Abstract
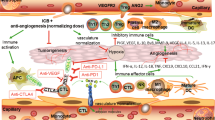
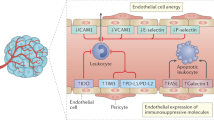
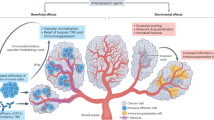
The combination of immune checkpoint inhibitors and anti-angiogenic agents is a promising new approach in cancer treatment. Immune checkpoint inhibitors block the signals that help cancer cells evade the immune system, while anti-angiogenic agents target the blood vessels that supply the tumour with nutrients and oxygen, limiting its growth. Importantly, this combination triggers synergistic effects based on molecular and cellular mechanisms, leading to better response rates and longer progression-free survival than treatment alone. However, these combinations can also lead to increased side effects and require close monitoring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Braun DA, Bakouny Z, Hirsch L, Flippot R, Van Allen EM, Wu CJ, et al. Beyond conventional immune-checkpoint inhibition—novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol. 2021;18:199–214. https://doi.org/10.1038/s41571-020-00455-z
Vukadin S, Khaznadar F, Kizivat T, Vcev A, Smolic M. Molecular mechanisms of resistance to immune checkpoint inhibitors in melanoma treatment: an update. Biomedicines. 2021;9:835. https://doi.org/10.3390/biomedicines9070835
Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60. https://doi.org/10.1186/s12943-019-0974-6
Duda DG, Jain RK. Revisiting antiangiogenic multikinase inhibitors in the era of immune checkpoint blockade: the case of sorafenib. Cancer Res. 2022;82:3665–7. https://doi.org/10.1158/0008-5472.CAN-22-2639
Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, Heymach JV. Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin Cancer Res. 2023;29:30–9. https://doi.org/10.1158/1078-0432.CCR-22-1366
Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–86. https://doi.org/10.1182/blood-2002-07-1956
Zheng W, Qian C, Tang Y, Yang C, Zhou Y, Shen P, et al. Manipulation of the crosstalk between tumor angiogenesis and immunosuppression in the tumor microenvironment: Insight into the combination therapy of anti-angiogenesis and immune checkpoint blockade. Front Immunol. 2022;13:1–24. https://doi.org/10.3389/fimmu.2022.1035323
Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–11. https://doi.org/10.1038/nri3064
Orr BA, Eberhart CG. Molecular pathways: not a simple tube-the many functions of blood vessels. Clin Cancer Res. 2015;21:18–23. https://doi.org/10.1158/1078-0432.CCR-13-1641
Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632–42. https://doi.org/10.1158/2326-6066.CIR-14-0053
Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40. https://doi.org/10.1038/nrclinonc.2018.29
Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell. 2020;37:471–84. https://doi.org/10.1016/j.ccell.2020.03.007
Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. https://doi.org/10.1016/j.tig.2008.10.012
Li Z, Seehawer M, Polyak K. Untangling the web of intratumour heterogeneity. Nat Cell Biol. 2022;24:1192–201. https://doi.org/10.1038/s41556-022-00969-x
Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1–targeted therapies is CD28-dependent. Science (80-). 2017;355:1423–7. https://doi.org/10.1126/science.aaf0683
Liu X, Hoft DF, Peng G. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. J Clin Invest. 2020;130:1073–83. https://doi.org/10.1172/JCI133679
Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, et al. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–31. https://doi.org/10.1182/blood-2012-03-416040
Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, et al. Tumor-derived γδ regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol. 2013;190:2403–14. https://doi.org/10.4049/jimmunol.1202369
Liu X-D, Hoang A, Zhou L, Kalra S, Yetil A, Sun M, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res. 2015;3:1017–29. https://doi.org/10.1158/2326-6066.CIR-14-0244
Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol. 2019;9:1–18. https://doi.org/10.3389/fimmu.2018.03176
Wang Y, Xiang Y, Xin VW, Wang X-W, Peng X-C, Liu X-Q, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13:107 https://doi.org/10.1186/s13045-020-00939-6.
Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–24. https://doi.org/10.1007/s00262-007-0441-x
Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. https://doi.org/10.4049/jimmunol.160.3.1224
Long J, Hu Z, Xue H, Wang Y, Chen J, Tang F, et al. Vascular endothelial growth factor (VEGF) impairs the motility and immune function of human mature dendritic cells through the VEGF receptor 2‐RhoA‐cofilin1 pathway. Cancer Sci. 2019;110:2357–67. https://doi.org/10.1111/cas.14091
Malo CS, Khadka RH, Ayasoufi K, Jin F, AbouChehade JE, Hansen MJ, et al. Immunomodulation mediated by anti-angiogenic therapy improves CD8 T cell immunity against experimental glioma. Front Oncol. 2018;8:1–6. https://doi.org/10.3389/fonc.2018.00320
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8++ T cells in tumors. J Exp Med. 2015;212:139–48. https://doi.org/10.1084/jem.20140559
Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–71. https://doi.org/10.1158/0008-5472.CAN-11-3687
Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475–85. https://doi.org/10.1038/s12276-020-00500-y
Tamura R, Tanaka T, Ohara K, Miyake K, Morimoto Y, Yamamoto Y, et al. Persistent restoration to the immunosupportive tumor microenvironment in glioblastoma by bevacizumab. Cancer Sci. 2019;110:499–508. https://doi.org/10.1111/cas.13889
Wang Y, Dong J, Quan Q, Liu S, Chen X, Cai X, et al. Immune cell infiltration of the primary tumor microenvironment predicted the treatment outcome of chemotherapy with or without bevacizumab in metastatic colorectal cancer patients. Front Oncol. 2021; 10. https://doi.org/10.3389/fonc.2020.581051.
Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. https://doi.org/10.1158/0008-5472.CAN-13-1196
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. https://doi.org/10.1016/j.ccr.2010.11.009
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. https://doi.org/10.1158/1078-0432.CCR-08-1332
Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–49. https://doi.org/10.1158/0008-5472.CAN-12-2325
Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med. 2014;12:294. https://doi.org/10.1186/s12967-014-0294-y
Lee C-H, Shah AY, Rasco D, Rao A, Taylor MH, Di Simone C, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22:946–58. https://doi.org/10.1016/S1470-2045(21)00241-2
Motzer R, Alekseev B, Rha S-Y, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300. https://doi.org/10.1056/nejmoa2035716
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1103–15. https://doi.org/10.1056/NEJMoa1816047
Choueiri TK, Eto M, Motzer R, De Giorgi U, Buchler T, Basappa NS, et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023a;24:228–38. https://doi.org/10.1016/S1470-2045(23)00049-9
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019a;380:1116–27. https://doi.org/10.1056/NEJMoa1816714
Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019b;393:2404–15. https://doi.org/10.1016/S0140-6736(19)30723-8
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl J Med. 2021;384:829–41. https://doi.org/10.1056/NEJMoa2026982
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. https://doi.org/10.1056/NEJMoa1915745
Makker V, Aghajanian C, Cohn AL, Romeo M, Bratos R, Brose MS, et al. A phase Ib/II study of lenvatinib and pembrolizumab in advanced endometrial carcinoma (Study 111/KEYNOTE-146): long-term efficacy and safety update. J Clin Oncol. 2023;41:974–9. https://doi.org/10.1200/JCO.22.01021
Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386:437–48. https://doi.org/10.1056/NEJMoa2108330
Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer. JAMA Oncol. 2019;5:1731. https://doi.org/10.1001/jamaoncol.2019.3343
Li S, Chen J, Sun Z. Improving antitumor immunity using antiangiogenic agents: Mechanistic insights, current progress, and clinical challenges. Cancer Commun. 2021;41:830–50. https://doi.org/10.1002/cac2.12183
Ren S, Xiong X, You H, Shen J, Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front Immunol. 2021;12:1–12. https://doi.org/10.3389/fimmu.2021.689132
Ikeuchi N, Igata F, Kinoshita E, Kawabata T, Tan I, Osaki Y, et al. Real-world efficacy and safety of atezolizumab plus bevacizumab, paclitaxel and carboplatin for first-line treatment of Japanese patients with metastatic non-squamous non-small cell lung cancer. Anticancer Res. 2023;43:713–24. https://doi.org/10.21873/anticanres.16210
Chen S, Wei H, Zhao W, Jiang W, Ning R, Zhou S, et al. PD-1/PD-L1 inhibitors plus anti-angiogenic agents with or without chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy as second or later-line treatment for patients with advanced non-small cell lung cancer: A real-world retrospective cohort study. Front Immunol. 2022;13:1–20. https://doi.org/10.3389/fimmu.2022.1059995
Provencio M, Ortega AL, Coves-Sarto J, Calvo V, Marsé-Fabregat R, Dómine M, et al. (2022) Atezolizumab Plus Bevacizumab as First-line Treatment for Patients With Metastatic Nonsquamous Non–Small Cell Lung Cancer With High Tumor Mutation Burden. JAMA Oncol. 1–9, https://doi.org/10.1001/jamaoncol.2022.5959.
Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, et al. Belzutifan for renal cell carcinoma in von Hippel–Lindau Disease. N Engl J Med. 2021;385:2036–46. https://doi.org/10.1056/nejmoa2103425
Choueiri TK, McDermott DF, Merchan J, Bauer TM, Figlin R, Heath EI, et al. (2023b) Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: an open-label, single-arm, phase 2 study. Lancet Oncol. 2045: 1–10, https://doi.org/10.1016/S1470-2045(23)00097-9.
Hamuro L, Hu Z, Passarell J, Barcomb H, Zhang J, Goldstein S, et al. Exposure-response analysis to support nivolumab once every 4 weeks dosing in combination with cabozantinib in renal cell carcinoma. Clin Cancer Res. 2022;28:1603–13. https://doi.org/10.1158/1078-0432.CCR-21-3149
Patil VM, Noronha V, Menon N, Rai R, Bhattacharjee A, Singh A, et al. Low-dose immunotherapy in head and neck cancer: a randomized Study. J Clin Oncol. 2023;41:222–32. https://doi.org/10.1200/JCO.22.01015
Laenens D, Yu Y, Santens B, Jacobs J, Beuselinck B, Bechter O, et al. Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J Clin Oncol. 2022;40:3430–8. https://doi.org/10.1200/JCO.21.01808
Hertz DL, McShane LM, Hayes DF. Defining clinical utility of germline indicators of toxicity risk: a perspective. J Clin Oncol. 2022;40:1721–31. https://doi.org/10.1200/JCO.21.02209
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–92. https://doi.org/10.1038/s41591-020-0856-x
Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26:693–8. https://doi.org/10.1038/s41591-020-0860-1
Chen Y, Li X, Liu G, Chen S, Xu M, Song L, et al. ctDNA concentration, MIKI67 mutations and hyper-progressive disease related gene mutations are prognostic markers for camrelizumab and apatinib combined multiline treatment in advanced NSCLC. Front Oncol. 2020;10:1–11. https://doi.org/10.3389/fonc.2020.01706
Palasantzas VEJM, Tamargo-Rubio I, Le K, Slager J, Wijmenga C, Jonkers IH, et al. iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends Genet. 2023;39:268–84. https://doi.org/10.1016/j.tig.2023.01.002
Schuster B, Junkin M, Kashaf SS, Romero-Calvo I, Kirby K, Matthews J, et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat Commun. 2020;11:1–12. https://doi.org/10.1038/s41467-020-19058-4
Tatarova Z, Blumberg DC, Korkola JE, Heiser LM, Muschler JL, Schedin PJ, et al. A multiplex implantable microdevice assay identifies synergistic combinations of cancer immunotherapies and conventional drugs. Nat Biotechnol. 2022;40:1823–33. https://doi.org/10.1038/s41587-022-01379-y
Yang C, Lee H, Pal S, Jove V, Deng J, Zhang W, et al. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLoS ONE. 2013;8:e64159. https://doi.org/10.1371/journal.pone.0064159
Montemagno C, Pagès G. Resistance to anti-angiogenic therapies: a mechanism depending on the time of exposure to the drugs. Front Cell Dev Biol. 2020;8:1–21. https://doi.org/10.3389/fcell.2020.00584
Dufies M, Giuliano S, Ambrosetti D, Claren A, Ndiaye PD, Mastri M, et al. Sunitinib stimulates expression of VEGFC by tumor cells and promotes lymphangiogenesis in clear cell renal cell carcinomas. Cancer Res. 2017;77:1212–26. https://doi.org/10.1158/0008-5472.CAN-16-3088
Funding
Funding is acknowledged from the French Government (Agence Nationale de Recherche, ANR) through the ‘Investments for the Future’ LABEX SIGNALIFE (ANR-11-LABX-0028-01 and IDEX UCAJedi ANR-15-IDEX-01) and [AD-ME project R19162DD]; CANC’AIR Genexposomic project, Canceropole PACA; DREAL PACA, ARS PACA, Région Sud, INSERM cancer; INCA Plan Cancer; ITMO Cancer, Children Medical Safety Research Institute (CMSRI, Vaccinophagy project R17033DJA), The Fondation Max et Yvonne de Foras, La Ligue contre le Cancer-Equipe labellisée 2019, Fondation ARC de la Recherche contre le Cancer Programme labellisé 2022, Fondation Amgen, Fondation Flavien.
Author information
Authors and Affiliations
Contributions
GM, PB: conceptualisation; investigation; writing—original draft; GP, BM, PH: visualisation; writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brest, P., Mograbi, B., Pagès, G. et al. Checkpoint inhibitors and anti-angiogenic agents: a winning combination. Br J Cancer 129, 1367–1372 (2023). https://doi.org/10.1038/s41416-023-02437-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02437-1
This article is cited by
-
Immune check points in cancer treatment: current challenges and perspectives
British Journal of Cancer (2023)