Abstract
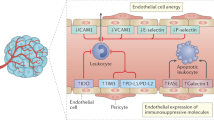
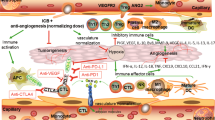
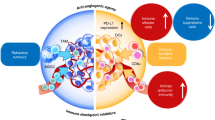
Antiangiogenic agents, generally antibodies or tyrosine-kinase inhibitors that target the VEGF–VEGFR pathway, are currently among the few combination partners clinically proven to improve the efficacy of immune-checkpoint inhibitors (ICIs). This benefit has been demonstrated in pivotal phase III trials across different cancer types, some with practice-changing results; however, numerous phase III trials have also had negative results. The rationale for using antiangiogenic drugs as partners for ICIs relies primarily on blocking the multiple immunosuppressive effects of VEGF and inducing several different vascular-modulating effects that can stimulate immunity, such as vascular normalization leading to increased intratumoural blood perfusion and flow, and inhibition of pro-apoptotic effects of endothelial cells on T cells, among others. Conversely, VEGF blockade can also cause changes that suppress antitumour immunity, such as increased tumour hypoxia, and reduced intratumoural ingress of co-administered ICIs. As a result, the net clinical benefits from antiangiogenic–ICI combinations will be determined by the balance between the opposing effects of VEGF signalling and its inhibition on the antitumour immune response. In this Perspective, we summarize the results from the currently completed phase III trials evaluating antiangiogenic agent–ICI combinations. We also discuss strategies to improve the efficacy of these combinations, focusing on aspects that include the deleterious functions of VEGF–VEGFR inhibition on antitumour immunity, vessel co-option as a driver of non-angiogenic tumour growth, clinical trial design, or the rationale for drug selection, dosing and scheduling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249 (2021).
Blasutig, I. M. et al. The phoenix rises: the rebirth of cancer immunotherapy. Clin. Chem. 63, 1190–1195 (2017).
Abi-Aad, S. J., Zouein, J., Chartouni, A., Naim, N. & Kourie, H. R. Simultaneous inhibition of PD-1 and LAG-3: the future of immunotherapy? Immunotherapy 15, 611–618 (2023).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386, 24–34 (2022).
Syn, N. L., Teng, M. W. L., Mok, T. S. K. & Soo, R. A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 18, e731–e741 (2017).
Jenkins, R. W., Barbie, D. A. & Flaherty, K. T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 118, 9–16 (2018).
Gide, T. N., Wilmott, J. S., Scolyer, R. A. & Long, G. V. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin. Cancer Res. 24, 1260–1270 (2018).
Andrews, A. Treating with checkpoint inhibitors – figure $1 million per patient. Am. Health Drug. Benefits 8, 9 (2015).
Baxi, S. et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 360, k793 (2018).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Carreau, N. A. & Pavlick, A. C. Nivolumab and ipilimumab: immunotherapy for treatment of malignant melanoma. Future Oncol. 15, 349–358 (2019).
Nikoo, M. et al. Nivolumab plus ipilimumab combination therapy in cancer: current evidence to date. Int. Immunopharmacol. 117, 109881 (2023).
Baas, P. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397, 375–386 (2021).
Wang, C. et al. The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis. J. Cell Physiol. 235, 4913–4927 (2020).
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L. & Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17, 725–741 (2020).
Cao, Y., Langer, R. & Ferrara, N. Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug. Discov. 22, 476–495 (2023).
Schmidt, E. V. Developing combination strategies using PD-1 checkpoint inhibitors to treat cancer. Semin. Immunopathol. 41, 21–30 (2019).
Motz, G. T. & Coukos, G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat. Rev. Immunol. 11, 702–711 (2011).
Heijmen, L. et al. Monitoring hypoxia and vasculature during bevacizumab treatment in a murine colorectal cancer model. Contrast Media Mol. imaging 9, 237–245 (2014).
Franco, M. et al. Targeted anti-VEGFR-2 therapy leads to short and long term impairment of vascular function and increases in tumor hypoxia. Cancer Res. 66, 3639–3648 (2006).
Chang, W. H. & Lai, A. G. The hypoxic tumour microenvironment: a safe haven for immunosuppressive cells and a therapeutic barrier to overcome. Cancer Lett. 487, 34–44 (2020).
Kopecka, J. et al. Hypoxia as a driver of resistance to immunotherapy. Drug. Resist. Updat. 59, 100787 (2021).
Wang, B. et al. Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy. J. Exp. Clin. Cancer Res. 40, 24 (2021).
Khan, K. A. & Kerbel, R. S. Improving immunotherapy outcomes with antiangiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 15, 310–324 (2018).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T-cells in tumors. J. Exp. Med. 212, 139–148 (2015).
Kim, C. G. et al. VEGF-A drives TOX-dependent T-cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci. Immunol. 4, eaay0555 (2019).
Huinen, Z. R., Huijbers, E. J. M., van Beijnum, J. R., Nowak-Sliwinska, P. & Griffioen, A. W. Anti-angiogenic agents – overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 18, 527–540 (2021).
Huijbers, E. J. M. et al. Tumors resurrect an embryonic vascular program to escape immunity. Sci. Immunol. 7, eabm6388 (2022).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Allen, E. et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak9679 (2017).
Jain, R. K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Baluk, P., Hashizume, H. & McDonald, D. M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 15, 102–111 (2005).
Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001).
Singleton, D. C., Macann, A. & Wilson, W. R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 18, 751–772 (2021).
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012).
Goel, S. et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121 (2011).
Lin, Y. Y. et al. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: implication for the future of immunotherapy. Semin. Liver Dis. 38, 379–388 (2018).
Wietecha, M. S., Cerny, W. L. & DiPietro, L. A. Mechanisms of vessel regression: toward an understanding of the resolution of angiogenesis. Curr. Top. Microbiol. Immunol. 367, 3–32 (2013).
Kikuchi, H. et al. Increased CD8+ T-cell infiltration and efficacy for multikinase inhibitors after PD-1 blockade in hepatocellular carcinoma. J. Natl Cancer Inst. 114, 1301–1305 (2022).
Manning, E. A. et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin. Cancer Res. 13, 3951–3959 (2007).
Martinez-Usatorre, A. et al. Overcoming microenvironmental resistance to PD-1 blockade in genetically engineered lung cancer models. Sci. Transl. Med. 13, eabd1616 (2021).
Hoffmann, E. et al. Vascular response patterns to targeted therapies in murine breast cancer models with divergent degrees of malignancy. Breast Cancer Res. 25, 56 (2023).
Sorensen, A. G. et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 72, 402–407 (2012).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004).
Batchelor, T. T. et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl Acad. Sci. USA 110, 19059–19064 (2013).
Lee, E. et al. An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone. Exp. Mol. Med. 55, 470–484 (2023).
Khan, K. A., Wu, F. T., Cruz-Munoz, W. & Kerbel, R. S. Ang2 inhibitors and Tie2 activators: potential therapeutics in perioperative treatment of early stage cancer. EMBO Mol. Med. 13, e08253 (2021).
Schmittnaegel, M. et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 9, eaak9670 (2017).
Gengenbacher, N. et al. Timed Ang2-targeted therapy identifies the angiopoietin–tie pathway as key regulator of fatal lymphogenous metastasis. Cancer Discov. 11, 424–445 (2021).
Kim, J. et al. Tie2 activation promotes choriocapillary regeneration for alleviating neovascular age-related macular degeneration. Sci. Adv. 5, eaau6732 (2019).
Park, H. R. et al. Angiopoietin-2-dependent spatial vascular destabilization promotes T-cell exclusion and limits immunotherapy in melanoma. Cancer Res. 83, 1968–1983 (2023).
Zhao, Y. et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. EClinicalMedicine 62, 102106 (2023).
Tzuri, N. et al. Developing a dual VEGF/PDL1 inhibitor based on high-affinity scFv heterodimers as an anti-cancer therapeutic strategy. Sci. Rep. 13, 11923 (2023).
Hassanzadeh Eskafi, A. et al. Investigation of the therapeutic potential of recombinant bispecific bivalent anti-PD-L1/VEGF nanobody in inhibition of angiogenesis. Immunopharmacol. Immunotoxicol. 45, 197–202 (2023).
Lai, X. & Friedman, A. How to schedule VEGF and PD-1 inhibitors in combination cancer therapy? BMC Syst. Biol. 13, 30 (2019).
Ferrara, N., Hillan, K. J., Gerber, H. P. & Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug. Discov. 3, 391–400 (2004).
Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021).
Zandberg, D. P. et al. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J. Immunother. Cancer 9, e002088 (2021).
Rapisarda, A. et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol. Cancer Ther. 8, 1867–1877 (2009).
Schito, L. et al. Metronomic chemotherapy offsets HIFα induction upon maximum-tolerated dose in metastatic cancers. EMBO Mol. Med. 12, e11416 (2020).
Conley, S. J. et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc. Natl Acad. Sci. USA 109, 2784–2789 (2012).
Riesterer, O. et al. Ionizing radiation antagonizes tumor hypoxia induced by antiangiogenic treatment. Clin. Cancer Res. 12, 3518–3524 (2006).
Rapisarda, A. & Melillo, G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat. Rev. Clin. Oncol. 9, 378–390 (2012).
Matsumoto, S. et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 71, 6350–6359 (2011).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Zhang, Z. et al. “γδT-cell-IL17A-neutrophil” axis drives immunosuppression and confers breast cancer resistance to high-dose anti-VEGFR2 therapy. Front. Immunol. 12, 699478 (2021).
Shigeta, K. et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 71, 1247–1261 (2020).
Ma, J. & Waxman, D. J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 7, 3670–3684 (2008).
Ou, D. L. et al. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J. Immunother. Cancer 9, e001657 (2021).
Abbott, B. Cancer doctors rethink aggressive treatments: cervical, rectal cancer patients responded as well to less invasive care, studies show. The Wall Street Journal www.wsj.com/articles/for-some-cancers-less-treatment-is-a-better-bet-ed94d8ef (2023).
Kerbel, R. S. & Andre, N. Commentary: adjuvant metronomic chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Lancet 398, 278–279 (2021).
Chen, Y. P. et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet 398, 303–313 (2021).
Wang, X. et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA 325, 50–58 (2021).
Bisogno, G. et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 20, 1566–1575 (2019).
Patil, V. M. et al. Low-dose immunotherapy in head and neck cancer: a randomized study. J. Clin. Oncol. 41, 222–232 (2023).
Mitchell, A. P. & Goldstein, D. A. Cost savings and increased access with ultra-low-dose immunotherapy. J. Clin. Oncol. 41, 170–173 (2022).
Liao, M. Z. et al. Model-informed therapeutic dose optimization strategies for antibody-drug conjugates in oncology: what can we learn from US Food and Drug Administration-approved antibody–drug conjugates? Clin. Pharmacol. Ther. 110, 1216–1230 (2021).
Ueda, S., Saeki, T., Osaki, A., Yamane, T. & Kuji, I. Bevacizumab induces acute hypoxia and cancer progression in patients with refractory breast cancer: multimodal functional imaging and multiplex cytokine analysis. Clin. Cancer Res. 23, 5769–5778 (2017).
Arjaans, M. et al. VEGF pathway targeting agents, vessel normalization and tumor drug uptake: from bench to bedside. Oncotarget 7, 21247–21258 (2016).
Heskamp, S. et al. Bevacizumab reduces tumor targeting of antiepidermal growth factor and anti-insulin-like growth factor 1 receptor antibodies. Int. J. Cancer 133, 307–314 (2013).
Pastuskovas, C. V. et al. Effects of anti-VEGF on pharmacokinetics, biodistribution, and tumor penetration of trastuzumab in a preclinical breast cancer model. Mol. Cancer Ther. 11, 752–762 (2012).
Dobosz, M., Ntziachristos, V., Scheuer, W. & Strobel, S. Multispectral fluorescence ultramicroscopy: three-dimensional visualization and automatic quantification of tumor morphology, drug penetration, and antiangiogenic treatment response. Neoplasia 16, 1–13 (2014).
Arjaans, M. et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 73, 3347–3355 (2013).
Thomas, V. A. & Balthasar, J. P. Sorafenib decreases tumor exposure to an anti-carcinoembryonic antigen monoclonal antibody in a mouse model of colorectal cancer. AAPS J. 18, 923–932 (2016).
Chung, T. K., Warram, J., Day, K. E., Hartman, Y. & Rosenthal, E. L. Time-dependent pretreatment with bevacuzimab increases tumor specific uptake of cetuximab in preclinical oral cavity cancer studies. Cancer Biol. Ther. 16, 790–798 (2015).
Goldstein, D. A. & Ratain, M. J. Alternative dosing regimens for atezolizumab: right dose, wrong frequency. Cancer Chemother. Pharmacol. 84, 1153–1155 (2019).
Deng, R. et al. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. mAbs 8, 593–603 (2016).
Paulsen, E. E. et al. Impact of microvessel patterns and immune status in NSCLC: a non-angiogenic vasculature is an independent negative prognostic factor in lung adenocarcinoma. Front. Oncol. 13, 1157461 (2023).
Hendrix, M. J., Seftor, E. A., Hess, A. R. & Seftor, R. E. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat. Rev. Cancer 3, 411–421 (2003).
López-Camarillo, C., Ruiz-García, E., Starling, N. & Marchat, L. A. Editorial: neovascularization, angiogenesis and vasculogenic mimicry in cancer. Front. Oncol. 10, 1140 (2020).
Ali, Z. et al. Intussusceptive vascular remodeling precedes pathological neovascularization. Arterioscler. Thromb. Vasc. Biol. 39, 1402–1418 (2019).
Pezzella, F. et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am. J. Pathol. 151, 1417–1423 (1997).
Kuczynski, E. A., Vermeulen, P. B., Pezzella, F., Kerbel, R. S. & Reynolds, A. R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 16, 469–493 (2019).
Bridgeman, V. L. et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J. Pathol. 241, 362–374 (2016).
Cuypers, A., Truong, A. K., Becker, L. M., Saavedra-García, P. & Carmeliet, P. Tumor vessel co-option: the past & the future. Front. Oncol. 12, 965277 (2022).
Donnem, T. et al. Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer 18, 323–336 (2018).
Vermeulen, P. B. et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol. 195, 336–342 (2001).
Kusters, B. et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 62, 341–345 (2002).
Ribatti, D. & Pezzella, F. Vascular co-option and other alternative modalities of growth of tumor vasculature in glioblastoma. Front. Oncol. 12, 874554 (2022).
van Dam, P. J. et al. Histopathological growth patterns as a candidate biomarker for immunomodulatory therapy. Semin. Cancer Biol. 52, 86–93 (2018).
Höppener, D. J. et al. Enrichment of the tumour immune microenvironment in patients with desmoplastic colorectal liver metastasis. Br. J. Cancer 123, 196–206 (2020).
Frentzas, S. et al. Vessel co-option mediates resistance to antiangiogenic therapy in liver metastases. Nat. Med. 22, 1294–1302 (2016).
Guerin, E., Man, S., Xu, P. & Kerbel, R. S. A model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res. 73, 2743–2748 (2013).
Cuypers, A. et al. Generation of vessel co-option lung metastases mouse models for single-cell isolation of metastases-derived cells and endothelial cells. Star. Protoc. 3, 101691 (2022).
Teuwen, L. A. et al. Tumor vessel co-option probed by single-cell analysis. Cell Rep. 35, 109253 (2021).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Motzer, R. et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384, 1289–1300 (2021).
Choueiri, T. K. et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 24, 228–238 (2023).
Choueiri, T. K. et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 384, 829–841 (2021).
Rini, B. I. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393, 2404–2415 (2019).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Ren, Z. et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 22, 977–990 (2021).
Qin, S. et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet 402, 1133–1146 (2023).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Tomita, Y. et al. Efficacy and safety of avelumab plus axitinib in elderly patients with advanced renal cell carcinoma: extended follow-up results from JAVELIN Renal 101. ESMO open. 7, 100450 (2022).
Kelley, R. K. et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 23, 995–1008 (2022).
Finn, R. S. et al. Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC) [abstract LBA34]. Ann. Oncol. 33, S1401 (2022).
Moore, K. N. et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. 39, 1842–1855 (2021).
Merck. Merck and Eisai provide update on two phase 3 trials evaluating KEYTRUDA® (pembrolizumab) plus LENVIMA® (lenvatinib) in patients with certain types of metastatic non-small cell lung cancer. Merck www.merck.com/news/merck-and-eisai-provide-update-on-two-phase-3-trials-evaluating-keytruda-pembrolizumab-plus-lenvima-lenvatinib-in-patients-with-certain-types-of-metastatic-non-small-cell-lung-cancer/ (2023).
Yang, J. C. H. et al. Pembrolizumab (Pembro) with or without lenvatinib (Lenva) in first-line metastatic NSCLC with PD-L1 TPS≥1% (LEAP-007): a phase III, randomized, double-blind study [abstract 120O]. Ann. Oncol. 32, S1429–S1430 (2021).
Loriot, Y. et al. First-line pembrolizumab (pembro) with or without lenvatinib (lenva) in patients with advanced urothelial carcinoma (LEAP-011): a phase 3, randomized, double-blind study [abstract]. J. Clin. Oncol. 40 (Suppl. 6), 432 (2022).
Merck. Merck and Eisai provide update on phase 3 LEAP-010 trial evaluating KEYTRUDA® (pembrolizumab) plus LENVIMA® (lenvatinib) in patients with certain types of recurrent or metastatic head and neck squamous cell carcinoma. Merck www.merck.com/news/merck-and-eisai-provide-update-on-phase-3-leap-010-trial-evaluating-keytruda-pembrolizumab-plus-lenvima-lenvatinib-in-patients-with-certain-types-of-recurrent-or-metastatic-head-and-ne/ (2023).
Makker, V. et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 386, 437–448 (2022).
Merck. Merck and Eisai provide update on phase 3 LEAP-001 trial evaluating KEYTRUDA (pembrolizumab) plus LENVIMA (lenvatinib) as first-line treatment for patients with advanced or recurrent endometrial carcinoma. Merck www.merck.com/news/merck-and-eisai-provide-update-on-phase-3-leap-001-trial-evaluating-pembrolizumab-plus-lenvima-lenvatinib-as-first-line-treatment-for-patients-with-advanced-or-recurrent-endometrial-carcinom/ (2023).
Ipsen. Exelixis and Ipsen announce positive results from phase 3 CONTACT-02 pivotal trial evaluating cabozantinib in combination with atezolizumab in metastatic castration-resistant prostate cancer. Ipsen www.ipsen.com/press-releases/exelixis-and-ipsen-announce-positive-results-from-phase-3-contact-02-pivotal-trial-evaluating-cabozantinib-in-combination-with-atezolizumab-in-metastatic-castration-resistant-prostate-cancer/ (2023).
Fojo, T. & Grady, C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J. Natl Cancer Inst. 101, 1044–1048 (2009).
Anagnostou, V. et al. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin. Cancer Res. 23, 4959–4969 (2017).
Tran, T. T. et al. Lenvatinib or anti-VEGF in combination with anti-PD-1 differentially augments antitumor activity in melanoma. JCI insight 8, e157347 (2023).
McDermott, D. F. et al. Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J. Clin. Oncol. 39, 1020–1028 (2021).
Verset, G. et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: data from the open-label, phase II KEYNOTE-224 trial. Clin. Cancer Res. 28, 2547–2554 (2022).
Kurtz, J. E. et al. Atezolizumab combined with bevacizumab and platinum-based therapy for platinum-sensitive ovarian cancer: placebo-controlled randomized phase III ATALANTE/ENGOT-ov29 trial. J. Clin. Oncol. 41, Jco2300529 (2023).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378, 2288–2301 (2018).
West, H. J. et al. Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: subgroup results from the phase III IMpower150 trial. J. Immunother. Cancer 10, e003027 (2022).
Mettu, N. B. et al. Assessment of capecitabine and bevacizumab with or without atezolizumab for the treatment of refractory metastatic colorectal cancer: a randomized clinical trial. JAMA Netw. Open. 5, e2149040 (2022).
Fukuoka, S. et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J. Clin. Oncol. 38, 2053–2061 (2020).
Fakih, M. et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine 58, 101917 (2023).
Yukami, H. et al. Updated efficacy outcomes of anti-PD-1 antibodies plus multikinase inhibitors for patients with advanced gastric cancer with or without liver metastases in clinical trials. Clin. Cancer Res. 28, 3480–3488 (2022).
Oaknin, A. et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial. Lancet 403, 31–43 (2024).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Atkins, M. B. et al. Phase II study of nivolumab and salvage nivolumab/ipilimumab in treatment-naive patients with advanced clear cell renal cell carcinoma (HCRN GU16-260-Cohort A). J. Clin. Oncol. 40, 2913–2923 (2022).
Duan, J. et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol. 6, 375–384 (2020).
Cheng, A. L. et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76, 862–873 (2022).
Merck. Merck and Eisai provide update on phase 3 trials of KEYTRUDA® (pembrolizumab) plus LENVIMA® (lenvatinib) in certain patients with advanced melanoma (LEAP-003) and metastatic colorectal cancer (LEAP-017). Merck www.merck.com/news/merck-and-eisai-provide-update-on-phase-3-trials-of-keytruda-pembrolizumab-plus-lenvima-lenvatinib-in-certain-patients-with-advanced-melanoma-leap-003-and-metastatic-colorectal-cance/ (2023).
Neal, J. et al. CONTACT-01: efficacy and safety from a phase III study of atezolizumab (atezo) + cabozantinib (cabo) vs docetaxel (doc) monotherapy in patients (pts) with metastatic NSCLC (mNSCLC) previously treated with checkpoint inhibitors and chemotherapy [abstact 60]. J. Thorac. Oncol. 18, S39–S40 (2023).
Mirati Therapeutics. Mirati Therapeutics provides update on the phase 3 SAPPHIRE study evaluating sitravatinib in combination with OPDIVO®. PR Newswire www.prnewswire.com/news-releases/mirati-therapeutics-provides-update-on-the-phase-3-sapphire-study-evaluating-sitravatinib-in-combination-with-opdivo-301833958.html (2023).
Pal, S. K. et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): a multicentre, randomised, open-label, phase 3 trial. Lancet 402, 185–195 (2023).
Reck, M. et al. Anti-angiogenic agents for NSCLC following first-line immunotherapy: rationale, recent updates, and future perspectives. Lung Cancer 179, 107173 (2023).
Jonasch, E. et al. Belzutifan for renal cell carcinoma in von Hippel–Lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021).
Iliopoulos, O. et al. Belzutifan treatment for von Hippel–Lindau (VHL) disease-associated central nervous system (CNS) hemangioblastomas (HBs) in the phase 2 LITESPARK-004 study [abstract]. J. Clin. Oncol. 41, 2008 (2023).
Albiges, L. et al. Belzutifan plus lenvatinib for patients (pts) with advanced clear cell renal cell carcinoma (ccRCC) after progression on a PD-1/L1 and VEGF inhibitor: preliminary results of arm B5 of the phase 1/2 KEYMAKER-U03B study [abstract]. J. Clin. Oncol. 41, 4553 (2023).
Albiges, L. et al. Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): randomized open-label phase III LITESPARK-005 study [abstract LBA88]. Ann. Oncol. 34, S1329–S1330 (2023).
Choueiri, T. K. et al. Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: an open-label, single-arm, phase 2 study. Lancet Oncol. 24, 553–562 (2023).
Andre, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Santos, L. V., Cruz, M. R., Lopes Gde, L. & Lima, J. P. VEGF-A levels in bevacizumab-treated breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res. Treat. 151, 481–489 (2015).
Greten, T. F. et al. Biomarkers for immunotherapy of hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 20, 780–798 (2023).
Zhu, A. X. et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 28, 1599–1611 (2022).
Rosellini, M. et al. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 20, 133–157 (2023).
Akalu, Y. T., Rothlin, C. V. & Ghosh, S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol. Rev. 276, 165–177 (2017).
Vargas-Leal, V. et al. Expression and function of glial cell line-derived neurotrophic factor family ligands and their receptors on human immune cells. J. Immunol. 175, 2301–2308 (2005).
Maiorano, B. A., Ciardiello, D., Maiello, E. & Roviello, G. Comparison of anti-programmed cell death ligand 1 therapy combinations vs sunitinib for metastatic renal cell carcinoma: a meta-analysis. JAMA Netw. Open. 6, e2314144 (2023).
Kim, C. et al. Association of high levels of antidrug antibodies against atezolizumab with clinical outcomes and T-cell responses in patients with hepatocellular carcinoma. JAMA Oncol. 8, 1825–1829 (2022).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Zheng, X. et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J. Clin. Invest. 128, 2104–2115 (2018).
Kammertoens, T. et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature 545, 98–102 (2017).
Liu, S. et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 11, 309 (2020).
Reckamp, K. L. et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-lung-MAP S1800A. J. Clin. Oncol. 40, 2295–2306 (2022).
McCoach, C. E. et al. Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann. Oncol. 28, 2707–2714 (2017).
Grünwald, V. et al. Depth of remission is a prognostic factor for survival in patients with metastatic renal cell carcinoma. Eur. Urol. 67, 952–958 (2015).
Heinemann, V. et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur. J. Cancer 51, 1927–1936 (2015).
Saijo, K. et al. Depth of response may predict clinical outcome in patients with recurrent/metastatic head and neck cancer treated with pembrolizumab-containing regimens. Front. Oncol. 13, 1230731 (2023).
Schreiber, R. D. et al. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020).
Albiges, L. et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5, e001079 (2020).
Yau, T. et al. Nivolumab plus cabozantinib with or without ipilimumab for advanced hepatocellular carcinoma: results from cohort 6 of the CheckMate 040 trial. J. Clin. Oncol. 41, 1747–1757 (2023).
Merle, P. et al. Ipilimumab with atezolizumab-bevacizumab in patients with advanced hepatocellular carcinoma: the PRODIGE 81-FFCD 2101-TRIPLET-HCC trial. Dig. Liver Dis. 55, 464–470 (2023).
Choueiri, T. K. et al. Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N. Engl. J. Med. 388, 1767–1778 (2023).
Rini, B. I. et al. Prospective cardiovascular surveillance of immune checkpoint inhibitor-based combination therapy in patients with advanced renal cell cancer: data from the phase III JAVELIN Renal 101 trial. J. Clin. Oncol. 40, 1929–1938 (2022).
Acknowledgements
H.-Y.K. is the recipient of grants from the National Science and Technology Council, Taiwan (R.O.C.) (111-2917-I-002-008) and the National Taiwan University Hospital. R.S.K. receives grant support from the Canadian Institutes for Health Research (CIHR). The authors are grateful to clinical colleagues, especially G. Bjarnason, (Sunnybrook Health Sciences Centre, Odette Cancer Centre, Toronto), and to basic research colleagues for critical reading of the manuscript. We also thank C. Cheng for her excellent secretarial assistance.
Author information
Authors and Affiliations
Contributions
All the authors contributed to all aspects related to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.S.K. has received research funding from Genentech and Novelty Nobility, and is a consultant for Novelty Nobility, OncoHost and PharmAbcine. H.-Y.K. and K.A.K. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks M. De Palma, A. Dudley, D. Fukumura, who co-reviewed with V. Salameti, and B. Rini for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuo, HY., Khan, K.A. & Kerbel, R.S. Antiangiogenic–immune-checkpoint inhibitor combinations: lessons from phase III clinical trials. Nat Rev Clin Oncol (2024). https://doi.org/10.1038/s41571-024-00886-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41571-024-00886-y