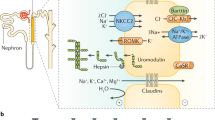
Abstract
Alkaptonuria is a rare inborn error of metabolism caused by the deficiency of homogentisate 1,2-dioxygenase activity. The consequent homogentisic acid (HGA) accumulation in body fluids and tissues leads to a multisystemic and highly debilitating disease whose main features are dark urine, ochronosis (HGA-derived pigment in collagen-rich connective tissues), and a painful and severe form of osteoarthropathy. Other clinical manifestations are extremely variable and include kidney and prostate stones, aortic stenosis, bone fractures, and tendon, ligament and/or muscle ruptures. As an autosomal recessive disorder, alkaptonuria affects men and women equally. Debilitating symptoms appear around the third decade of life, but a proper and timely diagnosis is often delayed due to their non-specific nature and a lack of knowledge among physicians. In later stages, patients’ quality of life might be seriously compromised and further complicated by comorbidities. Thus, appropriate management of alkaptonuria requires a multidisciplinary approach, and periodic clinical evaluation is advised to monitor disease progression, complications and/or comorbidities, and to enable prompt intervention. Treatment options are patient-tailored and include a combination of medications, physical therapy and surgery. Current basic and clinical research focuses on improving patient management and developing innovative therapies and implementing precision medicine strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Garrod, A. The incidence of alkaptonuria. A study in chemical individuality. Lancet 160, 1616–1620 (1902).
Fernândez-Canon, J. M. et al. The molecular basis of alkaptonuria. Nat. Genet. 14, 19–24 (1996). The authors describe the cloning of the human HGD gene and demonstrate for the first time, to our knowledge, that it harbours missense variants that co-segregate with the disease in the families.
Vilboux, T. et al. Mutation spectrum of homogentisic acid oxidase (HGD) in alkaptonuria. Hum. Mutat. 30, 1611–1619 (2009).
Zannoni, V. G., Seegmiller, J. E. & La Du, B. N. Nature of the defect in alcaptonuria. Nature 193, 952–953 (1962).
Phornphutkul, C. et al. Natural history of alkaptonuria. N. Engl. J. Med. 347, 2111–2121 (2002). This is the reference study for the definition of the natural history of AKU.
Gallagher, J. A., Ranganath, L. R. & Zatkova, A. in Brenner’s Encyclopedia of Genetics 2nd edn (eds Malay, S. & Hughes, K.) 71–75 (Elsevier, 2013).
Chow, W. Y. et al. Pigmentation chemistry and radical-based collagen degradation in alkaptonuria and osteoarthritic cartilage. Angew. Chem. Int. Ed. 59, 11937–11942 (2020). The authors propose that collagen degradation in AKU occurs via transient glycyl radicals, and this process is enhanced due to the redox environment generated by pigmentation.
Bernini, A. et al. A molecular spectroscopy approach for the investigation of early phase ochronotic pigment development in alkaptonuria. Sci. Rep. 11, 22562 (2021).
Barrios, P. C. & Font, R. L. Pigmented conjunctival lesions as initial manifestation of ochronosis. Arch. Ophthalmol. 122, 1060–1063 (2004).
Ranganath, L. R. et al. Reversal of ochronotic pigmentation in alkaptonuria following nitisinone therapy: analysis of data from the United Kingdom National Alkaptonuria Centre. JIMD Rep. 55, 75–87 (2020).
Dewan, K., MacDonald, C. B. & Shires, C. B. Blue man: ochronosis in otolaryngology. Clin. Case Rep. 10, e05717 (2022).
Mannoni, A. et al. Ochronosis, and ochronotic arthropathy. Semin. Arthritis Rheum. 33, 239–248 (2004).
Millucci, L. et al. Amyloidosis, inflammation, and oxidative stress in the heart of an alkaptonuric patient. Mediators Inflamm. 2014, 258471 (2014).
Millucci, L. et al. Diagnosis of secondary amyloidosis in alkaptonuria. Diagn. Pathol. 9, 185 (2014).
Millucci, L. et al. Secondary amyloidosis in an alkaptonuric aortic valve. Int. J. Cardiol. 172, e121–e123 (2014).
Křížek, V. Urolithiasis and prostatolithiasis in alcaptonuria with ochronosis. Int. Urol. Nephrol. 3, 245–250 (1971).
Introne, W. J. et al. Exacerbation of the ochronosis of alkaptonuria due to renal insufficiency and improvement after renal transplantation. Mol. Genet. Metab. 77, 136–142 (2022).
Lazaro, J. S., Lutz, R. & Deirmengian, G. K. Bilateral patellar tendon rupture following total knee arthroplasty in a patient with alkaptonuria: a case report. Cureus 15, e38597 (2023).
Zatkova, A., Ranganath, L. & Kadasi, L. Alkaptonuria: current perspectives. Appl. Clin. Genet. 13, 37–47 (2020).
Ranganath, L. R. & Cox, T. F. Natural history of alkaptonuria revisited: analyses based on scoring systems. J. Inherit. Metab. Dis. 34, 1141–1151 (2011). The authors applied advanced statistical methods to describe the natural history by quantitating the features of AKU, revealing distinct phases of the disease: a pre-ochronotic phase and an ochronotic phase.
Introne, W. J., Perry, M. & Chen, M. Alkaptonuria. GeneReviews [Internet] www.ncbi.nlm.nih.gov/books/NBK1454/ (updated 10 June 2021).
Milch, R. A. Studies of alcaptonuria: inheritance of 47 cases in eight highly inter-related Dominican kindreds. Am. J. Hum. Genet. 12, 76–85 (1960).
Al-Sbou, M., Mwafi, N. & Lubad, M. A. Identification of forty cases with alkaptonuria in one village in Jordan. Rheumatol. Int. 32, 3737–3740 (2012).
Al-Sbou, M. & Mwafi, N. Nine cases of alkaptonuria in one family in southern Jordan. Rheumatol. Int. 32, 621–625 (2012).
Sakthivel, S. et al. Mutation screening of the HGD gene identifies a novel alkaptonuria mutation with significant founder effect and high prevalence. Ann. Hum. Genet. 78, 155–164 (2014).
Srsen, S. & Varga, F. Screening for alkaptonuria in the newborn in Slovakia. Lancet 2, 576 (1978).
Zatková, A. et al. High frequency of alkaptonuria in Slovakia: evidence for the appearance of multiple mutations in HGO involving different mutational hot spots. Am. J. Hum. Genet. 67, 1333–1339 (2000).
Zatkova, A. An update on molecular genetics of alkaptonuria (AKU). J. Inherit. Metab. Dis. 34, 1127–1136 (2011).
Zatkova, A. et al. Identification of 11 novel homogentisate 1,2 dioxygenase variants in alkaptonuria patients and establishment of a novel LOVD-based HGD mutation database. JIMD Rep. 4, 55–65 (2012). The authors report on the establishment of a disease-specific HGD mutation database that summarizes all AKU gene variants identified so far in patients with AKU. This database is still active and regularly updated.
Soltysova, A., Kuzin, A., Samarkina, E. & Zatkova, A. Alkaptonuria in Russia. Eur. J. Hum. Genet. 30, 237–242 (2022).
Cox, T. F. & Ranganath, L. A quantitative assessment of alkaptonuria: testing the reliability of two disease severity scoring systems. J. Inherit. Metab. Dis. 34, 1153–1162 (2011).
Perry, M. B., Suwannarat, P., Furst, G. P., Gahl, W. A. & Gerber, L. H. Musculoskeletal findings and disability in alkaptonuria. J. Rheumatol. 33, 2280–2285 (2006).
Ranganath, L. R., Khedr, M., Vinjamuri, S. & Gallagher, J. A. Frequency, diagnosis, pathogenesis and management of osteoporosis in alkaptonuria: data analysis from the UK National Alkaptonuria Centre. Osteoporos. Int. 32, 927–938 (2021).
Ranganath, L. R. et al. Homogentisic acid is not only eliminated by glomerular filtration and tubular secretion but also produced in the kidney in alkaptonuria. J. Inherit. Metab. Dis. 43, 737–747 (2020).
Mistry, J. B., Bukhari, M. & Taylor, A. M. Alkaptonuria. Rare Dis. 1, e27475 (2013).
Kitahara, Y., Kaku, N., Tagomori, H. & Tsumura, H. Alkaptonuria with rapidly destructive arthropathy of the hip: a case report and literature review. Acta Orthop. Traumatol. Turc. 55, 563–568 (2021).
Ranganath, L. R., Khedr, M., Mistry, A., Vinjamuri, S. & Gallagher, J. A. Treatment of osteoporotic fractures in alkaptonuria by teriparatide stimulates bone formation and decreases fracture rate – a report of two cases. Bone Rep. 15, 101151 (2021).
Gómez-Lechón Quirós, L. et al. Family history of ochronotic arthropathy. Rheumatol. Int. 41, 1869–1874 (2021).
Taylor, A. M. et al. The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum. 63, 3887–3896 (2011).
Taylor, A. M. et al. Identification of trabecular excrescences, novel microanatomical structures, present in bone in osteoarthropathies. Eur. Cell Mater. 23, 300–309 (2012).
Ebrahim, I. C., Hoang, T. D., Vietor, N. O., Schacht, J. P. & Shakir, M. K. M. Dilemmas in the diagnosis and management of osteoporosis in a patient with alkaptonuria: successful treatment with teriparatide. Clin. Case Rep. 10, e6729 (2022).
Genovese, F. et al. Investigating the robustness and diagnostic potential of extracellular matrix remodelling biomarkers in alkaptonuria. JIMD Rep. 24, 29–37 (2015).
Brunetti, G. et al. Mechanisms of enhanced osteoclastogenesis in alkaptonuria. Am. J. Pathol. 188, 1059–1068 (2018).
Cox, T. et al. Subclinical ochronosis features in alkaptonuria: a cross-sectional study. BMJ Innov. 5, 82–91 (2019).
Genovese, F. et al. Nitisinone treatment affects biomarkers of bone and cartilage remodelling in alkaptonuria patients. Int. J. Mol. Sci. 24, 10996 (2023).
Avadhanula, S. et al. Assessment of thyroid function in patients with alkaptonuria. JAMA Netw. open. 3, e201357 (2020).
Kujawa, M. J. et al. Clinical presentation of 13 children with alkaptonuria. J. Inherit. Metab. Dis. 46, 916–930 (2023).
Ranganath, L. et al. Increased prevalence of Parkinson’s disease in alkaptonuria. JIMD Rep. 64, 282–292 (2023).
Cannon Homaei, S. et al. ADHD symptoms in neurometabolic diseases: underlying mechanisms and clinical implications. Neurosci. Biobehav. Rev. 132, 838–856 (2022).
Mitchell, J. J., Trakadis, Y. J. & Scriver, C. R. Phenylalanine hydroxylase deficiency. Genet. Med. 13, 607–617 (2011).
Arıcı, A. & Altun, H. Successful treatment of attention-deficit/hyperactivity disorder accompanying to alkaptonuria with methylphenidate and risperidone. Psychiatry Clin. Psychopharmacol. 29, 110–113 (2019).
Urbánek, T. & Rovenský, J. in Alkaptonuria and Ochronosis (eds Rovenský, J., Urbánek, T., Oľga, B. & Gallagher, J. A.) 91–98 (Springer, 2015). This monograph is a comprehensive review of clinical experience and research in the field of alkaptonuria and ochronosis.
Bernardini, G. et al. Homogentisate 1,2 dioxygenase is expressed in brain: implications in alkaptonuria. J. Inherit. Metab. Dis. 38, 807–814 (2015).
Braconi, D., Millucci, L., Spiga, O. & Santucci, A. Cell and tissue models of alkaptonuria. Drug. Discov. Today Dis. Model. 31, 3–10 (2020).
Millucci, L. et al. Amyloidosis in alkaptonuria. J. Inherit. Metab. Dis. 38, 797–805 (2015).
Millucci, L. et al. Alkaptonuria is a novel human secondary amyloidogenic disease. Biochim. Biophys. Acta - Mol. Basis Dis. 1822, 1682–1691 (2012). This paper defines for the first time to our knowledge AKU as a novel type II AA amyloidosis and suggests the use of low doses of methotrexate to treat this comorbidity.
Millucci, L. et al. Histological and ultrastructural characterization of alkaptonuric tissues. Calcif. Tissue Int. 101, 50–64 (2017).
Geminiani, M. et al. Cytoskeleton aberrations in alkaptonuric chondrocytes. J. Cell. Physiol. 232, 1728–1738 (2017).
Braconi, D. et al. Inflammatory and oxidative stress biomarkers in alkaptonuria: data from the DevelopAKUre project. Osteoarthr. Cartil. 26, 1078–1086 (2017).
Braconi, D. et al. Comparative proteomics in alkaptonuria provides insights into inflammation and oxidative stress. Int. J. Biochem. Cell Biol. 81, 271–280 (2016).
Braconi, D. et al. Effects of nitisinone on oxidative and inflammatory markers in alkaptonuria: results from SONIA1 and SONIA2 studies. Cells 11, 3668 (2022).
Pollak, M. R. et al. Homozygosity mapping of the gene for alkaptonuria to chromosome 3q2. Nat. Genet. 5, 201–204 (1993).
Janocha, S. et al. The human gene for alkaptonuria (AKU) maps to chromosome 3q. Genomics 19, 5–8 (1994).
Fernandez-Canon, J. M. & Penalva, M. A. Molecular characterization of a gene encoding a homogentisate dioxygenase from Aspergillus nidulans and identification of its human and plant homologues. J. Biol. Chem. 270, 21199–21205 (1995).
Granadino, B., De Bernabé, D. B. V., Fernández-Cañón, J. M., Peñalva, M. A. & De Córdoba, S. R. The human homogentisate 1,2-dioxygenase (HGO) gene. Genomics 43, 115–122 (1997).
Nemethova, M. et al. Twelve novel HGD gene variants identified in 99 alkaptonuria patients: focus on “black bone disease” in Italy. Eur. J. Hum. Genet. 24, 66–72 (2016).
Soltysova, A., Sekelska, M. & Zatkova, A. Breakpoints characterisation of the genomic deletions identified by MLPA in alkaptonuria patients. Eur. J. Hum. Genet. 31, 485–489 (2022).
Ascher, D. B. et al. Homogentisate 1,2-dioxygenase (HGD) gene variants, their analysis and genotype-phenotype correlations in the largest cohort of patients with AKU. Eur. J. Hum. Genet. 27, 888–902 (2019). The authors summarize data on AKU-causing variants in the largest cohort of clinically well described patients with AKU and in the first genotype–phenotype correlations study also demonstrate that the severity of AKU does not significantly depend on the residual activity of mutated HGD enzyme.
Lai, C. Y. et al. A novel deep intronic variant strongly associates with alkaptonuria. NPJ Genom. Med. 6, 89 (2021).
Bychkov, I. et al. Alkaptonuria in Russia: mutational spectrum and novel variants. Eur. J. Med. Genet. 64, 104165 (2021).
Titus, G. P. et al. Crystal structure of human homogentisate dioxygenase. Nat. Struct. Biol. 7, 542–546 (2000).
Karmakar, M. et al. HGDiscovery: an online tool providing functional and phenotypic information on novel variants of homogentisate 1,2-dioxigenase. Curr. Res. Struct. Biol. 4, 271–277 (2022).
Rodríguez, J. M. et al. Structural and functional analysis of mutations in alkaptonuria. Hum. Mol. Genet. 9, 2341–2350 (2000).
Lequeue, S. et al. A robust bacterial high-throughput screening system to evaluate single nucleotide polymorphisms of human homogentisate 1,2-dioxygenase in the context of alkaptonuria. Sci. Rep. 12, 19452 (2022).
Zatkova, A., Olsson, B., Ranganath, L. R. & Imrich, R. Analysis of the phenotype differences in siblings with alkaptonuria. Metabolites 12, 990 (2022).
Gupta, V. et al. Dominant negative mutations affect oligomerization of human pyruvate kinase M2 isozyme and promote cellular growth and polyploidy. J. Biol. Chem. 285, 16864–16873 (2010).
Stoner, R. & Blivaiss, B. B. Reaction of quinone of homogentisic acid with biological amines. Arthritis Rheum. 10, 53–60 (1967).
Martin, J. P. J. & Batkoff, B. Homogentisic acid autoxidation and oxygen radical generation: implications for the etiology of alkaptonuric arthritis. Free. Radic. Biol. Med. 3, 241–250 (1987).
Tokuhara, Y. et al. Detection of novel visible-light region absorbance peaks in the urine after alkalization in patients with alkaptonuria. PLoS ONE 9, 4–6 (2014).
Tokuhara, Y. et al. Absorbance measurements of oxidation of homogentisic acid accelerated by the addition of alkaline solution with sodium hypochlorite pentahydrate. Sci. Rep. 8, 11364 (2018).
Eslami, M., Namazian, M. & Zare, H. R. Electrooxidation of homogentisic acid in aqueous and mixed solvent solutions: experimental and theoretical studies. J. Phys. Chem. B 117, 2757–2763 (2013).
Eslami, M., Zare, H. R. & Namazian, M. The effect of solvents on the electrochemical behavior of homogentisic acid. J. Electroanal. Chem. 720–721, 76–83 (2014).
David, C., Daro, A., Szalai, E., Atarhouch, T. & Mergeay, M. Formation of polymeric pigments in the presence of bacteria and comparison with chemical oxidative coupling – II. Catabolism of tyrosine and hydroxyphenylacetic acid by Alcaligenes eutrophus CH34 and mutants. Eur. Polym. J. 32, 669–679 (1996).
Turick, C. E., Knox, A. S., Becnel, J. M., Ekechukwu, A. A. & Millike, C. E. in Biopolymers (ed. Elnashar, M.) 449–472 (InTech, 2010).
Galeb, H. A. et al. The polymerization of homogentisic acid in vitro as a model for pyomelanin formation. Macromol. Chem. Phys. 223, 2100489 (2022).
Galeb, H. A. et al. Phenolic polymers as model melanins. Macromol. Chem. Phys. 224, 2300025 (2023).
Braconi, D. et al. Redox-proteomics of the effects of homogentisic acid in an in vitro human serum model of alkaptonuric ochronosis. J. Inherit. Metab. Dis. 34, 1163–1176 (2011).
Braconi, D. et al. Evaluation of anti-oxidant treatments in an in vitro model of alkaptonuric ochronosis. Rheumatology 49, 1975–1983 (2010).
Braconi, D., Millucci, L., Ghezzi, L. & Santucci, A. Redox proteomics gives insights into the role of oxidative stress in alkaptonuria. Exp. Rev. Proteom. 10, 521–535 (2013).
Spreafico, A. et al. Antioxidants inhibit SAA formation and pro-inflammatory cytokine release in a human cell model of alkaptonuria. Rheumatology 52, 1667–1673 (2013).
Tinti, L. et al. Evaluation of antioxiodant drugs for the treatment of ochronotic alkaptonuria in an in vitro human cell model. J. Cell. Physiol. 225, 84–91 (2010).
Millucci, L. et al. Chondroptosis in alkaptonuric cartilage. J. Cell. Physiol. 230, 1148–1157 (2015).
Braconi, D. et al. Homogentisic acid induces aggregation and fibrillation of amyloidogenic proteins. Biochim. Biophys. Acta Gen. Subj. 1861, 135–146 (2017).
Braconi, D. et al. Biochemical and proteomic characterization of alkaptonuric chondrocytes. J. Cell. Physiol. 227, 3333–3343 (2012).
Braconi, D. et al. Proteomic and redox-proteomic evaluation of homogentisic acid and ascorbic acid effects on human articular chondrocytes. J. Cell. Biochem. 111, 922–932 (2010).
Millucci, L. et al. Angiogenesis in alkaptonuria. J. Inherit. Metab. Dis. 39, 801–806 (2016).
Gambassi, S. et al. Smoothened-antagonists reverse homogentisic acid-induced alterations of Hedgehog signaling and primary cilium length in alkaptonuria. J. Cell. Physiol. 232, 3103–3111 (2017).
Thorpe, S. D. et al. Reduced primary cilia length and altered Arl13b expression are associated with deregulated chondrocyte Hedgehog signaling in alkaptonuria. J. Cell. Physiol. 232, 2407–2417 (2017).
Galderisi, S. et al. Homogentisic acid induces autophagy alterations leading to chondroptosis in human chondrocytes: implications in alkaptonuria. Arch. Biochem. Biophys. 717, 109137 (2022).
Gallagher, J. A., Dillon, J. P., Sireau, N., Timmis, O. & Ranganath, L. R. Alkaptonuria: an example of a “fundamental disease” – A rare disease with important lessons for more common disorders. Semin. Cell Dev. Biol. 52, 53–57 (2016). In this paper, the authors present the ‘exposed collagen hypothesis’ to describe the mechanism of ochronotic pigmentation of the joint cartilages, and describe AKU as a ‘fundamental disease’ — that is, a rare genetic disease that is a gateway to understanding common conditions and human physiology.
Hughes, J. H. et al. Anatomical distribution of ochronotic pigment in alkaptonuric mice is associated with calcified cartilage chondrocytes at osteochondral interfaces. Calcif. Tissue Int. 108, 207–218 (2021).
Gallagher, J. A., Dillon, J. P. & Ranganath, L. R. Development of an effective therapy for alkaptonuria – lessons for osteoarthritis. Rheumatol. Immunol. Res. 2, 79–85 (2021).
Srsen, S. & Srsnová K, L. A. Clinical manifestation of alkaptonuria in relation to age. Bratisl. Lek. List. 77, 662–669 (1982).
Srsen S, S. K. Diagnosis of alkaptonuria in children. Padiatr. Padol. 14, 163–167 (1979).
Zibolena, M., Srsnovab, K. & Srsenb, S. Increased urolithiasis in patients with alkaptonuria in childhood. Clin. Genet. 58, 79–80 (2000).
Gucev, Z. S. et al. Early-onset ocular ochronosis in a girl with alkaptonuria (AKU) and a novel mutation in homogentisate 1,2-dioxygenase (HGD). Prilozi 32, 305–311 (2011).
Taylor, A. M. et al. Calculi and intracellular ochronosis in the submandibular tissues from a patient with alkaptonuria. J. Clin. Pathol. 63, 186–188 (2010).
Davison, A. S., Milan, A. M., Gallagher, J. A. & Ranganath, L. R. Acute fatal metabolic complications in alkaptonuria. J. Inherit. Metab. Dis. 39, 203–210 (2016).
Faria, B., Vidinha, J., Pêgo, C., Correia, H. & Sousa, T. Impact of chronic kidney disease on the natural history of alkaptonuria. CKJ Clin. Kidney J. 5, 352–355 (2012).
Heng, A. E. et al. Hemolysis in a patient with alkaptonuria and chronic kidney failure. Am. J. Kidney Dis. 56, e1–e4 (2010).
Ranganath, L. R., Jarvis, J. C. & Gallagher, J. A. Recent advances in management of alkaptonuria (invited review; best practice article). J. Clin. Pathol. 66, 367–373 (2013).
Lang, J., Narendrula, A., El-Zawahry, A., Sindhwani, P. & Ekwenna, O. Global trends in incidence and burden of urolithiasis from 1990 to 2019: an analysis of global burden of disease study data. Eur. Urol. Open. Sci. 35, 37–46 (2022).
Hyun, J. S. Clinical significance of prostatic calculi: a review. World J. Mens. Health 36, 15 (2018).
Davison, A. S., Luangrath, E., Selvi, E. & Ranganath, L. R. Fatal acute haemolysis and methaemoglobinaemia in a man with renal failure and alkaptonuria – is nitisinone the solution? Mol. Genet. Metab. Rep. 23, 100588 (2020).
Hugar, S. B., Shulman, J., Yanta, J. & Nine, J. Ochronosis presenting as methemoglobinemia. J. Forensic Sci. 64, 913–916 (2019).
Freeman, A. R. & Wills, S. M. Fatal methemoglobinemia complicating alkaptonuria (ochronosis): a rare presentation. Forensic Sci. Med. Pathol. 14, 236–240 (2018).
Isa, Y. et al. A rare case of acquired methemoglobinemia associated with alkaptonuria. Intern. Med. 53, 1797–1800 (2014).
Ahuja, V., Atter, P., Mundotiya, S. & Garg, S. Perioperative anesthetic challenges in alkaptonuria patient with comorbid conditions. J. Anaesthesiol. Clin. Pharmacol. 38, 152–153 (2022).
Pandey, R., Kumar, A., Garg, R., Khanna, P. & Darlong, V. Perioperative management of patient with alkaptonuria and associated multiple comorbidities. J. Anaesthesiol. Clin. Pharmacol. 27, 259–261 (2011).
Ranganath, L. R., Khedr, M., Vinjamuri, S. & Gallagher, J. A. Characterizing the alkaptonuria joint and spine phenotype and assessing the effect of homogentisic acid lowering therapy in a large cohort of 87 patients. J. Inherit. Metab. Dis. 44, 666–676 (2021).
Ranganath, L. R., Gallagher, J. A., Davidson, J. & Vinjamuri, S. Characterising the arthroplasty in spondyloarthropathy in a large cohort of eighty-seven patients with alkaptonuria. J. Inherit. Metab. Dis. 44, 656–665 (2021).
Rudebeck, M., Scott, C., Sireau, N. & Ranganath, L. A patient survey on the impact of alkaptonuria symptoms as perceived by the patients and their experiences of receiving diagnosis and care. JIMD Rep. 53, 71–79 (2020).
Ranganath, L. R., Heseltine, T., Khedr, M. & Fisher, M. F. Evaluating the aortic stenosis phenotype before and after the effect of homogentisic acid lowering therapy: analysis of a large cohort of eighty-one alkaptonuria patients. Mol. Genet. Metab. 133, 324–331 (2021).
Ahmad, M. S. Z. et al. Association of alkaptonuria and low dose nitisinone therapy with cataract formation in a large cohort of patients. JIMD Rep. 63, 351–360 (2022).
Srsen, S., Vondrácek, J. & Srsnová K, S. J. Analysis of the life span of alkaptonuric patients. Cas. Lek. Ces 124, 1288–1291 (1985).
Foot, C. L. & Fraser, J. F. Uroscopic rainbow: modern matula medicine. Postgrad. Med. J. 82, 126–129 (2006).
Davison, A. S., Milan, A. M., Hughes, A. T., Dutton, J. J. & Ranganath, L. R. Serum concentrations and urinary excretion of homogentisic acid and tyrosine in normal subjects. Clin. Chem. Lab. Med. 53, e81–e83 (2015).
Ranganath, L. R. et al. Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): an international, multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 8, 762–772 (2020). To our knowledge, this is the first long-term randomized trial to show that nitisinone is effective in treating alkaptonuria, partially reversing the ochronotic process and reducing the rate of disease progression.
Jacomelli, G., Micheli, V., Bernardini, G., Millucci, L. & Santucci, A. Quick diagnosis of alkaptonuria by homogentisic acid determination in urine paper spots. JIMD Rep. 31, 51 (2017).
Oláh, A. V. et al. Urinary homogentisic acid in alkaptonuric and healthy children. Clin. Chem. Lab. Med. 41, 356–359 (2003).
Hughes, A. T. et al. Serum markers in alkaptonuria: simultaneous analysis of homogentisic acid, tyrosine and nitisinone by liquid chromatography tandem mass spectrometry. Ann. Clin. Biochem. 52, 597–605 (2015).
Rovenský, J., Krátka, M. & Urbánek, T. in Alkaptonuria and Ochronosis (eds Rovenský, J, Urbánek, T., Oľga, B. & Gallagher, J. A.) 53–57 (Springer, 2015).
Imrich, R. et al. Radiological evolution of spinal disease in alkaptonuria and the effect of nitisinone. RMD Open 8, e002422 (2022).
Rovenský, J., Krátka, M. & Urbánek, T. in Alkaptonuria and Ochronosis (eds Rovenský, J., Urbánek, T., Oľga, B. & Gallagher, J. A.) 73–77 (Springer, 2015).
Hannoush, H. et al. Aortic stenosis and vascular calcifications in alkaptonuria. Mol. Genet. Metab. 105, 198–202 (2012).
Fincke, M. L. Inborn errors of metabolism. J. Am. Diet. Assoc. 46, 280–284 (1995).
Blachier, F. et al. Tolerable amounts of amino acids for human supplementation: summary and lessons from published peer-reviewed studies. Amino Acids 53, 1313–1328 (2021).
De Haas, V. et al. The success of dietary protein restriction in alkaptonuria patients is age-dependent. J. Inherit. Metab. Dis. 21, 791–798 (1998).
Arnoux, J. B. et al. Old treatments for new insights and strategies: proposed management in adults and children with alkaptonuria. J. Inherit. Metab. Dis. 38, 791–796 (2015).
Judd, S. et al. The nutritional status of people with alkaptonuria: an exploratory analysis suggests a protein/energy dilemma. JIMD Rep. 53, 45–60 (2020).
Morava, E., Kosztolányi, G., Engelke, U. F. H. & Wevers, R. A. Reversal of clinical symptoms and radiographic abnormalities with protein restriction and ascorbic acid in alkaptonuria. Ann. Clin. Biochem. 40, 108–111 (2003).
Ranganath, L. R. et al. Temporal adaptations in the phenylalanine/tyrosine pathway and related factors during nitisinone-induced tyrosinaemia in alkaptonuria. Mol. Genet. Metab. https://doi.org/10.1016/j.ymgme.2022.05.006 (2022).
Hughes, J. H. et al. Dietary restriction of tyrosine and phenylalanine lowers tyrosinemia associated with nitisinone therapy of alkaptonuria. J. Inherit. Metab. Dis. 43, 259–268 (2020).
Sealock, R. R., Galdston, M. & Steele, J. M. Administration of ascorbic acid to an alkaptonuric patient. Proc. Soc. Exp. Biol. Med. 1940 44, 580–583 (1940).
Lustberg, T. J., Schulman, J. D. & Seegmiller, J. E. Decreased binding of 14C-homogentisic acid induced by ascorbic acid in connective tissues of rats with experimental alcaptonuria. Nature 228, 770–771 (1970).
Wolff, J. A. et al. Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr. Res. 26, 140–144 (1989).
Lock, E. A. From weed killer to wonder drug. Adv. Exp. Med. Biol. 959, 175–185 (2017).
Lock, E. A. The discovery of the mode of action of nitisinone. Metabolites 12, 902 (2022). This monograph is a comprehensive review of the discovery of the mode of action of nitisinone and its use for the treatment of inborn errors of tyrosine metabolism.
Anikster, Y., Nyhan, W. L. & Gahl, W. A. NTBC and alkaptonuria. Am. J. Hum. Genet. 63, 920 (1998).
Suwannarat, P. et al. Use of nitisinone in patients with alkaptonuria. Metabolism 54, 719–728 (2005).
Introne, W. J. et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol. Genet. Metab. 103, 307–314 (2011).
Ranganath, L. R. et al. Suitability of nitisinone In alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid. Ann. Rheum. Dis. 75, 362–367 (2016).
European Medicines Agency. Assessment report: Orfadin. European Medicines Agency https://www.ema.europa.eu/en/documents/variation-report/orfadin-h-c-555-ii-0071-epar-assessment-report-variation_en.pdf (2020).
Sloboda, N. et al. Efficacy of low dose nitisinone in the management of alkaptonuria. Mol. Genet. Metab. 127, 184–190 (2019).
Spiga, O. et al. A new integrated and interactive tool applicable to inborn errors of metabolism: application to alkaptonuria. Comput. Biol. Med. 103, 1–7 (2018).
Bernini, A., Spiga, O. & Santucci, A. Structure-function relationship of homogentisate 1,2-dioxygenase: understanding the genotype-phenotype correlations in the rare genetic disease alkaptonuria. Curr. Protein Pept. Sci. 24, 380–392 (2023).
Spiga, O., Cicaloni, V., Bernini, A., Zatkova, A. & Santucci, A. ApreciseKUre: an approach of precision medicine in a rare disease. BMC Med. Inform. Decis. Mak. 17, 42 (2017).
Rossi, A. et al. AKUImg: a database of cartilage images of alkaptonuria patients. Comput. Biol. Med. 122, 103863 (2020).
Cicaloni, V. et al. Interactive alkaptonuria database: investigating clinical data to improve patient care in a rare disease. FASEB J. 33, 12696–12703 (2019).
Spiga, O. et al. Machine learning application for development of a data-driven predictive model able to investigate quality of life scores in a rare disease. Orphanet J. Rare Dis. 15, 46 (2020). The authors present a proof-of-principle study for the application of machine learning in AKU and rare diseases in general.
Spiga, O. et al. Machine learning application for patient stratification and phenotype/genotype investigation in a rare disease. Brief. Bioinform. 22, bbaa434 (2021).
Spiga, O. et al. Towards a precision medicine approach based on machine learning for tailoring medical treatment in alkaptonuria. Int. J. Mol. Sci. 22, 1187 (2021).
Langford, B. et al. Alkaptonuria severity score index revisited: analysing the AKUSSI and its subcomponent features. JIMD Rep. 41, 53–62 (2018).
Cant, H. E. O. et al. Improving the clinical accuracy and flexibility of the alkaptonuria severity score index. JIMD Rep. 63, 361–370 (2022).
Laxon, S., Ranganath, L. & Timmis, O. Living with alkaptonuria. BMJ 343, d5155 (2011). The authors offer a first-hand patient’s perspective potentially relevant for both patients and physicians.
Sestini, S. et al. Social and medical needs of rare metabolic patients: results from a MetabERN survey. Orphanet J. Rare Dis. 16, 336 (2021).
Kılavuz, S. et al. Demographic, phenotypic and genotypic features of alkaptonuria patients: a single centre experience. J. Pediatr. Res. 5, 7–11 (2018).
Akbaba, A. I., Ozgül, R. K. & Dursun, A. Presentation of 14 alkaptonuria patients from Turkey. J. Pediatr. Endocrinol. Metab. 33, 289–294 (2020).
Kisa, P. T. et al. Alkaptonuria in Turkey: clinical and molecular characteristics of 66 patients. Eur. J. Med. Genet. 64, 104197 (2021).
Ranganath, L. R. et al. Comparing nitisinone 2 mg and 10 mg in the treatment of alkaptonuria – an approach using statistical modelling. JIMD Rep. 2022 63, 80–92 (2022).
Khedr, M. et al. Nitisinone causes acquired tyrosinosis in alkaptonuria. J. Inherit. Metab. Dis. 43, 1014–1023 (2020).
Couce, M. L. et al. Evolution of tyrosinemia type 1 disease in patients treated with nitisinone in Spain. Medicine 98, e17303 (2019).
Barone, H. et al. Tyrosinemia type 1 and symptoms of ADHD: biochemical mechanisms and implications for treatment and prognosis. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 183, 95–105 (2020).
Van Vliet, K. et al. Emotional and behavioral problems, quality of life and metabolic control in NTBC-treated tyrosinemia type 1 patients. Orphanet J. Rare Dis. 14, 285 (2019).
Ellaway, C. J. et al. Outcome of tyrosinaemia type III. J. Inherit. Metab. Dis. 24, 824–832 (2001).
Laschi, M. et al. Inhibition of para-hydroxyphenylpyruvate dioxygenase by analogues of the herbicide nitisinone as a strategy to decrease homogentisic acid levels, the causative agent of alkaptonuria. ChemMedChem 11, 674–678 (2016).
Governa, P. et al. Survey on the recent advances in 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibition by diketone and triketone derivatives and congeneric compounds: structural analysis of HPPD/inhibitor complexes and structure-activity relationship considerations. J. Agric. Food Chem. 70, 6963–6981 (2022).
Santucci, A., Bernardini, G., Braconi, D., Petricci, E. & Manetti, F. 4-Hydroxyphenylpyruvate dioxygenase and its inhibition in plants and animals: small molecules as herbicides and agents for the treatment of human inherited diseases. J. Med. Chem. 60, 4101–4125 (2017).
Zaib, S. et al. Identification of potential inhibitors for the treatment of alkaptonuria using an integrated in silico computational strategy. Molecules 28, 2623 (2023).
Puurunen, M. K. et al. Safety and pharmacodynamics of an engineered E. coli Nissle for the treatment of phenylketonuria: a first-in-human phase 1/2a study. Nat. Metab. 3, 1125–1132 (2021).
Yadav, A. et al. Novel chemical scaffolds to inhibit the neutral amino acid transporter B0AT1 (SLC6A19), a potential target to treat metabolic diseases. Front. Pharmacol. 11, 140 (2020).
Bernini, A. et al. Toward a generalized computational workflow for exploiting transient pockets as new targets for small molecule stabilizers: application to the homogentisate 1,2-dioxygenase mutants at the base of rare disease alkaptonuria. Comput. Biol. Chem. 70, 133–141 (2017).
Bernini, A., Galderisi, S., Spiga, O., Amarabom, C. O. & Santucci, A. Transient pockets as mediators of gas molecules routes inside proteins: the case study of dioxygen pathway in homogentisate 1,2-dioxygenase and its implication in alkaptonuria development. Comput. Biol. Chem. 88, 107356 (2020).
Kobak, A. C., Oder, G., Kobak, Ş., Argin, M. & Inal, V. Ochronotic arthropathy: disappearance of alkaptonuria after liver transplantation for hepatitis B-related cirrhosis. J. Clin. Rheumatol. 11, 323–325 (2005).
Oishi, K., Arnon, R., Wasserstein, M. P. & Diaz, G. A. Liver transplantation for pediatric inherited metabolic disorders: considerations for indications, complications, and perioperative management. Pediatr. Transplant. 20, 756–769 (2016).
Gardin, A., Remih, K., Gonzales, E., Andersson, E. R. & Strnad, P. Modern therapeutic approaches to liver-related disorders. J. Hepatol. 76, 1392–1409 (2022).
Hughes, J. H. et al. Conditional targeting in mice reveals that hepatic homogentisate 1,2-dioxygenase activity is essential in reducing circulating homogentisic acid and for effective therapy in the genetic disease alkaptonuria. Hum. Mol. Genet. 28, 3928–3939 (2019).
Lequeue, S. et al. An AAV-mediated liver-directed gene therapy metabolically corrects alkaptonuria in an Hgd deficient mouse model [abstract 941]. J. Inher Metab. Dis. 46 (Suppl. 1), 284 (2023).
Hegedus, Z. L. & Nayak, U. Homogentisic acid and structurally related compounds as intermediates in plasma soluble melanin formation and in tissue toxicities. Arch. Physiol. Biochem. 102, 175–181 (1994).
Tinti, L., Spreafico, A., Chellini, F., Galeazzi, M. & Santucci, A. A novel ex vivo organotypic culture model of alkaptonuria-ochronosis. Clin. Exp. Rheumatol. 29, 693–696 (2011).
Taylor, A. M. et al. Ultrastructural examination of tissue in a patient with alkaptonuric arthropathy reveals a distinct pattern of binding of ochronotic pigment. Rheumatology 49, 1412–1414 (2010).
Angeles, A. P., Badger, R., Gruber, H. E. & Seegmiller, J. E. Chondrocyte growth inhibition induced by homogentisic acid and its partial prevention with ascorbic acid. J. Rheumatol. 16, 512–517 (1989).
Galderisi, S. et al. Homogentisic acid induces cytoskeleton and extracellular matrix alteration in alkaptonuric cartilage. J. Cell. Physiol. 236, 6011–6024 (2021).
Schiavone, M. L. et al. Homogentisic acid affects human osteoblastic functionality by oxidative stress and alteration of the Wnt/β-catenin signaling pathway. J. Cell. Physiol. 235, 6808–6816 (2020).
Tinti, L. et al. Development of an in vitro model to investigate joint ochronosis in alkaptonuria. Rheumatology 50, 271–277 (2011).
Mitri, E. et al. A new light on alkaptonuria: a Fourier-transform infrared microscopy (FTIRM) and low energy X-ray fluorescence (LEXRF) microscopy correlative study on a rare disease. Biochim. Biophys. Acta Gen. Subj. 1861, 1000–1008 (2017).
Bernardini, G. et al. Homogentisic acid induces morphological and mechanical aberration of ochronotic cartilage in alkaptonuria. J. Cell. Physiol. 234, 6696–6708 (2019).
Schiavone, M. L. et al. Mechanisms involved in the unbalanced redox homeostasis in osteoblastic cellular model of alkaptonuria. Arch. Biochem. Biophys. 690, 108416 (2020).
Moran, T. J. & Yunis, E. J. Studies on ochronosis: 2. Effects of injection of homogentisic acid and ochronotic pigment in experimental animals. Am. J. Pathol. 40, 359–369 (1962).
Blivaiss, B. B., Rosenberg, E. F., Kutuzov, H. & Stoner, R. Experimental ochronosis. Induction in rats by long-term feeding with L-tyrosine. Arch. Pathol. 82, 45–53 (1966).
Fayette, M. A. et al. Biochemical and molecular confirmation of alkaptonuria in a Sumatran orangutan (Pongo abelii). Mol. Genet. Metab. 139, 1076288 (2023).
Montagutelli, X. et al. aku, a mutation of the mouse homologous to human alkaptonuria, maps to chromosome 16. Genomics 19, 9–11 (1994).
Taylor, A. M. et al. Ochronosis in a murine model of alkaptonuria is synonymous to that in the human condition. Osteoarthr. Cartil. 20, 880–886 (2012).
Preston, A. J. et al. Ochronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone. Ann. Rheum. Dis. 73, 284–289 (2014).
Hughes, J. H., Bou-Gharios, G., Ranganath, L. R. & Gallagher, J. A. The contribution of mouse models in the rare disease alkaptonuria. Drug. Discov. Today Dis. Model. 31, 37–43 (2020).
Acknowledgements
A.S. acknowledges funding from Ministero dell’Università e della Ricerca (MUR) for her research (grants P2022RYR5W-PRIN 2022 PNRR and Tuscany Health Ecosystem (THE)-PNRR). W.J.I. is supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. The authors thank the anonymous patient for their contribution in Box 3.
Author information
Authors and Affiliations
Contributions
Introduction (G.B. and D.B.); Epidemiology (W.J.I. and N.S.); Mechanisms/pathophysiology (A.Z., G.B., D.B., A.S., L.R.R. and M.J.K.); Diagnosis, screening and prevention (M.J.K. and W.J.I.); Management (L.R.R., W.J.I., J.A.G., O.S. and M.G.); Quality of life (L.R.R. and N.S.); Outlook (G.B., D.B., A.S. and A.Z.); Overview of the Primer (G.B.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks F. Maillot and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Informed consent
The authors affirm that patient participants provided informed consent for publication of their experiences.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bernardini, G., Braconi, D., Zatkova, A. et al. Alkaptonuria. Nat Rev Dis Primers 10, 16 (2024). https://doi.org/10.1038/s41572-024-00498-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-024-00498-x