Abstract
Alkaptonuria is characterized by the accumulation of homogentisic acid (HGA), part of which is excreted in the urine but the excess HGA forms a dark brown ochronotic pigment that deposits in the connective tissue (ochronosis), eventually leading to early-onset severe arthropathy. We analyzed a cohort of 48 Russian AKU families by sequencing all 14 exons (including flanking intronic sequences) of the homogentisate 1,2-dioxygenase gene (HGD) and Multiplex Ligation-dependent Probe Amplification (MLPA) analysis. Nine novel likely pathogenic HGD variants were identified, which have not been reported previously in any other country. Recently, Bychkov et al. [1] reported on the variant spectrum in another cohort of 49 Russian AKU patients. Here we summarize complete data from both cohorts that include 82 Russian AKU families. Taken together, 31 different HGD variants were found in these patients, of which 14 are novel and found only in Russia. The most common variant was c.481G>A (p.(Gly161Arg)), present in almost 54% of all AKU alleles.
Similar content being viewed by others
Results and discussion
Alkaptonuria is characterized by the accumulation of homogentisic acid (HGA), of which large amounts are excreted in the urine [2]. Excess HGA forms a dark brown ochronotic pigment that deposits in the connective tissue (ochronosis), mainly in the skin, sclera, spine, and large-joint cartilage, as well as in the heart valves, where it causes aortic stenosis. Starting in their early 30s, Alkaptonuria (AKU) patients usually suffer from early-onset severe arthropathy (described in detail in ref. [3]). This disease is caused by the variants within a gene coding for homogentisate 1,2-dioxygenase (HGD) [4]. Worldwide, DNA sequencing has been performed in about 720 AKU patients and 249 different HGD variants have been reported, as summarized in the HGD mutation database (http://hgddatabase.cvtisr.sk/, June 2021).
To our knowledge, until recently there have been no reports on the AKU variant spectrum in Russia. We analyzed 48 Russian AKU families (Supplementary Table 1) by sequencing all 14 exons of the HGD gene (including flanking intronic sequences) and by Multiplex Ligation-dependent Probe Amplification (MLPA) analysis (as describe before [5]), and our results were submitted to the HGD mutation database [6]. We found nine novel HGD variants, most likely AKU-causing, which have not been reported previously in any other country. Early this year, Bychkov et al. [1] reported on their study in 49 Russian AKU families. In 37 patients, they identified homozygous or compound heterozygous variants within the HGD gene responsible for AKU. In their cohort, the variant spectrum comprised 12 missense variants, 3 splicing, and 2 small indels, of which 9 were novel, and the most common variant was c.481G>A (p.(Gly161Arg)), present in almost 73% of all AKU alleles [1].
After communication with the authors, we discovered that there was a partial overlap of two cohorts: 15 of their patients had already been analyzed by us and submitted to the database. There was also a partial overlap in novel variants: four of nine novel variants observed in our study (c.131T>C (p.(Leu44Pro)), c.127C>G (p.(Gln43Glu)), c. (p.(Ile179Ser)), and c.753C>T (p.(Gly251Gly))) were reported in detail by Bychkov et al. [1] as well.
We summarize in Table 1 complete data on the variant spectrum observed in all 82 Russian AKU families reported by two groups. In summary, 31 different HGD variants were found in Russia so far, of which 14 are novel and found only in this country. However, there are still 12 AKU patients from the cohort of Bychkov et al. [1], in which the second variant has not been identified; thus, even more variants can be found, indicating that in Russia AKU shows rather high allelic heterogeneity.
We can confirm that the c.481G>A (p.(Gly161Arg)) missense variant in exon 8 is the most frequent one in Russia but it is present on 53.7% out of 164 AKU chromosomes, not in 70% as observed in a previous smaller cohort [1]. This variant is rather frequent also in Slovakia, where it represents 68% AKU alleles (Table 1, the HGD mutation database).
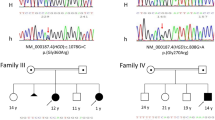
Table 2 summarizes five additional novel HGD variants found by us and their characterization according to ACMG guidelines [7]. Three of them are missense changes: c.174A>T (p.(Arg58Ser)), c.184T>A (p.(Tyr62Asn)), and c.791A>G (p.(Asn264Ser)). These amino acid substitutions were predicted to cause Protomer destablization by mCSM tool that has been recently shown to be the most effective in predicting the effect of HGD missense variants on the HGD protein function in AKU [5].
Another novel variant is c.470-1G>A, a substitution that abolishes donor splice site in intron 7 and most likely leads into exon 8 skipping (c.470_549del), thus to a frameshift and premature stop codon (p.(Val157Glufs*11), Table 1).
The last novel variant is an intronic variant c.1188 + 8T>A in intron 13 that is predicted to diminish a constitutive donor splicing site score and to increase a score of the cryptic donor site. During splicing event, an inclusion of 4 nucleotides of intron 13 into the HGD transcript can occur (c.1189_1190insTAAG), thus to cause a frameshift and preliminary stop codon (p.(Ala397Valfs*6), Table 1). In this patient, no other variants have been identified in any of the 14 exons of the HGD gene by sequencing and MLPA, and no RNA was available for the analysis.
Silent variant c.753C>T (p.(Gly251Gly)) found in 6.1% of Russian AKU chromosomes (Table 1) was shown to affect splicing [1]. In our cohort, it was present in seven AKU chromosomes/families. Interestingly, in one of these families (AKU_DB_287), there were three patients/sibs who carried each three AKU variants: c.753C>T (p.(Gly251Gly)) in one copy and c.16-1G>A (ivs1-1G>A) in two copies. All three patients carried two additional silent variants as well: c.372C>T (p.(Asp124Asp), exon 6, rs140977117, frequency 0.0232) and c.1191A>C (p.(Ala397Ala), exon 14, rs137923025, frequency 0.0231) (Supplementary Table 1), which are both listed in gnomAD Exomes and gnomAD Genomes databases as benign polymorphisms. They have one healthy sister who does not carry any of the mentioned variants. We were not able to follow the segregations of the variants in the family, as DNA of the parents was not available. The same silent variants were present also in the patient AKU_DB_333, in combination with different variants (c.481G>A (p.(Gly161Arg)) and c.536T>G (p.(Ile179Ser))), indicating that they are not associated with specific AKU allele/haplotype (Supplementary Table 1).
AKU shows a rather high phenotype heterogeneity, even within one family, and it is believed that disease severity is highly dependent upon the total load of non-excreted HGA throughout the life and increases with the patient’s age [3]. It has been shown by us and others that HGD proteins carrying AKU variants show reduced activity when compared to the wild-type HGD, ranging from <1% up to 34% of wild type [5, 8]. In our recent genotype–phenotype correlation study, we also showed that there was a small but statistically significant difference in urinary HGA excretion (corrected for dietary protein intake) in patients who carried variants with 1% residual HGD activity (such as c.481G>A (p.(Gly161Arg)) frequent in Russia) compared to those with >30% residual HGD activity (namely c.1102A>G (p.(Met368Val)) and c.365C>T (p.(Ala122Val))) [5]. However, serum levels or absolute urinary excretion of HGA showed no difference and there were also no differences in the tested AKU symptoms [5]. This indicates that there is no direct effect of HGD variant type on the variability of the AKU phenotype. Most likely, AKU variability can depend on the diet and the effectivity of renal function, which is crucial to the elimination of accumulated HGA from the patient’s body [5, 9]. It is also possible/likely that the differences in tissue characteristics between subjects play also a part in the development/progression/worsening of ochronosis. High variability in the levels of HGA (u-HGA (mmol/24 h) measured by gas chromatography and mass spectrometric analysis) was observed also in our cohort, even among the patients carrying the same mutations (Supplementary Table 1).
Distribution of different HGD variants in different populations does not reflect the severity of AKU; still, it is interesting from population genetics point of view. As can be seen in Table 1, several variants found in Russia are found with higher prevalence also in other countries, such as Slovakia (c.481G>A, c.16-1G>A, c.342+1G>A, c.457dupG, c.688C>T, c.808G>A), Turkey (c.175delA, c.808G>A, c.1007-2A>T), or in USA and Germany (c.1102A>G) (Table 1). It might be interesting to investigate the origin of these AKU alleles using haplotype analysis but it is beyond the focus of this report.
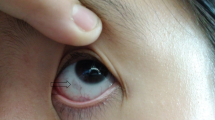
Until recently, painkillers and joint replacement surgery in advanced stages have been the only palliative treatments available for patients suffering from AKU [10]. Based on the recently published results of the Suitability Of Nitisinone In Alkaptonuria 2 clinical study [11], the European Medicines Agency authorized Swedish Orphan BIovitrum to use their product Orfadin® (Nitisinone) in AKU; on 22 October 2020, the European Commission approved it for the treatment of adult patients with AKU. Currently, nitisinone represents the only promising HGA-lowering therapy. Occasionally, several young individuals are reported with early signs of AKU, usually ocular ochronosis, but also early arthritis has been observed in 15-year-old patients [12,13,14]. As ochronosis might start at an early age, nitisinone should be administrated early as well; however, a pediatric study is needed to assess its safety and the earliest age at which it can be taken. Our cohorts include several Russian child/young age patients; they might be good candidates for such a study in the future.
Data availability
The datasets/sequences generated and analysed during the current study are available from the corresponding author on reasonable request. All patients and their variants identified during the current study were inserted in the HGD mutation database (http://hgddatabase.cvtisr.sk/) and can be identified based on the specific allele/family codes indicated in Supplementary Table 1. GnomAD database: gnomAD_v2.1.1_ENSG00000113924_(2021.03.24).
References
Bychkov I, Kamenets E, Kurkina M, Rychkov G, Ilyushkina A, Filatova A, et al. Alkaptonuria in Russia: mutational spectrum and novel variants. Eur J Med Genet. 2021;64:104165.
Zannoni VG, Lomtevas N, Goldfinger S. Oxidation of homogentisic acid to ochronotic pigment in connective tissue. Biochim Biophys Acta. 1969;177:94–105.
Zatkova A, Ranganath L, Kadasi L. Alkaptonuria: current perspectives. Appl Clin Genet. 2020;13:37–47.
Granadino B, Beltrán-Valero de Bernabé D, Fernández-Cañón JM, Peñalva MA, Rodríguez de Córdoba S. The human homogentisate 1,2-dioxygenase (HGO) gene. Genomics. 1997;43:115–22.
Ascher DB, Spiga O, Sekelska M, Pires DEV, Bernini A, Tiezzi M, et al. Homogentisate 1,2-dioxygenase (HGD) gene variants, their analysis and genotype-phenotype correlations in the largest cohort of patients with AKU. Eur J Hum Genet. 2019;27:888–902.
Zatkova A, Sedlackova T, Radvansky J, Polakova H, Nemethova M, Aquaron R, et al. Identification of 11 novel homogentisate 1,2 dioxygenase variants in alkaptonuria patients and establishment of a novel LOVD-based HGD mutation database. JIMD Rep. 2012;4:55–65.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Rodriguez JM, Timm DE, Titus GP, Beltran-Valero De Bernabe D, Criado O, Mueller HA, et al. Structural and functional analysis of mutations in alkaptonuria. Hum Mol Genet. 2000;9:2341–50.
Ranganath LR, Milan AM, Hughes AT, Khedr M, Davison AS, Shweihdi E, et al. Homogentisic acid is not only eliminated by glomerular filtration and tubular secretion but also produced in the kidney in alkaptonuria. J Inherit Metab Dis. 2020;43:737–47.
Ranganath LR, Jarvis JC, Gallagher JA. Recent advances in management of alkaptonuria (invited review; best practice article). J Clin Pathol. 2013;66:367–73.
Ranganath LR, Psarelli EE, Arnoux JB, Braconi D, Briggs M, Broijersen A, et al. Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): an international, multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8:762–72.
Akbaba AI, Ozgul RK, Dursun A. Presentation of 14 alkaptonuria patients from Turkey. J Pediatr Endocrinol Metab. 2020;33:289–94.
Gucev Z, Slaveska N, Laban N, Tasic V, Danilovski D, Popjordanova N, et al. Early-onset ocular ochronosis in a girl with alkaptonuria (AKU) and a novel mutation in homogentisate 1,2-dioxygenase (HGD). Prilozi. 2011;32:305–11.
Kisa PT, Gunduz M, Dorum S, Uzun OU, Cakar NE, Yildirim GK, et al. Alkaptonuria in Turkey: Clinical and molecular characteristics of 66 patients. Eur J Med Genet. 2021;64:104197.
den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12.
den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37:564–9.
Acknowledgements
This work was supported by Slovak grant agency VEGA (grant number 02/0040/20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethic committee at V.A. Nasonova Research Institute of Rheumatology approved the study (the approval number N18-2019) and all patients provided written informed consent prior to inclusion. All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Soltysova, A., Kuzin, A., Samarkina, E. et al. Alkaptonuria in Russia. Eur J Hum Genet 30, 237–242 (2022). https://doi.org/10.1038/s41431-021-00955-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00955-1
This article is cited by
-
Alkaptonuria
Nature Reviews Disease Primers (2024)
-
A new system for variant classification?
European Journal of Human Genetics (2022)