Abstract
Nanotechnology could improve our understanding of the pathophysiology of atherosclerosis and contribute to the development of novel diagnostic and therapeutic strategies to further reduce the risk of cardiovascular disease. Macrophages have key roles in atherosclerosis progression and, therefore, macrophage-associated pathological processes are important targets for both diagnostic imaging and novel therapies for atherosclerosis. In this Review, we highlight efforts in the past two decades to develop imaging techniques and to therapeutically manipulate macrophages in atherosclerotic plaques with the use of rationally designed nanoparticles. We review the latest progress in nanoparticle-based imaging modalities that can specifically target macrophages. Using novel molecular imaging technology, these modalities enable the identification of advanced atherosclerotic plaques and the assessment of the therapeutic efficacy of medical interventions. Additionally, we provide novel perspectives on how macrophage-targeting nanoparticles can deliver a broad range of therapeutic payloads to atherosclerotic lesions. These nanoparticles can suppress pro-atherogenic macrophage processes, leading to improved resolution of inflammation and stabilization of plaques. Finally, we propose future opportunities for novel diagnostic and therapeutic strategies and provide solutions to challenges in this area for the purpose of accelerating the clinical translation of nanomedicine for the treatment of atherosclerotic vascular disease.
Key points
-
Because macrophages have key roles in atherosclerosis progression, macrophage-mediated pro-atherosclerotic processes are important targets for both diagnostic imaging and novel therapies for atherosclerosis.
-
Nanotechnology is particularly advantageous in its capacity to substantially improve the pharmacokinetic profile and chemical stability of encapsulated diagnostic and therapeutic agents for atherosclerosis management.
-
The rational design of nanoparticle-based imaging agents that can specifically target inflammatory macrophages in atherosclerotic plaques offers diagnostic potential to non-invasively quantify atherosclerosis plaque burden, evaluate the efficacy of medical interventions and serve as surrogate end points.
-
Targeted nanotherapeutics that can modulate plaque macrophage functions by the activation or suppression of specific signalling pathways have shown great promise in preclinical models by improving therapeutic efficacy and reducing off-target and systemic adverse effects.
-
Rapid advances in nanotechnology and bioengineering and an improved understanding of atherosclerotic pathophysiology have accelerated the development of novel nanotherapeutics for atherosclerosis diagnosis and treatment.
-
The success of various nanomedicine-based approaches in preclinical studies of atherosclerosis and their use in human cancer bode well for their future application in the diagnosis and treatment of patients with cardiovascular disease.
Similar content being viewed by others
Introduction
Atherosclerotic cardiovascular disease is one of the most common causes of morbidity and mortality worldwide1. Atherosclerosis is considered a chronic inflammatory disease, initiated by the retention of plasma apolipoprotein B (apoB)-containing lipoproteins in focal areas of the arterial tree2,3. The cholesterol and oxidized phospholipids in these lipoproteins induce the activation of endothelial cells, which subsequently recruit monocytes into the subendothelial space4,5. In the arterial intima, monocytes differentiate into pro-inflammatory macrophages that locally amplify the inflammatory response6. In addition, macrophages engulf lipoproteins in the intima to form lipid-laden foam cells, giving rise to early atherosclerotic lesions. If the pro-inflammatory state persists, the atherosclerotic lesions progress to an advanced stage characterized by increased macrophage apoptosis and defective clearance of apoptotic cells7,8,9. This catastrophic combination promotes plaque necrosis, a key feature of ‘vulnerable’ plaques that can trigger occlusive luminal thrombosis and its consequences, namely, myocardial infarction, stroke and sudden cardiac death1,10. The risk of these cardiovascular events remains fairly high in the general population despite treatment with lipid-lowering therapies, notably statins but also PCSK9 inhibitors11,12. In theory, cardiovascular disease could be eliminated if apoB-containing lipoproteins could be decreased to very low levels early in life. However, this strategy is currently not practical for widespread application mainly because of low compliance and adverse effects in certain individuals. Moreover, the safety concerns of using cholesterol-lowering drugs in childhood have not yet been fully evaluated. For this reason, novel anti-inflammatory therapies are being developed to further reduce the risk of cardiovascular disease; however, these efforts are hampered by the low bioavailability, poor target specificity and high toxicity of conventional anti-inflammatory drugs. Furthermore, identifying which individuals are at risk of developing clinically dangerous vulnerable plaques remains a challenge.
Nanotechnology is particularly advantageous for addressing these challenges. Specifically, extensive studies have shown that nanoparticles substantially improve the pharmacokinetic profile and chemical stability of the loaded therapeutics, such as small-molecule drugs, peptides, proteins, small interfering RNA (siRNA) and microRNA (miRNA)13. Nanotherapy could also reduce the off-target and systemic adverse effects compared with those of free drugs alone, thereby overcoming the key translational barriers for those drugs14,15,16. Additionally, nanoparticle-mediated delivery strategies have shown great promise, in mouse models and with high specificity, in suppressing certain pathological processes, including inflammation, that are associated with atherosclerosis progression13. More importantly, targeted nanoparticles have been designed to improve the delivery of imaging agents and therapeutics to inflammatory macrophages in atherosclerotic plaques. In this Review, we provide a fresh perspective on atherosclerosis nanomedicine. Specifically, we discuss how the convergence of nanotechnology and a deeper understanding of the pro-atherosclerotic mechanisms mediated by macrophages in atherosclerotic plaques could drive advances in cardiovascular disease diagnosis and therapy. Moreover, we discuss the opportunities and challenges of macrophage-targeting nanomedicines that will need to be considered before clinical translation. Although nanoparticles have not yet been used for the treatment of atherosclerosis in patients, we envision that the improved understanding of atherosclerotic pathobiology gained over the past decades, combined with the success of nanomedicines in cancer therapy, will accelerate their future clinical translation for cardiovascular disease.
Macrophage biology in atherosclerosis
In the early phase of atherogenesis, endothelial cells attract monocytes to the arterial wall through chemokine–receptor interactions and by expressing cell adhesion molecules such as intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1). This monocyte recruitment system has a high degree of redundancy, which explains scientific efforts to halt atherogenesis by simultaneously targeting multiple receptor–ligand interactions17,18. The combined inhibition of five key adhesion molecules through nanoparticle-based RNA interference was shown in a 2016 study to be very effective in reducing monocyte recruitment to atherosclerotic lesions in a mouse model of atherosclerosis18. However, this therapy might not be of tremendous benefit in advanced atherosclerosis, when monocytes have already infiltrated the plaque. Indeed, monocyte suppression was found to reduce early but not advanced atherosclerosis in mice19, underscoring the importance of early detection of subclinical disease in humans for monocyte-directed therapies.
After migrating into the arterial vessel wall, monocytes differentiate into macrophages and acquire functionally distinct phenotypes depending on signals from the local microenvironment20. Macrophages can either adopt a more pro-inflammatory (‘M1-like’) phenotype, characterized by a high production of pro-inflammatory cytokines, reactive oxygen species (ROS) and reactive nitrogen species, or a more pro-resolving (‘M2-like’) phenotype, distinguished by the capacity to dampen inflammation and promote tissue repair. Atherosclerotic lesions in humans contain various macrophage populations21 although their functional profile is poorly understood22. Further insight into the mechanisms that drive macrophage phenotypes in atherosclerosis could provide new therapeutic opportunities. Studies indicate that pro-inflammatory lesional macrophages ingest large quantities of LDL-derived cholesterol mediated by the increased activity of cholesterol-trafficking pathways in these cells23. Macrophage scavenger receptors facilitate the uptake of oxidized cholesterol, which can be stored in the cell to prevent cholesterol-induced cytotoxicity, resulting in the classical ‘foamy’ appearance of macrophages in atherosclerotic lesions. Alternatively, excess cholesterol can be transported to extracellular apolipoprotein A-I (apoA-I) and HDL through the macrophage ATP-binding cassette (ABC) transporters ABCA1 and ABCG1, respectively24,25,26,27. The expression of these membrane lipid translocases is induced by peroxisome proliferator-activated receptor-γ (PPARγ) and the sterol-regulated transcription factor liver X receptor-α (LXRα)28. By promoting macrophage cholesterol efflux, LXRα agonists ameliorate atherosclerosis in preclinical animal models29,30,31. However, systemic LXRα activation also promotes hepatic steatosis and hypertriglyceridaemia by acting on the liver, thereby complicating LXRα-targeted therapy32. Interestingly, macrophages have been found to transfer cholesterol directly to adjacent smooth muscle cells in vitro, providing an alternative for ABC transporter-mediated cholesterol efflux33. Whether this transcellular cholesterol movement is relevant to macrophages in atherosclerotic lesions remains to be investigated.
Cholesterol accumulation in macrophages promotes inflammatory responses, including activation of Toll-like receptor (TLR) signalling, NF-κB-mediated activation of the NLRP3 inflammasome and pro-inflammatory cytokine production, which exacerbate the chronic inflammatory state in atherosclerosis34. Accordingly, suppression of cholesterol efflux pathways through macrophage-specific deletion of Abca1 and Abcg1 promotes an inflammatory macrophage signature and accelerates atherosclerosis in mice35. TLR signalling and NF-κB are popular targets for anti-inflammatory therapy of atherosclerosis, although general inhibition of these mediators is risky considering their broad function in inflammation and immunity. The same is the case with inhibition of IL-1β, a pro-inflammatory cytokine product of the NLRP3 inflammasome. The CANTOS trial36 showed that canakinumab, a monoclonal antibody against IL-1β, reduces the risk of cardiovascular events in patients with previous myocardial infarction but was also associated with a higher incidence of fatal infection compared with placebo. Additionally, results from the COLCOT trial37 published in 2019 showed that systemic treatment of patients within 30 days after myocardial infarction with the anti-inflammatory drug colchicine significantly lowered the risk of ischaemic cardiovascular events by the attenuation of NLRP3 inflammasome activation and inhibition of the release of IL-1β and IL-18 from inflammatory macrophages. However, this systemic treatment strategy also produced an immunosuppressive effect and increased the incidence of pneumonia compared with the placebo group37. These findings indicate that the localized and targeted delivery of anti-inflammatory therapy might be preferred over systemic approaches to reduce adverse effects related to impaired host defence. This goal might be even more achievable with the use of nanoparticle-directed pro-resolving therapy instead of anti-inflammatory therapy (see discussion below).
Increased inflammation not only attracts more circulating monocytes to the atherosclerotic vessel wall but also promotes a vulnerable plaque phenotype38. Vulnerable plaques are characterized by a thin fibrous cap and a large necrotic core and are therefore more prone to rupture39. Plaque rupture exposes pro-thrombotic substances in the plaque, which trigger the activation of platelets, leading to thrombus formation and arterial occlusion10. How to identify those individuals who are susceptible to having vulnerable and rupture-prone plaques is an important area of future research. In addition, further research is warranted into the mechanisms through which macrophages influence the stability of atherosclerotic lesions. Pro-inflammatory macrophages are known to diminish lesion stability by inhibiting collagen production by smooth muscle cells and by producing matrix metalloproteinases (MMPs) that degrade the protective fibrous cap40,41,42,43. By contrast, pro-resolving macrophages promote lesion stability by clearing apoptotic cells through the process of efferocytosis, thereby diminishing plaque necrosis7,44. Efferocytosis also initiates an inflammation resolution response by macrophages, resulting in the production of resolving mediators, including IL-10, transforming growth factor-β (TGFβ) and metabolites of long-chain unsaturated fatty acids called specialized pro-resolving mediators, all of which restrain inflammation and promote tissue repair45. Importantly, macrophage-mediated resolution of inflammation is impaired in advanced atherosclerotic lesions8,9,46. Boosting efferocytosis and inflammation resolution is therefore a promising approach to halt atherosclerosis progression and induce atherosclerosis regression47 and was shown to be effective in mouse models of atherosclerosis48,49.
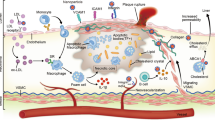
Considering the key role of macrophages in the progression and regression of atherosclerotic lesions (Fig. 1), these cells are an important therapeutic target in atherosclerotic cardiovascular disease. However, a high degree of overlap exists between macrophage-related mechanisms involved in atherosclerosis and host defence, which makes designing therapies that do not interfere with immunity difficult. Another complicating factor is the inability of current imaging modalities to non-invasively assess atherosclerotic plaque composition and stability or to identify individuals at high risk of developing acute coronary syndrome. In the past two decades, atherosclerotic macrophage-targeted nanoparticles have emerged as a potential solution to these problems, as further explained below.
a | Atherosclerosis development is initiated by the retention and aggregation of apolipoprotein B-containing lipoproteins (apoB-LPs) in the subendothelial space. ApoB-LPs activate endothelial cells, resulting in the upregulation of adhesion molecules (such as intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1)) that mediate monocyte adhesion to endothelial cells and migration into the arterial vessel wall. In the arterial intima, monocytes differentiate into macrophages, which engulf lipoprotein-derived cholesterol, leading to foam cell formation. Macrophages that cannot process the large amounts of cholesterol undergo cholesterol-induced cytotoxicity and apoptosis. b | If circulating apoB-LP levels remain elevated and the pro-inflammatory state persists, monocyte infiltration and macrophage apoptosis continue, producing so-called ‘vulnerable’ atherosclerotic plaques with large necrotic cores and thin fibrous caps. Vulnerable plaques are prone to rupture, which can lead to thrombus formation, arterial occlusion and sudden cardiac death. c | However, if the plasma cholesterol level is sufficiently lowered and/or the pro-inflammatory state subsides and resolution processes are activated, atherosclerosis regression is possible. Regressing atherosclerotic lesions are characterized by a high cholesterol efflux from macrophages to HDL, large numbers of pro-resolving macrophages that clear apoptotic cells through efferocytosis, reduced plaque necrosis and a thick protective fibrous cap. Regressing atherosclerotic plaques are relatively stable and less likely to rupture than vulnerable plaques and, most importantly, are associated with a lower risk of coronary artery disease in humans.
Journey of nanomedicines to macrophages
The aforementioned macrophage-mediated atherogenesis provides a plethora of compelling diagnostic and therapeutic targets for nanoparticle-assisted management of atherosclerosis. Consequently, a comprehensive investigation of the journey of nanoparticles, including their circulation in the bloodstream, extravasation into the atherosclerotic plaques, and macrophage targeting and intracellular trafficking, will aid in designing nanoparticles that specifically accumulate in lesional macrophages and thereby have more robust diagnostic and therapeutic efficacy.
Intravenous administration of nanotherapeutics is the most straightforward and effective delivery route for their systemic infiltration into atherosclerotic plaques. The diversity of nanoparticles is described in Box 1. Following intravenous administration, the adsorption of biomolecules (such as proteins) on the surface of nanoparticles gives rise to the formation of a ‘corona’, which has a crucial role in determining the pharmacokinetics, biodistribution and cellular uptake of nanoparticles50. For example, the adsorption of opsonin proteins, such as IgG, activates the clearance of administered nanoparticles by the mononuclear phagocyte system (MPS) in the liver and spleen50,51. To increase their accumulation in atherosclerotic plaques, nanoparticles are designed to evade MPS recognition by surface coating with polymers (such as polyethylene glycol (PEG))52 or biomimetic materials (such as erythrocyte membranes53, platelet membranes54, platelet-derived extracellular vesicles55, macrophage membranes56 and macrophage-derived exosomes57). In addition, other natural nanomaterials, such as HDL58,59, ferritin nanocages60 and heat shock protein61, which can evade MPS recognition and thereby prolong circulation in the blood, have been extensively used to deliver imaging and therapeutic agents to macrophages in atherosclerotic lesions. The size of nanoparticles also has an important role in their accumulation in plaques. For example, Tang and colleagues investigated how the size of HDL nanoparticles affects their accumulation in aortic plaques62. Using ex vivo near-infrared fluorescence imaging, the researchers discovered that the accumulation of nanoparticles with a diameter of 7–30 nm exceeded that of nanoparticles with a diameter of >70 nm. This size-dependent accumulation of nanoparticles is due to the fact that, when the nanoparticle size is >6 nm in diameter (the size threshold for renal clearance), smaller HDL nanoparticles have longer blood circulation times than larger HDL nanoparticles. Interestingly, a study published in 2020 showed that single-walled carbon nanotubes (SWNTs) accumulate in atherosclerotic lesion areas because they are taken up by circulating monocytes that subsequently migrate to the plaques63. Conversely, this monocyte-targeting approach could be used to prevent monocyte recruitment to inflammatory lesions by silencing Ccr2, which encodes a chemokine receptor, in pro-inflammatory monocytes through administration of siRNA-encapsulated lipid nanoparticles as shown in mice64.
After circulation in the blood, efficient accumulation of nanoparticles in lesional macrophages relies on extravasation through the leaky vasculature of atherosclerotic lesions, similar to nanoparticle accumulation in tumour tissues65,66. Specifically, circulating nanoparticles should first penetrate through the openings in the impaired vascular endothelium (on the lumen side) or in those of neovessels (on the adventitial side) to reach subendothelial atherosclerotic plaques13. In this area, attempts have been made to modify the nanoparticle surface with subendothelial collagen type IV-targeted peptides that can direct the nanoparticles to the plaque48,67,68. Interestingly, a 2017 study indicated that, after the nanoparticles infiltrate the leaky endothelium, restricted diffusion inside the atherosclerotic plaques makes the nanoparticles deposit only in the peripheral areas around the endothelial openings, achieving heterogeneous distribution69. Given this paradigm, nanoparticles with optimal physicochemical properties for deeper plaque penetration might improve their distribution profiles in plaques and improve the diagnostic and therapeutic efficacy. However, how the nanoparticle size, surface charge, shape, stiffness, chemical composition, and surface functional groups and ligands affect their distribution in the plaque microenvironment remains largely unexplored and warrants future systematic investigation. Nevertheless, evidence from a study in 2019 demonstrated that atherosclerotic plaque progression supports endothelial junction stabilization, thereby reducing nanoparticle infiltration70. This nanoparticle-aided characterization suggested that the accumulation of nanoparticles in advanced plaques might not be as efficient as in earlier-stage plaques and that the severity of atherosclerosis might not be characterized solely by infiltration-dominant nanoparticle accumulation because the imaging intensity produced by accumulated nanoparticles might decrease as atherosclerosis progresses. In addition to extravasation through the leaky endothelium mentioned above, accelerated dynamin-dependent transcytosis through endothelial cells that overlay the atherosclerotic lesions could improve the accumulation of ultrasmall iron oxide nanoparticles in plaques71, similar to discoveries of nanoparticle accumulation in tumour tissues72. The finding might provide a new strategy to design nanoparticles that can target endothelial cells and finally reach lesional macrophages by transcytosis.
After nanoparticles accumulate in atherosclerotic lesions, they can be taken up by various cell types in the microenvironment of the plaque, such as vascular smooth muscle cells63,69 and neutrophils58,73. Therefore, surface-targeted proteins or other ligands that can specifically recognize the epitopes overexpressed on macrophages52,61,74,75,76,77,78,79,80 have pivotal roles in increasing nanoparticle internalization by lesional macrophages and delivering therapeutics that specifically manipulate macrophages. The administration of macrophage-targeted nanoparticles is particularly crucial to identifying and modulating the functions of pro-inflammatory macrophages. In addition, efficient nanoparticle internalization has a key role in promoting therapeutic efficacy because many therapeutics carried by nanoparticles act on intracellular targets and compartments. This point is particularly crucial for biomolecular payloads, such as siRNA and miRNA, which are involved in the RNA-interference pathway and require endosomal escape and cytosolic delivery following nanoparticle uptake52,74,81,82,83,84. Polymer-based and ionizable lipid-based nanoparticles that can escape from endolysosomes have shown great promise in delivering those biomolecules to lesional macrophages52,74,81,82.
Nanoparticles for diagnostic imaging
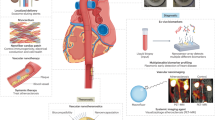
Molecular imaging technology facilitates the visualization of atherosclerotic plaques at high risk of rupture or erosion with high spatiotemporal resolution, addressing the pressing need for robust and reliable imaging agents to assist in the development of drugs for atherosclerosis. To this end, the rational design of nanomaterial-based imaging contrast agents that can specifically target macrophages in plaques can offer pivotal insights into plaque biology as well as help to quantify atherosclerosis burden and to evaluate the efficacy of therapies at the molecular, cellular and functional levels. In this section, we review and discuss state-of-the-art non-invasive imaging technologies, including MRI, CT, PET, fluorescence imaging, photoacoustic imaging (PAI) and combined imaging modalities, and the corresponding nanomaterial-based contrast agents for the identification of macrophage-rich atherosclerotic plaques at risk of rupture or erosion and to assess the efficacy of macrophage-specific anti-atherosclerotic therapies (Fig. 2).
Non-invasive bioimaging technologies facilitate the visualization of high-risk atherosclerotic plaques with high spatiotemporal resolution. a | The unique epitopes expressed on the macrophage surface can be recognized with the use of targeted nanoparticle-based imaging agents. Targeted nanoparticles have been designed to improve the delivery of imaging agents to inflammatory macrophages in atherosclerotic plaques, thereby improving imaging contrast. b | Various non-invasive bioimaging modalities, advantages, disadvantages and the associated nanoparticle-based imaging contrast agents for plaque visualization. The arrows and arrowheads show areas of high agent uptake. Nanoparticle-facilitated non-invasive bioimaging can provide insights into atherosclerotic plaque biology as well as help to quantify atherosclerosis burden and evaluate the efficacy of therapies at the molecular, cellular and functional levels. FI, fluorescence imaging; ICG, indocyanine green; LOX1, lectin-like oxidized LDL receptor 1; MARCO, macrophage receptor MARCO; MMR, macrophage mannose receptor; OPN, osteopontin; PAI, photoacoustic imaging; PS, phosphatidylserine. MRI image adapted with permission from ref.92, Elsevier. CT image adapted from ref.100, Springer Nature Limited. PET image adapted from ref.107, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). FI image adapted with permission from ref.79, American Chemical Society. PAI image adapted with permission from ref.112, American Chemical Society.
MRI
Atherosclerotic plaque burden and the accumulation of pro-inflammatory macrophages have been extensively characterized by MRI, which can generate images with high spatial resolution and excellent soft-tissue contrast. Additionally, systemic administration of nanoparticle-based imaging contrast agents, such as superparamagnetic iron oxide nanoparticles (SPIONs), which have superior safety profiles and can readily be phagocytosed by plaque macrophages, can induce signal loss in T2-weighted images and thereby improve the image contrast of plaques in the arterial walls85. More importantly, surface coating of SPIONs with targeting moieties, such as dextran86,87,88, human ferritin protein cage60, osteopontin (OPN)89 or annexin V90, can specifically target the SPIONs to the corresponding epitopes on the macrophage surface and increase their accumulation in vulnerable plaques in aortic or carotid arteries. In clinical settings, SPION-enhanced MRI has already been used to assess macrophage-associated inflammatory burdens87,91 and therapeutic outcomes of lipid-lowering therapy (ATHEROMA study92). Of note, the newly developed ultra-high-field MRI scanner (10.5 T), which can generate images with an exceptionally high signal-to-noise ratio, might provide more detailed data at the cellular and molecular levels for clinical cardiovascular research93.
Macrophage burden can also be characterized by systemic administration of Gd complexes, such as Gd-diethylenetriaminepentaacetic acid (Gd-DTPA)-containing nanoparticles54,94 or Gd inorganic nanocrystals95, which can increase the signal and improve the contrast of T1-weighted images. For instance, macrophage scavenger receptor-targeted immunomicelles94 and platelet membrane biomimetic nanoparticles54 that contain Gd-DTPA were used to study macrophage burden; the resulting increase in image contrast was proportional to the macrophage content of the plaques. Of note, the platelet membrane-coated nanoparticles can also target pre-atherosclerotic areas and thereby provide deeper mechanistic insights into atherogenesis beyond simply aiding in imaging the regions with clinically significant plaque formation. However, although these Gd-containing imaging agents seem to be promising, they might cause nephrogenic systemic fibrosis96, thereby hindering their clinical use.
In addition to conventional MRI, which detects the relaxation process of 1H, the use of 19F MRI in conjunction with the corresponding perfluoro-nanoemulsion contrast agents has been considered to be an attractive modality for the characterization of plaque inflammation owing to the minimal endogenous 19F signal in living organisms97. More intriguingly, after administration of perfluoro-nanoemulsions with distinctive chemical shifts98, 19F MRI-generated multiplexed images might be used to probe various pathological biomarkers of lesional macrophages and to study the pathophysiological processes of atherogenesis.
CT imaging
In clinical settings, the high spatial resolution and short acquisition time make CT a powerful tool to monitor atherosclerotic macrophages in coronary arteries99. Iodine-containing nanoparticles with a high X-ray attenuation coefficient are particularly advantageous in identifying pro-inflammatory macrophages in vulnerable plaques in coronary arteries and the abdominal aorta100,101. However, owing to the low sensitivity of CT, the administration of a high dose of nanoparticles (100 mg of iodine per kg for mice101 and 250 mg of iodine per kg for rabbits100) is required to achieve detectable image contrast enhancement. The potential toxicity of such a high dose of nanoparticles must be systematically investigated before future clinical translation. Despite this issue, the use of CT for atherosclerosis diagnosis is expanding because findings obtained through PET–CT dual-modality imaging have been extensively adopted as surrogate markers in several clinical trials102,103. Remarkably, the spectral multicolour CT, developed in the past decade, has shown great promise because this technology can distinguish between CT imaging agents with different X-ray attenuation coefficients and can be used to characterize the composition of advanced atherosclerotic plaques prone to rupture104,105.
PET
PET is particularly suitable for the non-invasive and quantitative characterization of atherosclerotic macrophage-mediated inflammation owing to the high tissue penetration and superior sensitivity (in the picomolar range) of PET. Because PET does not provide anatomical information, this imaging modality is usually coupled with MRI or CT for anatomical reference. 18F-fluoro-2-deoxyglucose (18F-FDG) radioactive tracers, which can be avidly taken up by macrophages and reflect the metabolic activity of inflamed plaques, have been extensively used to study the progression of atherosclerosis and the effectiveness of medical interventions in both animal models and patients58,106. However, imaging macrophages in coronary arteries with the use of 18F-FDG is particularly challenging because 18F-FDG lacks specificity for macrophages and can also be avidly taken up by cardiomyocytes, yielding false-positive signals13. Nanoparticle-based PET imaging agents can provide a solution to this problem.
PET radioactive tracer-labelled nanoparticles have favourable pharmacokinetics and can specifically and quantitatively monitor pro-inflammatory macrophages in the cardiovascular system. For example, Keliher and colleagues used 18F-labelled polyglucose nanoparticles (termed 18F-Macroflor) to non-invasively visualize atherosclerotic plaques and infarcted myocardial tissue in mouse and rabbit models because lesional macrophages take up these glucose-based nanoprobes with high efficiency107. Owing to this favourable macrophage uptake profile, 18F-Macroflor produced imaging patterns distinct from those of 18F-FDG, facilitating imaging in ischaemic heart disease in rabbits. In addition to 18F radionuclide, 89Zr-labelled nanoparticles have also been widely used to monitor pro-inflammatory macrophages and characterize the severity of atherosclerosis in animal models owing to their slower radioactive decay (89Zr, t1/2 = 78.4 h; 18F, t1/2 = 1.8 h), which can match the in vivo kinetics of long-circulating and macrophage-targeting nanoparticles such as those incorporating dextran73, hyaluronan77 or HDL108. In addition, owing to the long half-life of 89Zr, advantageous prolonged monitoring of macrophages becomes feasible108. Senders and colleagues studied atherosclerosis progression in a rabbit model by using 68Ga-labelled nanobody radiotracers that have an extremely short blood circulation time (<2 min) and can specifically target atherosclerosis-associated biomarkers such as VCAM1, macrophage mannose receptor (MMR) and lectin-like oxidized LDL receptor 1 (LOX1)75. These clinically translatable multiparametric nano-radiotracers might aid in the identification of high-risk atherosclerotic plaques and in anti-atherosclerosis drug development.
Fluorescence imaging
Owing to its high spatial resolution, fast acquisition, lack of radiation-associated risk and cost-effectiveness, non-invasive fluorescence imaging has been widely used to image macrophage-rich vascular lesions in preclinical studies. To achieve deep tissue penetration, current fluorophores, such as organic dyes or nanoparticle-based probes mainly emitting in the near-infrared window (700–900 nm), have been used to probe macrophages in atherosclerotic lesions61. For example, NaGdF4:Yb,Er@NaGdF4 upconversion nanoparticles modified with antibodies against OPN or macrophage receptor MARCO were used to fluorescently image pro-inflammatory foamy macrophages78 and M1-like macrophages109, respectively. This fluorescence imaging-facilitated diagnosis readily distinguished atherosclerotic plaques at risk of rupture from stable plaques, suggesting a possible solution to some of the current challenges in atherosclerosis management. Additionally, the early, developmental and late stages of atherosclerosis could be identified with fluorescence imaging with near-infrared-emissive, quantum dot-encapsulated, virus-like nanoparticles whose surfaces were modified with the corresponding targeting peptides79. In addition to quantitatively characterizing macrophage burden, macrophage-associated enzyme-activatable nanoparticles that can be switched from fluorescent off to on have also shown great promise in imaging vulnerable plaques76.
Photoacoustic imaging
As documented by findings from a clinical trial110 reported in 2020, non-invasive PAI, which combines the sensitivity of optical imaging with the acoustic resolution and imaging depth of ultrasonography, is an excellent imaging modality for the study of plaque composition in carotid arteries. Similar to other imaging modalities, the use of macrophage-targeting PAI nanoprobes could substantially improve the regional PAI intensity for vulnerable plaque visualization. For instance, intravenous administration of anti-OPN antibody-conjugated Ti3C2 nanosheet–indocyanine green nanocomposites that specifically target macrophage-derived foam cells markedly improved the PAI contrast of vulnerable plaques in the aortic arch in mice111. In addition, self-assembled bovine serum albumin-based nanoprobes that have distinct PAI responses to glutathione and H2O2 allowed the study of inflammation-related processes of macrophages in rupture-prone plaques in mice112. These imaging nanoprobes could help differentiate advanced vulnerable plaques from early-stage lesions on the basis of the image pattern and degree of contrast enhancement.
Dual-modality imaging
Each imaging modality has unique diagnostic characteristics and the combination of two or more imaging modalities provides complementary information from different angles and deepens our understanding of the complex pathophysiology of atherosclerosis. For example, the lack of anatomical reference of PET necessitates the integration of CT or MRI to synergistically characterize inflammation in atherosclerotic plaques and evaluate the therapeutic efficacy of medical interventions58,59,63,107,113,114. With a fully integrated MRI–PET scanner, the high sensitivity and specificity of PET, in combination with the superior soft-tissue contrast of MRI, yields functional, molecular and anatomical information useful to the study of plaque burden in the clinical setting75. In addition, the combination of MRI and fluorescence imaging enables the acquisition of images with both high spatial resolution and high sensitivity, which is particularly advantageous in monitoring inflammatory macrophages in carotid plaques78,109. As imaging technology continues to evolve, more powerful, combined-modality imaging will accelerate and improve the diagnosis of atherosclerosis.
Nanotherapeutics in atherosclerosis
The activation and suppression of specific signalling pathways (pro-inflammatory or anti-inflammatory) in macrophages has important roles in the progression of atherosclerosis. Consequently, the manipulation of macrophage functions with the use of exogenous small molecules or biomolecules (such as siRNA, miRNA, peptides and proteins) has therapeutic potential in atherosclerosis via the activation or silencing of signalling pathways in macrophages, including manipulations that promote the inflammation resolution roles of macrophages. In this section, we review and discuss the latest progress and developments in using macrophage-targeted nanotherapeutics for atherosclerosis treatment. Specifically, we discuss design and strategy considerations relevant to how nanomaterials could efficiently carry and stabilize encapsulated therapeutics. In addition, we focus on how to substantially improve the delivery efficiency and bioavailability of cargo molecules to macrophages with the use of rationally designed nanomaterials. Specific targeting moieties that could substantially increase the uptake efficiency of nanomaterials by macrophages are also discussed. The primary pathological processes involved in atherosclerosis include monocyte recruitment, macrophage accumulation and proliferation, defective efferocytosis, plaque inflammation, and cholesterol and oxidized LDL accumulation (Fig. 3). These processes have been considered compelling therapeutic targets for atherosclerotic plaque regression and stabilization (Table 1).
a | Monocyte recruitment to the atherosclerotic lesion areas can be reduced by delivering therapeutics to monocytes or to vascular endothelial cells by nanoparticles. b | The proliferation of inflammatory macrophages can be inhibited by nanoparticle-assisted delivery of therapeutics to the lesional macrophages. c | The restoration of efferocytosis in macrophages by nanotherapeutics can help to remove dead cells from atherosclerotic plaques, prevent secondary necrosis and elicit the production of anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGFβ). d | Inflammation can be ameliorated by modulating the functions of lesional macrophages via nanotherapeutics, increasing the secretion of pro-resolving cytokines (such as IL-10 and TGFβ) or inhibiting the secretion of pro-inflammatory cytokines (such as IL-6, IL-1β and TNF) from lesional macrophages. e | Induction of macrophage apoptosis by local heating or by toxic agents can reduce macrophage burden in atherosclerotic lesions. However, this strategy is suitable only for early lesions. In late atherosclerotic lesions, the impaired phagocytic clearance of apoptotic macrophages might lead to secondary necrosis of these cells and a pro-inflammatory response. f | Promotion of cholesterol efflux from the cholesterol-laden macrophage by nanotherapeutics can reduce foam cell formation.
Inhibition of monocyte recruitment and macrophage proliferation
Experimental data indicate that monocyte recruitment to the arterial wall is one of the earliest processes that support the progression of atherosclerotic plaques6,115,116. The suppression of monocyte recruitment has been considered a promising therapeutic target because it would prevent not only the ensuing macrophage accumulation and proliferation in the plaques but also the destabilization and rupture of atherosclerotic plaques117,118,119 (Fig. 3a). For instance, Lameijer and colleagues used reconstituted HDL nanoparticles to deliver a small-molecule inhibitor of the CD40–TRAF6 (tumour necrosis factor receptor-associated factor 6) interaction to circulating Ly6Chigh monocytes59, which are particularly pro-inflammatory. Short-term infusion (four infusions over a period of 1 week) of HDL nanoparticles loaded with the CD40–TRAF6 inhibitor rapidly decreased plaque inflammation by reducing Ly6Chigh monocyte recruitment and macrophage content in the lesion areas in atheroprone Apoe–/– mice as confirmed by transcriptome analysis59. Another study using the same mouse model showed that long-term administration (biweekly treatment for 6 weeks) of this nanoformulation increased plaque stability by increasing collagen cap areas and decreased plaque size while preserving the innate immunity of the mice120. Importantly, the safety of these HDL-based nanoimmunotherapeutics has been proven in non-human primates59, highlighting their potential for clinical translation. Furthermore, another study showed that intravenous administration of pitavastatin-encapsulated poly(d,l-lactic-co-glycolic acid) (PLGA) nanoparticles can reduce plaque destabilization by inhibiting monocyte recruitment in Apoe–/– mice121. Collectively, these findings demonstrate that targeted nanoparticles can effectively stabilize atherosclerotic plaques by acting on circulating monocytes and diminishing their recruitment to the vessel wall.
Aside from leukocytes, vascular endothelial cells that express VCAM1 and promote monocyte recruitment can also serve as therapeutic targets for atherosclerosis management. For instance, Sager and colleagues used siRNA-encapsulated polymeric nanoparticles to inhibit monocyte recruitment to atherosclerotic lesions in Apoe–/– mice after myocardial infarction and to reduce matrix-degrading protease activity in plaques by simultaneously silencing five endothelial cell adhesion molecules in the vascular endothelium18. In addition, the same group employed siRNA-encapsulated lipid-polymer nanoparticles to inhibit leukocyte release from the haematopoietic stem cell niche by silencing Ccl2 (also known as Mcp1) in bone-marrow endothelial cells, thereby reducing the supply of monocytes to the atherosclerotic lesions after myocardial infarction in mice122. These types of nanotherapeutics might provide numerous opportunities for the treatment of atherosclerosis.
Macrophage proliferation in the plaque after monocyte trafficking across the vessel walls and monocyte-to-macrophage differentiation specifically contributes to inflammation in advanced atherosclerotic plaques123 and, therefore, suppression of pro-inflammatory macrophage proliferation with the use of nanotherapeutics could stabilize vulnerable plaques (Fig. 3b). For example, targeted delivery of rapamycin (which is extensively used clinically as an immunosuppressant) with the use of biomimetic nanoparticles, such as leukosomes124 or erythrocyte membrane53, or with H2O2-responsive125 or dual-responsive126 cyclodextrin-based nanoparticles suppresses the proliferation of macrophages in atherosclerotic plaques and reduces the levels of key pro-inflammatory cytokines via rapamycin-mediated inhibition of the NF-κB pathway in mice and is associated with reduced toxicity compared with free rapamycin. Additionally, nanoparticle-assisted delivery of simvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor historically regarded as a blood LDL-cholesterol-lowering drug for atherosclerosis treatment127, can inhibit lesional macrophage proliferation and resolve local inflammation in atherosclerotic Apoe–/– mice128,129,130. A regimen of 1 week of high-dose (four infusions of 60 mg/kg simvastatin) and 12 weeks of low-dose (24 infusions of 15 mg/kg simvastatin) simvastatin-loaded reconstituted HDL (rHDL) nanoparticles reduced the local macrophage burden in the aorta and aortic roots and reduced the plaque area in the aortic sinus of atherosclerotic Apoe–/– mice, respectively128,129. In an effort to demonstrate the clinical translatability of this regimen, the same group used a microfluidizer-based high-pressure homogenization approach for the large-scale production of simvastatin–rHDL nanoimmunotherapeutics in a controlled and reproducible manner for anti-atherogenesis studies in larger animals, including rabbit and pig models of atherosclerosis58,131. Interestingly, a systematic comparison showed that the simvastatin-encapsulated PEGylated polymeric micelles have higher anti-atherosclerosis efficacy in Apoe–/– mice than simvastatin delivery by rHDL nanoparticles or liposomes132. These findings are probably due to the higher stability of the polymeric drug nanoformulation as well as the increased efficiency of targeted drug delivery to plaque macrophages132.
Removing inflammation-triggering cholesterol from the plaque microenvironment by the same type of nanoparticles might also yield therapeutic benefits. For instance, Kim and colleagues designed cargo-switching nanoparticles that can scavenge intracellular cholesterol while releasing simvastatin from the methyl-β-cyclodextrin phospholipid core-shell nanoparticles133. This cargo-switching strategy enabled not only the local delivery of simvastatin to lesional macrophages but also the depletion of pre-existing cholesterol in plaques, thereby achieving synergistic anti-atherogenic efficacy in Apoe–/– mice133. Of note, the cargo-switching nanoparticles did not reduce plasma cholesterol levels, indicating that the anti-atherogenic effects were not mediated by a systemic cholesterol-lowering effect133. Similar to cargo-switching nanoparticles, the administration of atorvastatin-encapsulated macrophage-biomimetic nanoparticles that can inhibit local macrophage proliferation and sequester pro-inflammatory cytokines in the lesions yielded synergistic therapeutic efficacy56. Targeted delivery of dual therapeutics by the same type of nanoparticle showed great promise in improving the therapeutic efficacy in mice56 owing to their capacity to act on complementary anti-atherogenic pathways.
Restoration of the efferocytic capacity of macrophages
Restoring the capacity of macrophages to carry out efferocytosis (the phagocytic clearance of apoptotic cells) has the potential to stabilize vulnerable plaques and reduce inflammation7,49 (Fig. 3c). A 2016 study reported that the upregulation of CD47, a ‘do-not-eat-me’ signal, in atherosclerotic plaques is one cause of defective efferocytosis by plaque macrophages, allowing the local accumulation of apoptotic cells and the promotion of atherosclerosis134. Indeed, although the first trial in humans of an anti-CD47 antibody therapy efficiently restored efferocytosis by macrophages, the antibody also resulted in anaemia owing to the removal of red blood cells from the spleen135. To reactivate efferocytosis while reducing adverse effects, Flores and colleagues developed a macrophage-specific nanotherapy, namely SWNTs that encapsulate a small-molecule inhibitor of signal-regulatory protein-α (SIRPα), the antiphagocytic target of CD47 (ref.63). The researchers showed that these nanoparticles reactivated macrophage efferocytosis in atherosclerotic plaques and substantially reduced plaque burden in Apoe–/– mice without inducing off-target adverse effects63.
Our findings reveal that the calcium-activated kinase CaMKIIγ is activated in lesional macrophages during atherosclerosis progression and this process was shown to reduce the expression of the efferocytosis receptor MERTK and to further impair efferocytosis and exacerbate plaque necrosis in aortic root lesions of Ldlr–/– mice fed a high-fat diet44,136. To restore efferocytosis in lesional macrophages, we employed macrophage-targeted siCamk2g nanoparticles (termed S2P-siCamk2g), which consist of a PLGA core with encapsulated siRNA–cationic lipid complex and surface-derivatized S2P targeting peptides52. Treatment of atherosclerotic Ldlr–/– mice with S2P-siCamk2g nanoparticles not only effectively restored efferocytosis in atherosclerotic lesional macrophages but also promoted plaque stabilization by increasing fibrous cap thickness and decreasing the necrotic core area.
Anti-inflammatory therapies
Given that chronic inflammation is a central pathological feature throughout all stages of atherosclerosis, inhibition of inflammation has been considered an essential therapeutic strategy137,138 (Fig. 3d). However, as revealed by the CIRT trial139, systemic treatment with a low dose of the anti-inflammatory drug methotrexate did not lower the plasma levels of pro-inflammatory cytokines or reduce cardiovascular events compared with placebo. These findings, combined with the results of the CANTOS36 and COLCOT37 studies, suggest that the targeted delivery of anti-inflammatory nanotherapeutics to lesional macrophages might be preferred over a systemic approach to effectively resolve inflammation while reducing adverse effects. Therefore, an ongoing phase II clinical trial140 has been designed to treat patients with stable coronary disease with methotrexate-loaded LDL-like nanoparticles, which can specifically target LDL receptors on the inflammatory macrophage surface. This approach might provide more therapeutic benefits by the targeted delivery of this anti-inflammatory drug to lesional macrophages.
Great efforts have been made in the past 10 years towards the targeted delivery of anti-inflammatory drugs in animal models of atherosclerosis. For example, attempts have been made to deliver IL-10, an inflammation-resolving cytokine that can temper inflammation, by long-circulating collagen type IV-targeted PLGA67 and cRGD peptide-conjugated pluronic-based141 nanocarriers. The targeted delivery of IL-10 not only significantly increased its systemic half-life but also substantially improved delivery to lesional macrophages compared with free IL-10, effectively improving atherosclerotic plaque stability in Ldlr–/– mice67 or Apoe–/– mice141 fed a high-fat diet. In addition, the same type of collagen type IV-targeted PLGA nanoparticles has been used to deliver a pro-resolving annexin A1 biomimetic Ac2-26 peptide to atherosclerotic macrophages in mice48,142. The targeted delivery of the Ac2-26 peptide by nanoparticles significantly increased its atheroprotective effects in Ldlr–/– mice fed a high-fat diet compared with those of free Ac2-26 peptide treatment48. In 2020, M2-like macrophage-derived exosomes with inflammation tropism were used to deliver hexyl 5-aminolevulinate hydrochloride, an FDA-approved drug that can promote the intracellular generation of anti-inflammatory carbon monoxide and bilirubin via the intrinsic biosynthesis pathway57. The anti-inflammatory cytokines released from M2-like macrophages and the anti-inflammatory bio-products synergistically promoted regression of atherosclerotic plaques in Apoe–/– mice. Interestingly, unlike findings with conventional drug-loaded nanotherapeutics, a study showed that hyaluronan nanoparticles can selectively target inflammatory macrophages in atherosclerotic mice and function as an anti-inflammatory agent on their own (without any payload) by directly interacting with macrophages to downregulate the inflammatory response77. The therapeutic effects were demonstrated by the reduced production of nitric oxide and tumour necrosis factor, two key pro-inflammatory mediators in atherosclerosis, by inflammatory macrophages77. This therapeutic strategy might simplify the preparation of nanotherapeutics without the concerns about drug loading and release behaviour.
The targeted delivery of inhibitors that can reduce the secretion of pro-inflammatory cytokines from plaque macrophages is a particularly promising approach for atherosclerosis treatment. For example, a study published in 2021 showed that inflammatory cell-targeted platelet-derived extracellular vesicles efficiently delivered an NLRP3 inflammasome inhibitor to macrophage-derived foam cells in atherosclerotic plaques in atherosclerotic mice55. This approach significantly reduced local inflammation (reflected by decreased IL-1β levels), atherosclerotic plaque area, and macrophage and T cell burdens in these mice55. Similar therapeutic effects were reported in mice after the targeted delivery of methotrexate with the use of polymeric nanoparticles143,144 and liposomes144. Remarkably, owing to the improved pharmacokinetic profile, bioavailability and drug accumulation in the lesional macrophages, the nanoparticle-facilitated targeted delivery approach significantly increased anti-inflammatory and anti-atherogenic efficacy and reduced systemic toxicity compared with the free drugs alone. Nakashiro and colleagues used PLGA nanoparticles to deliver pioglitazone, a potent agonist of PPARγ that can polarize macrophages towards a less-inflammatory, M2-like subtype in angiotensin II-infused Apoe–/– mice fed a high-fat diet145. This therapeutic strategy is particularly advantageous in advanced atherosclerotic plaques given that macrophages that differentiate from recruited monocytes in advanced lesions might favour the pro-inflammatory M1-like subtype over the M2-like subtype that participates in inflammation resolution138.
Lastly, efficient scavenging of overproduced ROS from lesional macrophages to attenuate ROS-induced inflammation and reduce the secretion of pro-inflammatory cytokines is of considerable interest for atherosclerosis treatment146. Indeed, a series of superoxide dismutase mimetic cyclodextrin-based nanoparticles that efficiently scavenge a broad spectrum of ROS and significantly reduce the serum and atherosclerotic plaque levels of ROS-induced pro-inflammatory cytokines and decreased cholesterol crystal formation in the lesions in Apoe–/– mice were developed147,148.
Macrophage apoptosis
Externally induced macrophage apoptosis can reduce macrophage burden and thereby reduce macrophage-associated inflammation and plaque burden in early atherosclerotic lesions (Fig. 3e). Owing to its high spatial resolution, light can be used to induce apoptosis specifically in lesional macrophages with the use of light-sensitive molecules and thereby avoid compromising the host defence. For instance, McCarthy and colleagues used macrophage-avid, crosslinked, dextran-coated iron oxide nanocrystals loaded with photosensitizers for targeted macrophage ablation149. When activated by 650-nm light, these photosensitizers produce cytotoxic ROS to induce extensive macrophage apoptosis in the carotid artery atheroma in Apoe–/– mice, reducing the macrophage burden and increasing plaque stability. The therapeutic effects shown in this study open new avenues for atherosclerosis treatment because externally modulated macrophage apoptosis could be used to promote plaque regression. Indeed, local hyperthermia generated by photo-activatable photothermal agents that induce macrophage apoptosis has also shown great promise for atherosclerosis treatment, as demonstrated in the Yucatan miniature swine model (PLASMONICS study)150. Encouraged by the superior therapeutic efficacy of the approach used in this study, investigators in a first-in-human trial (NANOM-FIM)151 delivered the same type of silica–gold-based nanoparticles by using a bioengineered on-artery patch followed by local photothermal ablation. This photothermal therapy showed great promise, yielding an average 37.8% reduction in atherosclerotic plaque burden compared with baseline and significantly reducing cardiovascular mortality compared with patients who were implanted with a stent. In addition to gold nanoparticles, tremendous efforts have been made in the past 10 years in designing photothermal agents for photothermal-mediated macrophage apoptosis in preclinical studies such as SWNTs152, gold nanorods153 and MoO2 nanoclusters154. The common advantages of these nanoparticles for efficient photothermal ablation are superior photothermal conversion efficiency, excellent biocompatibility and efficient phagocytic uptake by inflammatory macrophages. Of note, a non-invasive alternating magnetic field in conjunction with magnetic nanoparticles has been extensively studied for antitumour therapy155 and we envision that such a spatiotemporally controllable approach might offer a promising alternative strategy for hyperthermia-induced macrophage apoptosis. Nevertheless, the therapeutic outcomes of this strategy might vary significantly in early versus late atherosclerotic lesions because defective phagocytic clearance of apoptotic macrophages can lead to secondary necrosis, promote a pro-inflammatory response and exacerbate atherosclerosis in late lesions156.
Promotion of cholesterol efflux from macrophages
Lipid metabolism of macrophages is closely associated with the degree of macrophage foam cell formation and severity of atherosclerosis34. Promoting cholesterol efflux from lipid-laden macrophages by increasing ABCA1 or ABCG1 expression has been considered a promising strategy to prevent excess lipid accumulation in atherosclerotic macrophages35,157,158 (Fig. 3f). miRNA-mediated oligonucleotide-based therapeutic approaches that specifically target predefined gene sequences have been shown to upregulate the expression of ABCA1 in macrophages and increase cholesterol efflux159. Similar to siRNAs, this robust nanoparticle-mediated delivery approach is particularly practical to prevent the hydrolysis of delicate miRNA by nucleases in the circulation and peripheral tissues. For instance, chitosan nanoparticle-mediated delivery of efflux-promoting miR-206 or miR-223 markedly increased cholesterol efflux from macrophage-derived foam cells and reverse cholesterol transport in mice81. In addition, integrin-targeting and cyclodextrin-based nanoparticles were used to deliver an antisense oligonucleotide against miR-33, a miRNA that inhibits cholesterol efflux, to macrophages in atherosclerotic lesions and was shown to substantially increase the cholesterol efflux capacity of lesional macrophages and reduce plaque burden in atherosclerotic Apoe–/– mice compared with free antisense miR-33 alone74.
Another strategy that can upregulate ABCA1 and ABCG1 expression to promote cholesterol efflux from macrophages is the activation of LXRs30. However, systemic LXR activation is known to induce liver toxicity and increase plasma and hepatic triglyceride levels, resulting in the failure of this approach in clinical translation160. In the past decade, nanoparticle-mediated delivery of an LXR agonist has shown great promise in promoting ABCA1 expression and cholesterol efflux while reducing adverse liver toxicities62,161,162. Yu and colleagues used biodegradable collagen type IV-targeted poly(d,l-lactide) polymeric nanoparticles to deliver the LXR agonist GW3965 to atherosclerotic lesions in Ldlr–/– mice68. The primary therapeutic effects of this nanoparticle-mediated delivery included increased cholesterol efflux from lesional macrophages and decreased macrophage burden and inflammatory cytokine levels in atherosclerotic mice, while plasma triglyceride and cholesterol levels and hepatic lipid metabolism remained unaffected. Both the improved therapeutic effects and reduced adverse effects generated by nanoparticle-mediated delivery of GW3965 are preferable to free GW3965 treatment68. Additionally, cholesterol efflux was also promoted by synthetic HDL-mediated delivery of the LXRα agonist T1317 to plaque macrophages in Apoe–/– mice163.
In addition to cholesterol efflux, autophagy can prevent foam cell formation by using double membranes in macrophages to sequester and degrade lipid droplets through lysosomal acid lipase164. The activation of autophagy to regress plaques has been demonstrated in Apoe–/– mice by inhibiting mechanistic target of rapamycin (mTOR) through targeted and H2O2-responsive delivery of siRNA with the use of cerium oxide nanowires83 or by the targeted delivery of rapamycin through platelet membrane-coated PLGA nanoparticles165. Of note, local inflammation in atherosclerosis might activate and stimulate platelet attachment to endothelial cells, which makes this biomimetic platelet membrane-coated delivery strategy promising for atherosclerosis management.
Different from the abovementioned cholesterol efflux and lipid-sequestering approaches, a library of sugar-based amphiphilic nanoparticles was designed to block and reduce the expression of macrophage scavenger receptor 1 and CD36, two macrophage surface receptors for oxidized lipid uptake166. The competitive binding strategy and the downregulation of these two scavenger receptors by sugar-based amphiphilic nanoparticles synergistically reduced oxidized lipid accumulation in the arterial wall and atherosclerotic plaque formation in Apoe–/– mice166.
Challenges and perspectives
Over the past two decades, our improved understanding of the pathophysiological processes of atherogenesis and rapid advances in nanotechnology and bioengineering have robustly fuelled research into novel nanotherapeutic strategies to treat cardiovascular diseases. Although clinically approved nanotherapeutics are still scarce167, the shift in therapeutic target from lipid lowering to inflammation resolution has opened new avenues for nanomedical atherosclerosis management as highlighted in this Review (Fig. 4). Although modulation of the immunoprofile of lesional macrophages by nanoparticles has shown great promise in resolving inflammation in atherosclerosis, the concomitant hampering of the innate immune system (which forms the first line of defence against pathogens) needs to be carefully investigated before moving to clinical trials. In this regard, accurate modulation of the local immune responses is particularly crucial to reduce systemic adverse effects. On the basis of findings from the CANTOS36, CIRT168 and COLCOT37 trials, which had large study cohorts, targeted delivery of anti-inflammatory therapeutics is superior to systemic delivery in reducing atherosclerotic plaque inflammation without compromising the host defence. Additionally, the targeted delivery of nanotherapeutics to lesional macrophages could also diminish systemic adverse effects, which are frequently the result of treatment with free drugs. Of note, the targeted delivery of dual therapeutics that synergistically act on different anti-atherogenic pathways might also increase the efficacy of the nanotherapeutics.
Nevertheless, despite the great promise already shown, we should note that the success of nanotherapeutics in preclinical studies cannot guarantee their efficacy in humans169. This discrepancy might result from the substantial differences in atherosclerotic pathobiology between animal models and humans. In animal models, atherosclerosis is usually established in weeks, whereas atherosclerotic plaques in patients develop over a period of years or even decades and are therefore more complex and heterogeneous170. Additionally, animal models are often genetically manipulated in a way that is not necessarily relevant to humans. For example, Apoe–/– mice have a completely blunted clearance of apoE-containing lipoproteins and therefore develop atherosclerotic lesions independently of cholesterol feeding whereas, in humans, atherosclerosis usually develops in a multigenetic manner and in combination with environmental factors171. The use of mouse models, such as the Apoe*3-Leiden.CETP mouse, with a lipoprotein metabolism closer to that of humans, might have advantages172. Mice are naturally deficient in cholesteryl-ester transfer protein (CETP) and therefore have high levels of HDL and low levels of LDL and VLDL. The ApoE*3-Leiden.CETP mouse contains a human CETP transgene as well as a mutated human APOE gene, resulting in moderately reduced LDL receptor-mediated clearance of apoB-containing lipoproteins as well as a humanized lipoprotein cholesterol distribution. Although findings from ApoE*3-Leiden.CETP mice might translate better to humans compared with other mouse models, these models have not yet been used in preclinical studies on macrophage-targeted nanomedicine of atherosclerosis (Table 1). Furthermore, it is advisable to feed these mice a diet that only moderately increases plasma cholesterol levels and allow atherosclerosis to develop over a longer period of time, similar to atherosclerosis development in humans. Lastly, whenever possible, nanomedicine-mediated findings in mice should be validated by studies using human biobanks and the identification of novel therapeutic targets in mice should be paralleled by human genetic studies. Moreover, macrophage subtypes and plaque composition vary from patient to patient and during different stages of atherosclerosis22,173, making administration of personalized nanotherapeutics particularly crucial to achieving the desirable therapeutic outcomes.
Nanoparticle-facilitated bioimaging technologies can non-invasively characterize plaque composition, monitor inflammatory macrophages and assess therapeutic outcomes. Current imaging modalities have rapidly evolved and state-of-the-art imaging technologies are now being transformed from tools for imaging existing atherosclerosis in symptomatic patients to sophisticated means of non-invasively characterizing early-stage subclinical abnormalities. The clinically available integrated imaging systems in combination with two or even more imaging modalities can synergize the strengths of each individual technique and thereby provide more insights into atherogenesis at the molecular and functional levels. This imaging-facilitated diagnosis would help physicians to provide appropriate early medical interventions to prevent further complications. In addition, the importance of accurate bioimaging has been demonstrated, for example, by providing surrogate end points for novel atherosclerosis therapeutics in clinical settings92,103,174. Bioimaging-assisted characterization enables a dramatic reduction of the number of patients needed in studies and costs as well as shortening the observation period for assessing the efficacy of newly developed therapeutic interventions in clinical settings175. In addition to clinical imaging modalities, the recently developed ‘shortwave infrared’ (SWIR, 1,000–2,000 nm) imaging, also called ‘second window of near-infrared’ (900–1700 nm) imaging, acquired using an InGaAs camera, might provide an alternative method to non-invasively monitor inflammatory burdens in real time. More specifically, the SWIR region of the electromagnetic spectrum has advantages over the traditional near-infrared window (700–900 nm) owing to deeper tissue penetration, higher spatial resolution, decreased autofluorescence, and minimized photon absorption and scattering by tissues at these wavelengths176,177. In addition, the multiplexed SWIR images might provide more mechanistic insights into the interaction between different macrophage subsets (such as M1, M2a, M2b and M2c) and other cells (for example, T cells, B cells, neutrophils and vascular smooth muscle cells) or biomolecules in the microenvironments of atherosclerotic plaques when the target-specific nanoparticles are used.
If some nanoparticles for atherosclerosis diagnosis and therapy reached the clinic, it would be highly beneficial. However, before then, the many prerequisites include a detailed evaluation of the biosafety, biodistribution and clearance of the nanotherapeutics in preclinical studies, industrial-scale production of nanoparticles under good manufacturing practice (GMP) to guarantee reproducibility from batch to batch, and investigation of whether the nanotherapeutics show higher treatment efficacy and fewer adverse effects than those of free drugs alone. Nanoparticle-associated challenges and considerations are summarized in Box 2. For example, several nanoparticle platforms (such as HDL-like and LDL-like nanoparticles) that can target lesional macrophages for atherosclerosis treatment and diagnosis have entered clinical trials, as highlighted in Table 2. Of note, the ongoing AEGIS-II trial178 will test whether HDL-like nanoparticles can reduce the risk of major adverse cardiovascular events in patients with acute coronary syndrome. This nanoformulation, previously demonstrated to improve cholesterol efflux efficacy of lesional macrophages in both healthy individuals and patients with coronary or peripheral artery disease179, might provide therapeutic benefits for patients with acute coronary syndromes. In addition, an equally important but often overlooked consideration is that even though some nanocarriers have been proven to be biocompatible and to have superior safety profiles for drug delivery and bioimaging, they might not be suitable for studies on anti-atherosclerosis therapies. For example, the administration of amorphous silica nanoparticles would intensify macrophage infiltration, increase endoplasmic reticulum stress in atherosclerotic plaques, and increase the serum levels of LDL cholesterol and total triglycerides, thereby actually exacerbating atherosclerosis progression180. In addition, systemic administration of the FDA-approved iron supplement ferumoxytol nanoparticles might increase iron accumulation and the presence of pro-inflammatory M1-like macrophages in atherosclerotic lesions181,182. Consequently, the immune responses modulated by nanoparticles must be comprehensively investigated before the use of these strategies in clinical trials.
Thanks to the invention of advanced biological research techniques, such as single-cell RNA sequencing and flow cytometry, our understanding of the pathophysiological processes of atherosclerosis has evolved far beyond the former focus on lipid accumulation because more inflammation-associated pathological markers and the transcriptomic and epigenomic characteristics of lesional macrophages have been identified21,183. This transcriptome-based landscape of atherosclerotic plaque progression could accelerate the discovery of more diagnostic and therapeutic targets and, consequently, more nanoparticle-based interventions can be tailor-made against those cardiovascular disease processes. In addition, vaccination against atherosclerosis by selectively modulating atheroprotective and pro-atherogenic immunity has shown great promise over the past decade184,185,186. Modulation of the immune response using a vaccine containing antigens relevant to atherogenesis offers the potential to elicit a specific immune response against the relevant antigens without affecting host immunity. For example, the administration of vaccines containing apoB-100 peptides derived from oxidized LDL has shown therapeutic efficacy in an animal model of atherosclerosis by increasing the activation of regulatory T lymphocytes in the plaques. Indeed, the emerging use of mRNA as a therapeutic agent has been applied to modulate innate and adaptive immunogenicity by increasing intracellular protein translation and activating specific signalling pathways187. Indeed, such an effective nanoparticle-mediated mRNA delivery strategy has dramatically affected the landscape of vaccine development and mRNA vaccines have been applied to prevent coronavirus disease 2019 (COVID-19)188,189. We envision that nanoparticle-mediated mRNA vaccine technology might also be used to prevent the initiation and even progression of atherosclerosis.
Conclusions
Although lipid-lowering therapies have been considered effective strategies for atherosclerosis treatment, cardiovascular disease remains the leading cause of death worldwide1. Innovative nanotechnology and our improved understanding of atherosclerosis pathobiology have synergistically propelled the development of novel nanotherapeutics for atherosclerosis diagnosis and treatment. In this Review, we have highlighted progress in the past two decades in macrophage-targeted nanomedicine for atherosclerosis diagnosis and treatment and have addressed considerations for clinical translation. Despite the obvious challenges, we believe that the numerous successes of nanomedicine-based approaches in preclinical studies of atherosclerosis and their use in humans for cancer treatment bode well for their eventual use in the diagnosis and treatment of patients with cardiovascular disease.
References
Virani, S. S. et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation 143, e254–e743 (2021).
Tabas, I., Williams, K. J. & Borén, J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116, 1832–1844 (2007).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Mestas, J. & Ley, K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 18, 228–232 (2008).
Glass, C. K. & Witztum, J. L. Atherosclerosis. The road ahead. Cell 104, 503–516 (2001).
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011). This review discusses the central roles of monocyte-derived macrophages in both early atherogenesis and advanced plaque progression.
Doran, A. C., Yurdagul, A. & Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 20, 254–267 (2020).
Yurdagul, A., Doran, A. C., Cai, B., Fredman, G. & Tabas, I. A. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovasc. Med. 4, 86 (2018).
Kasikara, C., Doran, A. C., Cai, B. & Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Invest. 128, 2713–2723 (2018).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014). This review discusses mechanisms of atherosclerotic plaque initiation and progression and the concepts of plaque activity, burden and vulnerability.
Lieb, W., Enserro, D. M., Larson, M. G. & Vasan, R. S. Residual cardiovascular risk in individuals on lipid-lowering treatment: quantifying absolute and relative risk in the community. Open Heart 5, e000722 (2018).
Dhindsa, D. S., Sandesara, P. B., Shapiro, M. D. & Wong, N. D. The evolving understanding and approach to residual cardiovascular risk management. Front. Cardiovasc. Med. 7, 88 (2020).
Lobatto, M. E., Fuster, V., Fayad, Z. A. & Mulder, W. J. M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 10, 835–852 (2011).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotech. 2, 751–760 (2007).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Chen, W., Glackin, C. A., Horwitz, M. A. & Zink, J. I. Nanomachines and other caps on mesoporous silica nanoparticles for drug delivery. Acc. Chem. Res. 52, 1531–1542 (2019).
Combadière, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117, 1649–1657 (2008).
Sager, H. B. et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl Med. 8, 342ra80 (2016).
Stoneman, V. et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ. Res. 100, 884–893 (2007).
Hoeksema, M. A., Stöger, J. L. & De Winther, M. P. J. Molecular pathways regulating macrophage polarization: Implications for atherosclerosis. Curr. Atheroscler. Rep. 14, 254–263 (2012).
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. 127, 1437–1455 (2020).
Stöger, J. L. et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 225, 461–468 (2012).
Chinetti-Gbaguidi, G. et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ. Res. 108, 985–995 (2011).
Kennedy, M. A. et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1, 121–131 (2005).
Oram, J. F., Lawn, R. M., Garvin, M. R. & Wade, D. P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 275, 34508–34511 (2000).
Wang, N., Lan, D., Chen, W., Matsuura, F. & Tall, A. R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl Acad. Sci. USA 101, 9774–9779 (2004).
Wang, N., Silver, D. L., Costet, P. & Tall, A. R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275, 33053–33058 (2000).
Chawla, A. et al. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7, 161–171 (2001).
Tangirala, R. K. et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl Acad. Sci. USA 99, 11896–11901 (2002).
Naik, S. U. et al. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 113, 90–97 (2006).
Joseph, S. B. et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl Acad. Sci. USA 99, 7604–7609 (2002).
Fessler, M. B. The challenges and promise of targeting the liver X receptors for treatment of inflammatory disease. Pharmacol. Ther. 181, 1–12 (2018).
He, C. et al. Cultured macrophages transfer surplus cholesterol into adjacent cells in the absence of serum or high-density lipoproteins. Proc. Natl Acad. Sci. USA 117, 10476–10483 (2020).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Westerterp, M. et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ. Res. 112, 1456–1465 (2013).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017). Results of a clinical trial that shows that anti-inflammatory therapy against IL-1β can reduce the risk of cardiovascular events in patients with previous myocardial infarction.
Tardif, J.-C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Hansson, G. K., Libby, P. & Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 278, 483–493 (2015).
Virmani, R., Burke, A. P., Farb, A. & Kolodgie, F. D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 47, C13–C18 (2006).
Galis, Z. S., Sukhova, G. K., Lark, M. W. & Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 94, 2493–2503 (1994).
Quillard, T. et al. Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 2464–2472 (2011).
Herman, M. P. et al. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation 104, 1899–1904 (2001).
Boyle, J. J., Weissberg, P. L. & Bennett, M. R. Tumor necrosis factor-α promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler. Thromb. Vasc. Biol. 23, 1553–1558 (2003).
Doran, A. C. et al. CAMKIIγ suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J. Clin. Invest. 127, 4075–4089 (2017).
Bäck, M., Yurdagul, A., Tabas, I., Öörni, K. & Kovanen, P. T. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16, 389–406 (2019).
Schrijvers, D. M., De Meyer, G. R. Y., Kockx, M. M., Herman, A. G. & Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, 1256–1261 (2005).
Fredman, G. & Tabas, I. Boosting inflammation resolution in atherosclerosis: the next frontier for therapy. Am. J. Pathol. 187, 1211–1221 (2017).
Fredman, G. et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl Med. 7, 275ra20 (2015).
Yurdagul, A. et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 31, 518–533.e10 (2020).
Bertrand, N. et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 8, 777 (2017).
Walkey, C. D. & Chan, W. C. W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 41, 2780–2799 (2012).
Tao, W. et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl Med. 12, eaay1063 (2020). This study shows that macrophage-targeted siRNA nanoparticles can restore efferocytic capacity of atherosclerotic lesional macrophages and, therefore, reduce plaque burden in a preclinical model of advanced atherosclerosis.
Wang, Y. et al. Biomimetic nanotherapies: red blood cell based core–shell structured nanocomplexes for atherosclerosis management. Adv. Sci. 6, 1900172 (2019).
Wei, X. et al. Nanoparticle functionalization with platelet membrane enables multifactored biological targeting and detection of atherosclerosis. ACS Nano 12, 109–116 (2018).
Ma, Q. et al. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J. Control. Rel. 329, 445–453 (2021).
Gao, C. et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 11, 2622 (2020).
Wu, G. et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew. Chem. Int. Ed. 59, 4068–4074 (2020).
Binderup, T. et al. Imaging-assisted nanoimmunotherapy for atherosclerosis in multiple species. Sci. Transl Med. 11, eaaw7736 (2019).
Lameijer, M. et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng. 2, 279–292 (2018).
Terashima, M. et al. Human ferritin cages for imaging vascular macrophages. Biomaterials 32, 1430–1437 (2011).
Uchida, M. et al. Protein cage nanoparticles bearing the LyP-1 peptide for enhanced imaging of macrophage-rich vascular lesions. ACS Nano 5, 2493–2502 (2011).
Tang, J. et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl Acad. Sci. USA 113, E6731–E6740 (2016).
Flores, A. M. et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 15, 154–161 (2020). This study utilizes ‘Trojan horse’ single-walled carbon nanotubes to reactivate efferocytic capacity of macrophages by inhibiting the antiphagocytic CD47–SIRPα signalling axis.
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011).
Kim, Y. et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc. Natl Acad. Sci. USA 111, 1078–1083 (2014).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017).
Kamaly, N. et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano 10, 5280–5292 (2016).
Yu, M. et al. Targeted nanotherapeutics encapsulating liver X receptor agonist GW3965 enhance antiatherogenic effects without adverse effects on hepatic lipid metabolism in Ldlr−/− mice. Adv. Healthc. Mater. 6, 1700313 (2017).
Stein-Merlob, A. F. et al. Atheroma susceptible to thrombosis exhibit impaired endothelial permeability in vivo as assessed by nanoparticle-based fluorescence molecular imaging. Circ. Cardiovasc. Imaging 10, e005813 (2017).
Beldman, T. J. et al. Nanoparticle-aided characterization of arterial endothelial architecture during atherosclerosis progression and metabolic therapy. ACS Nano 13, 13759–13774 (2019).
Poller, W. C. et al. Uptake of citrate-coated iron oxide nanoparticles into atherosclerotic lesions in mice occurs via accelerated transcytosis through plaque endothelial cells. Nano Res. 9, 3437–3452 (2016).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Majmudar, M. D. et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 112, 755–761 (2013).
Li, C. et al. Site-specific microRNA-33 antagonism by pH-responsive nanotherapies for treatment of atherosclerosis via regulating cholesterol efflux and adaptive immunity. Adv. Funct. Mater. 30, 2002131 (2020).
Senders, M. L. et al. Nanobody-facilitated multiparametric PET/MRI phenotyping of atherosclerosis. JACC Cardiovasc. Imaging 12, 2015–2026 (2019).
Narita, Y. et al. Macrophage-targeted, enzyme-triggered fluorescence switch-on system for detection of embolism-vulnerable atherosclerotic plaques. J. Control. Rel. 302, 105–115 (2019).
Beldman, T. J. et al. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano 11, 5785–5799 (2017).
Qiao, R. et al. Molecular imaging of vulnerable atherosclerotic plaques in vivo with osteopontin-specific upconversion nanoprobes. ACS Nano 11, 1816–1825 (2017).
Sun, X. et al. In vivo targeting and imaging of atherosclerosis using multifunctional virus-like particles of simian virus 40. Nano Lett. 16, 6164–6171 (2016).
Nie, S. et al. Detection of atherosclerotic lesions and intimal macrophages using CD36-targeted nanovesicles. J. Control. Rel. 220, 61–70 (2015).
Nguyen, M. A. et al. Delivery of microRNAs by chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano 13, 6491–6505 (2019).
Lu, J. et al. Biofunctional polymer-lipid hybrid high-density lipoprotein-mimicking nanoparticles loading anti-miR155 for combined antiatherogenic effects on macrophages. Biomacromolecules 18, 2286–2295 (2017).
Gao, W. et al. H2O2-responsive and plaque-penetrating nanoplatform for mTOR gene silencing with robust anti-atherosclerosis efficacy. Chem. Sci. 9, 439–445 (2018).
Behzadi, S. et al. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 46, 4218–4244 (2017).
Weissleder, R., Nahrendorf, M. & Pittet, M. J. Imaging macrophages with nanoparticles. Nat. Mater. 13, 125–138 (2014).
Schmitz, S. A. et al. Magnetic resonance imaging of atherosclerotic plaques using superparamagnetic iron oxide particles. J. Magn. Reson. Imaging 14, 355–361 (2001).
Kool, M. E. et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107, 2453–2458 (2003).
Morishige, K. et al. High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation 122, 1707–1715 (2010).
Qiao, H. et al. MRI/optical dual-modality imaging of vulnerable atherosclerotic plaque with an osteopontin-targeted probe based on Fe3O4 nanoparticles. Biomaterials 112, 336–345 (2017).
Cheng, D. et al. Detection of vulnerable atherosclerosis plaques with a dual-modal single-photon-emission computed tomography/magnetic resonance imaging probe targeting apoptotic macrophages. ACS Appl. Mater. Interfaces 7, 2847–2855 (2015).
Trivedi, R. A. et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler. Thromb. Vasc. Biol. 26, 1601–1606 (2006). Together with Kool et al. (2003), this study shows that ultrasmall superparamagnetic particles of iron oxide-enhanced MRI can be used for in vivo detection of macrophages in human atherosclerotic plaques.
Tang, T. Y. et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 53, 2039–2050 (2009).
Nowogrodzki, A. The world’s strongest MRI machines are pushing human imaging to new limits. Nature 563, 24–26 (2018).
Amirbekian, V. et al. Detecting and assessing macrophages in vivo to evalute atherosclerosis noninvasively using molecular MRI. Proc. Natl Acad. Sci. USA 104, 961–966 (2007).
Xing, H. et al. Ultrasmall NaGdF4 nanodots for efficient MR angiography and atherosclerotic plaque imaging. Adv. Mater. 26, 3867–3872 (2014).
Grobner, T. & Prischl, F. C. Immune cell screening of a nanoparticle library improves. Kidney Int. 72, 260–264 (2007).
Van Heeswijk, R. B. et al. Fluorine MR imaging of inflammation in atherosclerotic plaque in vivo. Radiology 275, 421–429 (2015).
Akazawa, K. et al. Perfluorocarbon-based 19F MRI nanoprobes for in vivo multicolor imaging. Angew. Chem. Int. Ed. 57, 16742–16747 (2018).
Budoff, M. J. et al. Assessment of coronary artery disease by cardiac computed tomography. Circulation 114, 1761–1791 (2006).
Hyafil, F. et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 13, 636–641 (2007).
Ding, J. et al. CT/fluorescence dual-modal nanoemulsion platform for investigating atherosclerotic plaques. Biomaterials 34, 209–216 (2013).
Schwartz, G. G. et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367, 2089–2099 (2012).
Fayad, Z. A. et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 378, 1547–1559 (2011).
Cormode, D. P. et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology 256, 774–782 (2010).
Danad, I., Fayad, Z. A., Willemink, M. J. & Min, J. K. New applications of cardiac computed tomography. JACC Cardiovasc. Imaging 8, 710–723 (2015).
Rudd, J. H. F. et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105, 2708–2711 (2002).
Keliher, E. J. et al. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat. Commun. 8, 14064 (2017).
Pérez-Medina, C. et al. In vivo PET imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc. Imaging 9, 950–961 (2016).
Wang, Y. et al. Optical/MRI dual-modality imaging of M1 macrophage polarization in atherosclerotic plaque with MARCO-targeted upconversion luminescence probe. Biomaterials 219, 119378 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04237064 (2020).
Ge, X. et al. A non-invasive nanoprobe for in vivo photoacoustic imaging of vulnerable atherosclerotic plaque. Adv. Mater. 32, 2000037 (2020).
Gao, W. et al. A redox-responsive self-assembled nanoprobe for photoacoustic inflammation imaging to assess atherosclerotic plaque vulnerability. Anal. Chem. 91, 1150–1156 (2019).
Nahrendorf, M. et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 117, 379–387 (2008).
Withana, N. P. et al. Dual-modality activity-based probes as molecular imaging agents for vascular inflammation. J. Nucl. Med. 57, 1583–1590 (2016).
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Potteaux, S. et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe-/- mice during disease regression. J. Clin. Invest. 121, 2025–2036 (2011).
Hilgendorf, I., Swirski, F. K. & Robbins, C. S. Monocyte fate in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 35, 272–279 (2015).
Lutgens, E. et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J. Exp. Med. 207, 391–404 (2010).
Seijkens, T. T. P. et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J. Am. Coll. Cardiol. 71, 527–542 (2018). Together with Lameijer et al. (2018), this study shows that nanoimmunotherapy can inhibit monocyte recruitment and be used to treat atherosclerosis by targeting CD40–TRAF6 signalling.
Katsuki, S. et al. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation 129, 896–906 (2014).
Krohn-Grimberghe, M. et al. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nat. Biomed. Eng. 4, 1076–1089 (2020).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Boada, C. et al. Rapamycin-loaded biomimetic nanoparticles reverse vascular inflammation. Circ. Res. 126, 25–37 (2020).
Dou, Y. et al. Non-proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials 143, 93–108 (2017).
Zhang, R. et al. A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 230, 119605 (2020).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Tang, J. et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 1, e1400223 (2015).
Duivenvoorden, R. et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 5, 3065 (2014). Together with Tang et al (2015), this study presents data that demonstrate that statin-loaded HDL-like nanoparticles can effectively decrease macrophage burden in atherosclerotic plaques.
Hossaini Nasr, S. et al. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale 12, 9541–9556 (2020).
Kim, Y. et al. Single step reconstitution of multifunctional high-density lipoprotein-derived nanomaterials using microfluidics. ACS Nano 7, 9975–9983 (2013).
Alaarg, A. et al. A systematic comparison of clinically viable nanomedicines targeting HMG-CoA reductase in inflammatory atherosclerosis. J. Control. Rel. 262, 47–57 (2017).
Kim, H. et al. Affinity-driven design of cargo-switching nanoparticles to leverage a cholesterol-rich microenvironment for atherosclerosis therapy. ACS Nano 14, 6519–6531 (2020).
Kojima, Y. et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90 (2016).
Advani, R. et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N. Engl. J. Med. 379, 1711–1721 (2018).
Thorp, E., Cui, D., Schrijvers, D. M., Kuriakose, G. & Tabas, I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe-/- mice. Arterioscler. Thromb. Vasc. Biol. 28, 1421–1428 (2008).
Soehnlein, O. & Libby, P. Targeting inflammation in atherosclerosis — from experimental insights to the clinic. Nat. Rev. Drug Discov. 20, 589–610 (2021). This review describes the role of inflammation in atherogenesis and summarizes strategies of inflammation targeting for atherosclerosis treatment.
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 (2010).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380, 752–762 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04616872 (2020).
Kim, M. et al. Targeted delivery of anti-inflammatory cytokine by nanocarrier reduces atherosclerosis in Apo E-/- mice. Biomaterials 226, 119550 (2020).
Kamaly, N. et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc. Natl Acad. Sci. USA 110, 6506–6511 (2013).
Stigliano, C. et al. Methotraxate-loaded hybrid nanoconstructs target vascular lesions and inhibit atherosclerosis progression in ApoE−/− mice. Adv. Healthc. Mater. 6, 1601286 (2017).
Di Francesco, V. et al. Modulating lipoprotein transcellular transport and atherosclerotic plaque formation in ApoE−/− mice via nanoformulated lipid-methotrexate conjugates. ACS Appl. Mater. Interfaces 12, 37943–37956 (2020).
Nakashiro, S. et al. Pioglitazone-incorporated nanoparticles prevent plaque destabilization and rupture by regulating monocyte/macrophage differentiation in ApoE−/− Mice. Arterioscler. Thromb. Vasc. Biol. 36, 491–500 (2016).
Wang, Y., Wang, G. Z., Rabinovitch, P. S. & Tabas, I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ. Res. 114, 421–433 (2014).
Wang, Y. et al. Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano 12, 8943–8960 (2018).
Guo, J. et al. Cyclodextrin-derived intrinsically bioactive nanoparticles for treatment of acute and chronic inflammatory diseases. Adv. Mater. 31, 1904607 (2019).
McCarthy, J. R., Korngold, E., Weissleder, R. & Jaffer, F. A. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small 6, 2041–2049 (2010).
Kharlamov, A. N. & Gabinsky, J. L. Plasmonic photothermic and stem cell therapy of atherosclerotic plaque as a novel nanotool for angioplasty and artery remodeling. Rejuvenation Res. 15, 222–230 (2012).
Kharlamov, A. N. et al. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale 7, 8003–8015 (2015).
Kosuge, H. et al. Near infrared imaging and photothermal ablation of vascular inflammation using single-walled carbon nanotubes. J. Am. Heart Assoc. 1, e002568 (2012).
Qin, J. et al. Gold nanorods as a theranostic platform for in vitro and in vivo imaging and photothermal therapy of inflammatory macrophages. Nanoscale 7, 13991–14001 (2015).
Wang, X. et al. Differential phagocytosis-based photothermal ablation of inflammatory macrophages in atherosclerotic disease. ACS Appl. Mater. Interfaces 11, 41009–41018 (2019).
Chen, W., Cheng, C. A. & Zink, J. I. Spatial, temporal, and dose control of drug delivery using noninvasive magnetic stimulation. ACS Nano 13, 1292–1308 (2019).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: The importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Rosenson, R. S. et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919 (2012).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364, 127–135 (2011).
Feinberg, M. W. & Moore, K. J. MicroRNA regulation of atherosclerosis. Cir. Res. 118, 703–720 (2016).
Hong, C. & Tontonoz, P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 13, 433–444 (2014).
Zhang, X. Q. et al. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Adv. Healthc. Mater. 4, 228–236 (2015).
Gadde, S. et al. Development of therapeutic polymeric nanoparticles for the resolution of inflammation. Adv. Healthc. Mater. 3, 1448–1456 (2014).
Guo, Y. et al. Synthetic high-density lipoprotein-mediated targeted delivery of liver X receptors agonist promotes atherosclerosis regression. EBioMedicine 28, 225–233 (2018).
Liao, X. et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 15, 545–553 (2012).
Song, Y. et al. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE−/−) mice. Nanomedicine 15, 13–24 (2019).
Lewis, D. R. et al. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc. Natl Acad. Sci. USA 112, 2693–2698 (2015).
US Food and Drug Administration. Highlights of prescribing information: Fenoglide (fenofibrate) tablets (FDA, 1993).
Aday, A. W. & Ridker, P. M. Targeting residual inflammatory risk: a shifting paradigm for atherosclerotic disease. Front. Cardiovasc. Med. 6, 16 (2019).
van der Valk, F. M. et al. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomedicine 11, 1039–1046 (2015).
Bentzon, J. F. & Falk, E. Atherosclerotic lesions in mouse and man: Is it the same disease? Curr. Opin. Lipidol. 21, 434–440 (2010).
Zadelaar, S. et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 27, 1706–1721 (2007).
Westerterp, M. et al. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arter. Thromb. Vasc. Biol. 26, 2552–2559 (2006).
Marso, S. P. et al. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc. Imaging 5 (Suppl. 3), S42–S52 (2012).
Tawakol, A. et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose- positron emission tomography/computed tomography feasibility study. J. Am. Coll. Cardiol. 62, 909–917 (2013).
Mulder, W. J. M., Jaffer, F. A., Fayad, Z. A. & Nahrendorf, M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 6, 239sr1 (2014).
Cosco, E. D. et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 12, 1123–1130 (2020).
Chen, W. et al. Shortwave infrared imaging with J-aggregates stabilized in hollow mesoporous silica nanoparticles. J. Am. Chem. Soc. 141, 12475–12480 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03473223 (2021). This is an ongoing, large-scale, phase III clinical trial that will test whether HDL-like nanoparticles can reduce the risk of major adverse cardiovascular events in patients with acute coronary syndrome.
Gille, A., D’Andrea, D., Tortorici, M. A., Hartel, G. & Wright, S. D. CSL112 (apolipoprotein A-I [human]) enhances cholesterol efflux similarly in healthy individuals and stable atherosclerotic disease patients. Arter. Thromb. Vasc. Biol. 38, 953–963 (2018).
Ma, R. et al. Amorphous silica nanoparticles accelerated atherosclerotic lesion progression in ApoE-/-mice through endoplasmic reticulum stress-mediated CD36 up-regulation in macrophage. Part. Fibre Toxicol. 17, 50 (2020).
Zanganeh, S. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 11, 986–994 (2016).
Stadler, N., Lindner, R. A. & Davies, M. J. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler. Thromb. Vasc. Biol. 24, 949–954 (2004).
Williams, J. W. et al. Single cell RNA sequencing in atherosclerosis research. Circ. Res. 126, 1112–1126 (2020).
Nilsson, J. & Hansson, G. K. Vaccination strategies and immune modulation of atherosclerosis. Circ. Res. 126, 1281–1296 (2020).
Hansson, G. K. & Nilsson, J. Developing a vaccine against atherosclerosis. Nat. Rev. Cardiol. 17, 451–452 (2020).
Chyu, K. Y. & Shah, P. K. In pursuit of an atherosclerosis vaccine chasing the Holy Grail. Circ. Res. 123, 1121–1123 (2018).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug. Discov. 17, 261–279 (2018).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04148833 (2020).
Zheng, K. H. et al. No benefit of HDL mimetic CER-001 on carotid atherosclerosis in patients with genetically determined very low HDL levels. Atherosclerosis 311, 13–19 (2020).
Nicholls, S. J. et al. Effect of infusion of high-density lipoprotein mimetic containing recombinant apolipoprotein A-I Milano on coronary disease in patients with an acute coronary syndrome in the MILANO-PILOT trial: a randomized clinical trial. JAMA Cardiol. 3, 806–814 (2018).
Kharlamov, A. N. et al. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: long-term outcomes and safety in NANOM-FIM trial. Futur. Cardiol. 13, 345–363 (2017). Together with Kharlamov et al. (2015), this is the first-in-human trial that demonstrates that plasmonic photothermal therapy can reduce atherosclerotic plaque burden and major adverse cardiovascular events.
Nicholls, S. J. et al. Effect of serial infusions of CER-001, a pre-β high-density lipoprotein mimetic, on coronary atherosclerosis in patients following acute coronary syndromes in the CER-001 atherosclerosis regression acute coronary syndrome trial: a randomized clinical trial. JAMA Cardiol. 3, 815–822 (2018).
Tardif, J. C. et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur. Heart J. 35, 3277–3286 (2014).
Nissen, S. E. et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290, 2292–2300 (2003).
Zheng, K. H. et al. HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis 251, 381–388 (2016).
Zheng, K. H. et al. Plaque permeability assessed with DCE-MRI associates with USPIO uptake in patients with peripheral artery disease. JACC Cardiovasc. Imaging 12, 2081–2083 (2019).
Smits, L. P. et al. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 263, 211–218 (2017).
Tang, T. et al. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke 37, 2266–2270 (2006).
Howarth, S. P. S. et al. Utility of USPIO-enhanced MR imaging to identify inflammation and the fibrous cap: a comparison of symptomatic and asymptomatic individuals. Eur. J. Radiol. 70, 555–560 (2009).
Acknowledgements
The authors are supported by Harvard Medical School/Brigham and Women’s Hospital Department of Anaesthesiology-Basic Scientist Grant (2420 BPA075, W.T.), the US METAvivor Early Career Investigator Award (2018A020560, W.T.), the NIH grants R01HL127464 (I.T. and J.S.), R01HL087123 (I.T.), R35HL145228 (I.T.), R01HL159012 (J.S. and I.T.) and R01HL156362 (J.S.), and the Niels Stensen Fellowship (M.S.). W.T. is a recipient of the Khoury Innovation Award (2020A003219), The Gillian Reny Stepping Strong Center for Trauma Innovation Breakthrough Innovator Award (113548) and AHA Collaborative Sciences Award (2018A004190) and received a start-up package (for 3 years) from the Department of Anaesthesiology, Perioperative and Pain Medicine to establish his independent research laboratory at Harvard Medical School and Brigham and Women’s Hospital. The authors thank their department for this generous support.
Author information
Authors and Affiliations
Contributions
All the authors wrote the article and reviewed and edited the manuscript before submission. W.C. and M.S. designed the figures for initial submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks L. Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Apolipoprotein B (apoB)-containing lipoproteins
-
Cholesterol-rich lipoproteins, such as LDL and chylomicron remnants, that have a crucial role in atherogenesis.
- ‘Vulnerable’ plaques
-
A type of atherosclerotic plaque composed of a lipid-rich necrotic core and thin overlying fibrous cap. These plaques are highly unstable and their rupture can trigger acute thrombosis and its consequences, namely myocardial infarction and stroke.
- Bioavailability
-
The percentage of an administered drug or substance that enters the systemic circulation and reaches its target tissue and is therefore able to have a biological effect.
- Nanomedicine
-
Applications of nanotechnology for diagnosis, treatment and control of biological systems.
- Cholesterol efflux
-
Process by which cells transfer excess intracellular cholesterol to other cells through either passive diffusion or dedicated mechanisms involving ABC transporters. Cholesterol efflux is important to prevent cholesterol-induced cytotoxicity, as occurs in macrophage foam cells in the arterial wall, and is an essential step in reverse cholesterol transport.
- Efferocytosis
-
Process through which apoptotic cells are cleared by phagocytes, including pro-resolving macrophages. Impairments in efferocytosis drive atherosclerosis progression owing to secondary necrosis of uncleared apoptotic cells in the arterial wall.
- Inflammation resolution
-
A highly coordinated process to restore tissue homeostasis following infection or tissue damage. Inflammation resolution is regulated by macrophages with a resolving–repair phenotype that produce a variety of pro-resolving mediators to dampen inflammation, repair damaged tissues and improve efferocytosis.
- Transcytosis
-
Transcellular process through which nanoparticles are transported across the interior of a cell.
- Endosomal escape
-
Process through which nanoparticles escape from endosomes and enter the cytoplasm, either by crossing the endosome membrane or as a result of endosome rupture.
- Plaque burden
-
A measure of atherosclerotic plaque volume relative to the volume of the arterial wall.
- Plaque regression
-
In humans, plaque regression refers to atherosclerotic plaques becoming smaller and more stable, which is associated with a lower risk of coronary artery disease.
- Nano–bio interface
-
The interface between nanoparticles and biological systems, such as serum proteins and extracellular matrix; interactions at the nano–bio interface determine the biological fates of nanoparticles in living organisms.
- Bioimaging
-
Non-invasive imaging techniques to visualize biological processes.
Rights and permissions
About this article
Cite this article
Chen, W., Schilperoort, M., Cao, Y. et al. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol 19, 228–249 (2022). https://doi.org/10.1038/s41569-021-00629-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00629-x
This article is cited by
-
Immunomodulation and immunopharmacology in heart failure
Nature Reviews Cardiology (2024)
-
Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment
Signal Transduction and Targeted Therapy (2024)
-
Targeted therapies of inflammatory diseases with intracellularly gelated macrophages in mice and rats
Nature Communications (2024)
-
Selenopeptide nanomedicine ameliorates atherosclerosis by reducing monocyte adhesions and inflammations
Nano Research (2024)
-
Magnetic-optical dual-modality imaging monitoring chemotherapy efficacy of pancreatic ductal adenocarcinoma with a low-dose fibronectin-targeting Gd-based contrast agent
European Journal of Nuclear Medicine and Molecular Imaging (2024)