
Clinical Trial Results Offer Hope to DR-TB patients with short, effective treatment. Médecins Sans Frontières https://www.msf.org/clinical-trial-finds-short-effective-safe-DR-TB-treatment (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02333799 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03086486 (2023).
Drug-resistant Tuberculosis Clinical Trials Progress Report. RESIST-TB https://www.resisttb.org/clinical-trials-progress-report (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02619994 (2019).
Mok, J. et al. 9 months of delamanid, linezolid, levofloxacin, and pyrazinamide versus conventional therapy for treatment of fluoroquinolone-sensitive multidrug-resistant tuberculosis (MDR-END): a multicentre, randomised, open-label phase 2/3 non-inferiority trial in South Korea. Lancet 400, 1522–1530 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04062201 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02754765 (2023).
Guglielmetti, L. et al. Evaluating newly approved drugs for multidrug-resistant tuberculosis (endTB): study protocol for an adaptive, multi-country randomized controlled trial. Trials 22, 651 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03896685 (2022).
ShORRT Initiative. Tropical Disease Research https://tdr.who.int/activities/shorrt-research-package (2022).
Tuberculosis Treatment: 2023 Pipeline Report. Treatment Action Group https://www.treatmentactiongroup.org/resources/pipeline-report/2023-pipeline-report/ (2023).
WHO Operational Handbook on Tuberculosis. Module 4: Treatment — Drug-resistant Tuberculosis Treatment, 2022 Update. World Health Organization https://www.who.int/publications/i/item/9789240065116 (2022).
Maugans, C. et al. Best practices for the care of pregnant people living with TB. Int. J. Tuberc. Lung Dis. 27, 357–366 (2023).
Patankar, S. et al. Making the case for all-oral, shorter regimens for children with drug-resistant tuberculosis.Am. J. Respir. Crit. Care Med. 208, 130–131 (2023).
Turkova, A. et al. Shorter treatment for nonsevere tuberculosis in African and Indian children. N. Engl. J. Med. 386, 911–922 (2022).
Management of Multidrug-resistant Tuberculosis in Children: A Field Guide. Sentinel Project on Pediatric Drug-Resistant Tuberculosis https://sentinel-project.org/wp-content/uploads/2022/03/DRTB-Field-Guide-2021_v5.pdf (2022).
Furin, J., Tommasi, M. & Garcia-Prats, A. J. Drug-resistant tuberculosis: will grand promises fail children and adolescents? Lancet Child. Adolesc. Health 2, 237–238 (2018).
Karo, B. et al. Isoniazid (INH) mono-resistance and tuberculosis (TB) treatment success: analysis of European surveillance data, 2002 to 2014. Eur. Surveill. 24, 1800392 (2019).
Gegia, M., Winters, N., Benedetti, A., Soolingen, D. & Menzies, D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect. Dis. 17, 223–234 (2017).
Furin, J., Cox, H. & Pai, M. Tuberculosis. Lancet 393, 1642–1656 (2019).
WHO Consolidated Guidelines on Tuberculosis: Module 1: Prevention: Tuberculosis Preventive Treatment. World Health Organization https://www.who.int/publications/i/item/9789240001503 (2020).
Guidelines for Programmatic Management of Tuberculosis Preventive Treatment in India. National TB Elimination Programme https://tbcindia.gov.in/WriteReadData/l892s/Guidelines%20for%20Programmatic%20Management%20of%20Tuberculosis%20Preventive%20Treatment%20in%20India.pdf (2021).
Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. World Health Organization https://www.who.int/publications/i/item/9789241550239 (2018).
Kherabi, Y., Tunesi, S., Kay, A. & Guglielmetti, L. Preventive therapy for contacts of drug-resistant tuberculosis. Pathogens 11, 1189 (2022).
Huang, C. C. et al. Isoniazid preventive therapy in contacts of multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 202, 1159–1168 (2020).
Seddon, J. A. et al. Levofloxacin versus placebo for the prevention of tuberculosis disease in child contacts of multidrug-resistant tuberculosis: study protocol for a phase-III cluster randomised controlled trial (TB-CHAMP). Trials 19, 693 (2018).
Fox, G. J. et al. Levofloxacin versus placebo for the treatment of latent tuberculosis among contacts of patients with multidrug-resistant tuberculosis (the VQUIN MDR trial): a protocol for a randomised controlled trial. BMJ Open 10, e033945 (2020).
First Effective Treatment to Prevent Multidrug-Resistant TB. UCL News https://www.ucl.ac.uk/news/2023/nov/first-effective-treatment-prevent-multidrug-resistant-tb (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03568383 (2023).
WHO announces forthcoming updates on tuberculosis preventive treatment. World Health Organization https://www.who.int/news/item/13-02-2024-who-announces-forthcoming-updates-on-tuberculosis-preventive-treatment (2024).
Zimmerman, E., Smith, J., Banay, R., Kau, M. & Garfin, A. M. C. G. Behavioural barriers and perceived trade-offs to care-seeking for tuberculosis in the Philippines. Glob. Public Health 17, 210–222 (2022).
Daftary, A., Frick, M., Venkatesan, N., & Pai, M. Fighting TB stigma: we need to apply lessons learnt from HIV activism. BMJ Glob. Health 2, e000515 (2017).
Pradhan, A. et al. Internalized and perceived stigma and depression in pulmonary tuberculosis: do they explain the relationship between drug sensitivity status and adherence? Front. Psychiatry 13, 869647 (2022).
Redwood, L. et al. Depression, stigma and quality of life in people with drug-susceptible TB and drug-resistant TB in Vietnam. Int. J. Tuberc. Lung Dis. 25, 461–467 (2021).
Daftary, A., Padayatchi, N. & O’Donnell, M. Preferential adherence to antiretroviral therapy over tuberculosis treatment: a qualitative study of drug-resistant TB/HIV co-infected patients in South Africa. Glob. Public Health 9, 1107–1116 (2014).
Susanto, T. D. et al. Anxiety and depression level of patients with multidrug-resistant tuberculosis (MDR-TB) in two hospitals in Banten province, Indonesia. Dialogues Health 2, 100115 (2023).
Walker, I. et al. Depression and anxiety in patients with multidrug-resistant tuberculosis in Nepal: an observational study. Public Health Action 9, 42–48 (2019).
Sommerland, N. et al. Evidence-based interventions to reduce tuberculosis stigma: a systematic review. Int. J. Tuberc. Lung Dis. 21, S81–S86 (2017).
As’hab, P. P., Keliat, B. A. & Wardani, I. Y. The effects of acceptance and commitment therapy on psychosocial impact and adherence of multidrug-resistant tuberculosis patients. J. Public Health Res. 11, 2737 (2022).
Thomas, B. E. et al. Psycho-socio-economic issues challenging multidrug resistant tuberculosis patients: a systematic review. PLoS ONE 11, e0147397 (2016).
Udwadia, Z. & Furin, J. Quality of drug-resistant tuberculosis care: gaps and solutions. J. Clin. Tuberc. Mycobact. Dis. 16, 100101 (2019).
Pai, M., Dewan, P. K. & Swaminathan, S. Transforming tuberculosis diagnosis. Nat. Microbiol. 8, 756–759 (2023).
McKenna, L. et al. The 1/4/6x24 campaign to cure tuberculosis quickly. Nat. Med. 11, 2337 (2023).
Zaunbrecher, M. A., Sikes, R. D. Jr, Metchock, B., Shinnick, T. M. & Posey, J. E. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 106, 20004–20009 (2009).
Miotto, P. et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur. Respir. J. 50, 1701354 (2017).
Rifat, D. et al. Mutations in fbiD (Rv2983) as a novel determinant of resistance to pretomanid and delamanid in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 65, e01948–20 (2020).
Plinke, C. et al. embCAB sequence variation among ethambutol-resistant Mycobacterium tuberculosis isolates without embB306 mutation. J. Antimicrob. Chemother. 65, 1359–1367 (2010).
Safi, H. et al. Evolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-β-D-arabinose biosynthetic and utilization pathway genes. Nat. Genet. 45, 1190–1197 (2013).
Srivastava, S., Ayyagari, A., Dhole, T. N., Nyati, K. K. & Dwivedi, S. K. emb nucleotide polymorphisms and the role of embB306 mutations in Mycobacterium tuberculosis resistance to ethambutol. Int. J. Med. Microbiol. 299, 269–280 (2009).
Grant, S. S. et al. Baeyer-Villiger monooxygenases EthA and MymA are required for activation of replicating and non-replicating Mycobacterium tuberculosis inhibitors. Cell Chem. Biol. 23, 666–677 (2016).
Dover, L. G. et al. EthA, a common activator of thiocarbamide-containing drugs acting on different mycobacterial targets. Antimicrob. Agents Chemother. 51, 1055–1063 (2007).
Vilchèze, C. et al. Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol. Microbiol. 69, 1316–1329 (2008).
Zhang, Y., Dhandayuthapani, S. & Deretic, V. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc. Natl Acad. Sci. USA 93, 13212–13216 (1996).
Ando, H., Miyoshi-Akiyama, T., Watanabe, S. & Kirikae, T. A silent mutation in mabA confers isoniazid resistance on Mycobacterium tuberculosis. Mol. Microbiol. 91, 538–547 (2014).
Vilchèze, C. et al. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob. Agents Chemother. 49, 708–720 (2005).
Reeves, A. Z. et al. Aminoglycoside cross-resistance in Mycobacterium tuberculosis due to mutations in the 5′ untranslated region of whiB7. Antimicrob. Agents Chemother. 57, 1857–1865 (2013).
Liu, J. et al. Mutations in efflux pump Rv1258c (tap) cause resistance to pyrazinamide, isoniazid, and streptomycin in Mycobacterium tuberculosis. Front. Microbiol. 10, 216 (2019).
Gopal, P. et al. Pyrazinamide triggers degradation of its target aspartate decarboxylase. Nat. Commun. 11, 1661 (2020).
Scorpio, A. & Zhang, Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2, 662–667 (1996).
Wong, S. Y. et al. Functional role of methylation of G518 of the 16S rRNA 530 loop by GidB in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 57, 6311–6318 (2013).
Catalogue of Mutations in Mycobacterium tuberculosis Complex and their Association with Drug Resistance. World Health Organization https://www.who.int/publications/i/item/9789240028173 (2021).
Farhat, M. R. et al. Rifampicin and rifabutin resistance in 1003 Mycobacterium tuberculosis clinical isolates. J. Antimicrob. Chemother. 74, 1477–1483 (2019).
Nahid, P. et al. Treatment of drug-resistant tuberculosis. An Official ATS/CDC/ERS/IDSA clinical practice guideline. Am. J. Respir. Crit. Care Med. 200, e93–e142 (2019).
Author information
Authors and Affiliations
Contributions
M.P., M.F., H.C., C.M.D., J.F. and M.G. wrote the article and, together with C.R. and M.S.A.E.A. researched data for the article. All authors contributed substantially to the discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Clinicaltrials.gov: https://clinicaltrials.gov/
Supplementary information
Glossary
- Directly observed therapy
-
Refers to the delivery of anti-tuberculosis drug treatment under direct observation of health workers, community workers or family members with the goal of improving adherence.
- Extensively drug-resistant TB
-
(XDR-TB). Defined as multidrug-resistant or rifampicin-resistant tuberculosis with further resistance to fluoroquinolones and to either bedaquiline or linezolid or both (key second-line drugs).
- Inh-resistant and Rif-susceptible TB
-
(Hr-TB). Defined as resistance to isoniazid and susceptibility to rifampicin.
- Multidrug-resistant TB
-
(MDR-TB). Defined as resistance to rifampicin and isoniazid, the two most important first-line drugs used to treat tuberculosis (TB), regardless of resistance to other TB drugs.
- Pre-XDR-TB
-
Defined as multidrug-resistant or rifampicin-resistant tuberculosis with resistance to fluoroquinolones.
- Rif mono-resistant TB
-
(RMR-TB). Defined as resistance to rifampicin (Rif), with susceptibility to isoniazid.
- Rif-resistant TB
-
(RR-TB). Defined as resistance to rifampicin (Rif), regardless of resistance to other tuberculosis (TB) drugs. Individuals with RR-TB are treated with regimens similar to those for multidrug-resistant TB (MDR-TB) and are therefore grouped with MDR-TB as MDR/RR-TB.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farhat, M., Cox, H., Ghanem, M. et al. Drug-resistant tuberculosis: a persistent global health concern. Nat Rev Microbiol (2024). https://doi.org/10.1038/s41579-024-01025-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41579-024-01025-1