Abstract
The leading cause of death in the world, cardiovascular disease (CVD), remains a formidable condition for researchers, clinicians and patients alike. CVD comprises a broad collection of diseases spanning the heart, the vasculature and the blood that runs through and interconnects them. Limitations in CVD therapeutic and diagnostic landscapes have generated excitement for advances in nanomedicine, a field focused on improving patient outcomes through transformative therapies, imaging agents and ex vivo diagnostics. CVD nanomedicines are fundamentally shaped by their intended clinical application, including (1) cardiac or heart-related biomaterials, which can be functionally (for example, mechanically, immunologically, electrically) improved by incorporating nanomaterials; (2) the vasculature, involving systemically injected nanotherapeutics and imaging nanodiagnostics, nano-enabled biomaterials or tissue-nanoengineered solutions; and (3) improving the sensitivity and/or specificity of ex vivo diagnostic devices for patient samples. While immunotherapy has developed into a key pillar of oncology in the past dozen years, CVD immunotherapy and immunoimaging are recently emergent and likely to factor substantially in CVD management in the coming decade. The nanomaterials in CVD-related clinical trials and many promising preclinical strategies indicate that nanomedicine is on the cusp of greatly impacting patients with CVD. Here we review these recent advances, highlighting key clinical opportunities in the rapidly emerging field of CVD nanomedicine.
Similar content being viewed by others
Main
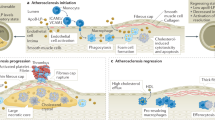
Tremendous advances in understanding the pathogenesis and biomolecular underpinnings of CVD over the past 2 decades have primed the field for explosive growth in medical breakthroughs. For CVD, the leading cause of death and morbidity worldwide, radically improved detection and treatment will require similarly revolutionary perspectives and technologies. Nanomedicine has begun to take a leading role in CVD treatment and diagnosis on the basis of nanomaterials’ extraordinary flexibility, including their targeting, signaling and delivery capabilities (Fig. 1). Flexibly nanoengineered physicochemical properties are critical to nanomaterials’ ability to support the varied needs of CVD. In this review, we consider the impact of nanomedicine on all CVDs, which span maladies of the heart (from electrical conduction and heart muscle diseases to congenital malformations) and blood vessels (for example, atherosclerotic plaque and strokes) to the blood itself (for example, thrombosis and pulmonary emboli).
Cardiovascular nanomedicine applications can be divided into four major application classes, spanning new therapeutics and diagnostics to theranostics and nanomaterials for physicochemical enhancements. Therapeutics and diagnostic imaging include both systemic and localized nanodelivery applications. Diagnostics may also involve ex vivo applications in which nanomaterials amplify signal by leveraging electrical, magnetic, acoustic and optical signal reporting and/or boosting capabilities, for example, to drive faster, more efficient or more highly parallelized screening and response-to-therapy assessment strategies. Theranostics integrates therapeutic and diagnostic modalities, typically into a single nanomaterial. Physicochemical enhancements encompass the application of nanomaterials to boost and/or beneficially modulate a bulk material’s electrical, structural, chemical, immunological and/or mechanical properties, often to support extended contact with in vivo biology. Highlighted in this schematic are examples from each CVD nanomedicine-application class, linked to the associated cardiovascular anatomy, selected from references in this review. Some portions of Fig. 1 adapted from refs. 19,50,149; reprinted from ref. 38, © 2017, with permission from Elsevier; adapted from ref. 150, © 2017, Keliher, E. et al., CC BY 4.0; adapted with permission from ref. 151, © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim; adapted with permission from ref. 152, © 2022, American Chemical Society.
Despite CVD’s heavier societal burden as compared to that of cancer and the surprising yet stark similarities between the diseases1,2, the CVD nanomedicine field has trailed shockingly far behind that for cancer. To wit: cancer nanotechnology produced more articles last year than cardiovascular nanotechnology has ever produced based on a PubMed keyword analysis (Fig. 2). The voluminous cancer nanotechnology research has likely been strongly influenced by the confluence of (1) focused, extensive funding efforts by the US National Cancer Institute (NCI)’s Center for Cancer Nanotechnology Excellence mechanisms, which enticed researchers from diverse fields toward the common goal of applying nanotechnology to beat cancer beginning in 2005 (ref. 3), paralleling the NCI’s provocative, controversial objective to control suffering and death due to cancer by 2015 (ref. 4); and (2) enthusiasm and momentum related to early nanomedical clinical trials successfully driving Doxil and Abraxane’s cancer nanotherapy regulatory approvals in the mid 1990s to 2000s. Intriguingly, advancements in cancer nanotechnology can often be applied to CVD due to similarities in their inflammatory disease pathogenesis1,2. Hence, despite cancer’s head start, CVD-related nanomaterials could eventually outpace cancer nanomaterials in clinical trials by evading the pitfalls learned from cancer nanotechnology over the past 2 decades. Reversing Fig. 2’s trend will require dedicated efforts, beginning with foundation-level grassroots efforts to spark sociocultural interest while amplifying scientific interest through CVD nanomedicine-focused scientific journals. These efforts could spawn (1) new private foundations, boosting the field’s funding and awareness, and (2) federal funding of CVD nanomedicine consortia (for example, the Centers for Cancer Nanotechnology Excellence) that will fundamentally transform the field by dedicating resources to large, disparate interdisciplinary groups to conceive new nanomedical paradigms. Equally important, it will spark the global conversations necessary to catalyze the urgency that regulatory agencies adopted for the coronavirus disease 2019 (COVID-19) pandemic (which caused far fewer annual deaths than CVD), shifting focus to CVD as befitting the world’s top killer. CVD nanomedicine also has the intriguing advantage of leveraging cutting-edge, high-dimensional data-driven technologies and advanced artificial intelligence (AI) as part of comprehensive regulatory data packages to shrink the time between bench discovery and clinical trials.
Using search terms related to cancer nanomedicine and to cardiovascular nanomedicine, PubMed-based searches indicate that the field of cancer nanomedicine (a) publishes approximately tenfold more articles per year than the field of cardiovascular nanomedicine (b). c, Searches of US- and European-based clinical trials using related search terms (shown with searches combined for https://clinicaltrials.gov and the EU Clinical Trials Register) keenly illustrate that these trends persist through clinical translation, with approximately an order of magnitude higher numbers of clinical trials involving cancer nanomedicine than cardiovascular nanomedicine-related clinical trials. A concerted focus on revealing the diverse lessons of the last 20 years of cancer nanomedicine could transform successful translational pathways for clinical cardiovascular disease-related nanomaterials, building on its early yet exponential preclinical growth. In sum, these trends suggest that cardiovascular nanomedicine remains in its infancy, with tremendous liberty to expand and impact patients once supported by the appropriate infrastructures and funding programs.
CVDs are an ideal platform from which to develop medical nanotechnologies. These diseases affect more people globally than any other; they are everywhere the dominant determinant of health and illness. This ubiquity partially derives from the reliance of every organ and tissue on perfusion, hence the remarkable mass of blood vessels and critical reliance on maintaining physiologic pressures and flow. Heart and vascular diseases affect and are manifested by aberrations in every organ. CVD’s impact also speaks to the spectrum of physiologic processes involved and sensitivity to pathologies. The heart is a pump, but it is also a densely vascularized, innervated endocrine organ under numerous neurochemical, immunological and electromechanical synchronization and modulation pathways. Cardiovascular dynamics are mediated by virtually every physiologic event, and every pathologic process affects the heart and blood vessels.
Nanotechnology targets organs and/or cells and mechanisms of disease, thereby heightening diagnostic precision and directing targeted therapy. Critical gaps in current CVD treatment include the ability to detect and target specific pathological areas, be it inflammation, thrombosis or proliferation within the heart or blood vessels, without affecting healthy tissues. The tools of nanomedicine offer provocative potential for resolution of site-specific pathology without systemic adverse side effects while also enabling platform nanotechnologies that deliver multifunctional diagnostic and/or therapeutic functionality and ‘all-in-one’-type theranostics5. Hence, targeting, accessing and intervening on the nanoscale is critical to the diagnosis, classification and treatment of CVDs.
In our review, we will focus on nanomaterials for in vivo cardiovascular therapy, imaging and ex vivo diagnostics across interconnected CVD tissues and organs (Fig. 1). We will describe nanomedicine’s advantages and disadvantages, where nanomedicine involves the diverse medical applications of nanomaterials, including nanomaterials injected into living trial participants for therapy and imaging, nanomaterials applied in or on implanted biomaterials or scaffolding for tissue-engineered solutions and nanomaterials as drivers of ex vivo strategies to evaluate patient liquid (for example, blood, urine, saliva) and tissue biopsies for diagnostic information in CVD. Throughout this review, we are translationally motivated by CVD nanomaterials’ eventual applications as complementary to, or replacing, current clinical CVD therapeutic and diagnostic options. We concentrate on the recent literature, reviewing nanomedicine articles with the largest impact on heart and vascular research in the past decade and those expected to have the greatest impact in the coming decade.
Nanomedicine
Our biological world resides and is defined on a nanometric scale. Cells are microns in dimension, but their surface-decorated and signaling compounds, deoxyribonucleic acid (DNA), proteins, metabolites and our cellular machinery are on the order of nanometers. Accordingly, imaging, targeting, diagnostics, materials, therapeutics and their intersection are increasingly designed to interact with our living universe on the nanoscale.
This review describes the nanoscale world, imaging thereof and intervention within this world using materials on the same scale. Nanomaterials are defined as engineered materials that are 1–100 nm in at least one dimension critical to the application. Nanomedicine follows as the application of nanomaterials and nanodevices to the prevention, diagnosis and/or sensing and treatment of disease. We make the case that nanomaterials’ multifunctionality and physicochemical tunability by materials, surface and biomolecular engineering enable their broad applicability to CVD. Nanomaterials’ electrical tunability, for example, permits their use in improving cardiac electrical functionality6,7. Their modularity, shape and size tunability enable them to selectively target tissue sites and cells within the body5,8, generating powerful vehicles for therapeutic modulation when combined with their high payload capacity. Moreover, their intrinsic sensor capabilities and/or payload capacity produce tremendous value as in vivo nano-contrast agents and ex vivo diagnostic strategies9. Indeed, nanomaterials are appealing for therapeutic and diagnostic applications based on one or more key attributes discussed herein, including their (1) highly modular, controllable physicochemical properties including size, material, density, shape, porosity, surface charge and chemical and/or electrical tunability; (2) size (large enough to transport bigger therapeutic or imaging contrast payloads than a small molecule, yet tiny enough to maintain systemic travel capabilities (through the bloodstream) throughout the body); (3) large surface area-to-volume ratios, enabling high binding avidity and payload capacity; and (4) multifunctional potential (for example, combined therapeutic–diagnostic payloads (‘theranostics’) and multiple diagnostic molecule types to enable multimodal imaging, such as positron emission tomography (PET) and magnetic resonance imaging (MRI))5. Applied intelligently, these attributes can be mixed and matched to forge formidable strategies for treating and diagnosing various forms of CVD. From an upstart field in the 1980–1990s, nanomedicine has progressed to a rapidly maturing discipline nourishing hundreds of clinical trials. Although efficiently targeted in vivo nanomaterial delivery remains a key bottleneck to achieving nanomedicine’s transformative promise, the field maintains unique advantages compared to related fields such as cell therapy and nuclear imaging diagnostics. These benefits include its modularity (ease of swapping out components to focus on different targets or imaging modalities) and ability to engineer a nanomaterial of defined complexity based on clinical need, from simple polymers to multimodal nanobots capable of multiple complex biological interactions.
CVD-related nanomaterials can be categorized into therapeutic, diagnostic, theranostic and physicochemical modulation (for example, enhancing other materials’ properties) (Fig. 1). Therapeutic nanomaterials are characterized by their ability to carry large payloads of curative molecules and release them acutely or chronically by sustained and/or triggered release. Diagnostic nanomaterials are typified by the ability to produce signal to visualize their site-specific accumulation representing a biomedical, cellular or molecular state (for example, inflammation), generally using imaging or ex vivo detection strategies5. Nanotheranostic strategies integrate nanotherapeutics and nanodiagnostics, ideally enhancing each (Box 1). Nanomaterials may also physically, mechanically or biochemically enhance CVD treatments, such as by tuning cardiac electrical conductivity, the immune response of implants or mimicking heart biomechanics6,10, collectively comprising ‘physicochemical modulatory’ nanomaterials. While a vast diversity of nanomaterials exist, those currently most used for cardiovascular nanomedicine are primarily ‘adaptable’5: carbon-based or organic materials that are typically polymeric, lipid- or protein-based including biomimetics as well as inorganic nanomaterials, for example, metallics, particularly gold and iron oxides (Table 1). These materials reduce toxicity fears and may thus curb common regulatory concerns for in vivo nanotechnologies. These nanomaterials’ ability to adaptably load therapeutic and/or imaging molecules or structures makes them amenable to application in multiple different CVDs with the modular exchange of a molecule and/or targeting ligand.
Cardiovascular targets
The heart
The heart has a wide variety of failure modes or processes, including myocardial injury and inflammation (for example, myocarditis), valve and structural issues and electrical problems. Myocarditis, for instance, may result from viral (including influenza and severe acute respiratory syndrome coronavirus 2), bacterial and other infections and even (rarely) from vaccines11,12,13. Recent developments enable nanomaterials to help resolve such issues, including by overcoming limitations of conventionally used single-phase biomaterials (for example, polymeric cardiac valve replacements) to modulate the mechanical, electrical and biological and/or immunological properties of these critical biomaterials to better support heart function. The biomaterials, regenerative medicine and tissue engineering fields have spearheaded new developments in heart-directed therapeutics and diagnostics. Frequently, nanomaterials can support the function of other (bulk) biomaterials to improve their efficiency, efficacy and durability for heart valves, cardiac patches and vascular grafts and stents, etc. Conversely, specialized nanomaterials for intravenous (i.v.) and intracardiac injections are increasingly developed to treat conditions such as ischemia–reperfusion injury.
Myocardium and electrophysiology
Heart muscle is a functional syncytium comprising cardiomyocytes that initiate and propagate electrical signals to contract and generate force. Cardiomyocytes must remain healthy, viable and in a proper three-dimensional (3D) structure to function. Given its complexity, the specialized nature of the cells and precise 3D spatiotemporal structure critical to its function, it is unsurprising that the myocardium suffers a wide variety of disorders and dysfunctions, from diverse cardiomyopathies to ischemias. Myocardial therapies typically comprise intracardiac or i.v. injections, or implants, the properties of which may feature or be substantially improved by nanomaterials.
The heart is minimally self-regenerative14,15,16. Thus, after heart muscle injury, the heart may be patched, including by implantable, injectable and nanofibrous or nano-patterned scaffold cardiac patch materials, to repair the tissue6. Successful regenerative biomaterials typically cannot be unifunctional, such as providing solely mechanical stability; rather, they must be multifunctional structures mimicking the native tissue, with appropriate electrical conductivity, growth factors, cellular scaffolding and non-immunogenicity as well as mechanical structure and durability. However, existing biomaterials have not yet succeeded in full-tissue regenerative potential with the requisite physicochemical and structural properties14. Nanomaterials can agilely be applied to compensate for key shortcomings in cardiac regenerative medical technology, providing, for example, sustained release of growth factors for stem cells and other cells to thrive, engineered non-immunogenicity, nano-patterned or nanofibrous scaffolding upon which cardiomyocytes may flourish, mechanical stability tuned to the native myocardium, electrical conductivity to mimic the heart’s syncytium and the potential to concurrently electronically monitor cardiac patch performance6,17,18,19. Gold nanomaterials have even been used to ‘weld’ functional cardiac patches safely onto the heart using heat derived from near-infrared light absorption, complementing their excellent conductivity7 and enabling efficient intercellular electrical coupling. Increasingly, 3D-printed CVD biomaterials are sought due to their flexibility and precision fabrication; to further improve their properties, nanomaterials can be embedded in 3D bioprinters’ ‘ink’ to mimic the highly organized, contractile, conductive and thermal functions of the human heart20.
Alternatively, nanomaterials free from other scaffolding can repair or protect the myocardium via intracardiac or systemic strategies. A variety of nanomaterials including lipidic, polymeric, biomimetic and silicon-based formulations have been used to deliver protein-, RNA- and small-molecule-based (including statins) therapies to reduce oxidative stress, improve myriad heart functions such as fractional shortening, increase wall thickness and/or improve vasculogenesis21,22,23,24,25,26,27,28,29. A recent strategy united stem cell therapy with nanotherapy by fusing cardiac-homing platelet nanovesicles (extracellular vesicles) to cardiac stem cells (CSCs) (which are intrinsically cardioregenerative) to overcome low intracardiac CSC retention to repair myocardial infarction injury30. Platelet surface markers naturally targeted fused CSCs to the heart, increasing their retention to provide time to fulfill their cardioregenerative potential, thereby reducing fibrosis and infarct size while improving pump function in rat and pig models.
Other key paradigms to heal the myocardium involve injecting nanoimmunotherapeutics to manipulate the immune system that subsequently treats the heart. The size, surface moieties and payloads provide substantial flexibility for nanotherapeutics to evade or actively target and modulate multiple aspects of and pathways within the immune system. These strategies may target the adaptive system, such as a systemic nano-based T cell-reprogramming agent that delivers mRNA to produce anti-fibrotic chimeric antigen receptor (CAR) T cells in situ to improve cardiac function31; cardioprotective immunomodulatory therapy can also be achieved through the innate immune system, including nanoparticles delivering microRNA (miRNA) to repolarize cardiac macrophages toward a reparative anti-inflammatory phenotype to reduce hypertrophy and fibrosis32 (Fig. 3) or nanomaterials to silence tumor necrosis factor (TNF)-α and deliver antioxidants in macrophage- and/or monocyte-targeted strategies to reduce inflammatory cell infiltration, decrease pro-inflammatory and oxidative stress and improve cardiac function (for example, fractional shortening and ejection fraction)33,34.
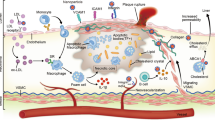
Summarizing the key components of nanomedicine applied toward imaging and treating CVD by leveraging the power of the immune system, this schematic distinguishes between nanomaterials applied to diseases of the vasculature and of the heart itself and between nanotherapeutic and nanoimaging applications. Systemically injected nanomaterials enter immune cells either already in the disease site (examples shown here: atherosclerotic plaque in the blood vessels and cardiac fibrosis) or circulating through the vasculature and are subsequently recruited into diseased tissue sites as part of native inflammatory processes. Immune cells are the conduit by which treatment and imaging are achieved, leveraging efficient delivery to diseased sites. These cells can thereby provide quantitative insights into local inflammation based on immune cell trafficking, numbers and activation by one or more imaging modalities, such as MRI, PET–CT and photoacoustic tomography. Nanotherapies are used to reprogram immune cell fates, such as restimulating macrophage efferocytosis or polarization or producing CAR T cells from circulating T cells in situ, to empower the body’s immune system to alleviate the disease state. M1 and M2 represent M1- and M2-polarized macrophages in the image. Images at bottom adapted with permission from ref. 153, © The Optical Society; and courtesy of Allen D. Elster, MRIQuestions.com.
Schematic of an idealized nanotheranostic strategy for CVD, using atherosclerosis as a model. Systemically injected nanotheranostic agents target atherosclerotic plaque. A CVD biomarker produced within the plaque simultaneously (1) triggers release of a therapeutic from the nanoparticles to treat the disease state, such as an immunomodulatory molecule for immune cells (shown here, for example, within atherosclerotic plaque) or other therapeutic molecules. The CVD biomarker, ideally chosen such that its levels quantitatively correlate with the severity of the disease state, could (2) unleash imageable signal from the nanomaterial. Signal produced upon recognition of the CVD biomarker could be imaged by an appropriate modality, such as MRI, PET or optical approaches, including photoacoustic imaging or fluorescence. The approach would preferably employ a linearly quantitative imaging strategy, for example, to support repeatedly monitoring the disease state in response to regular treatments. This paradigm would fulfill nanotheranostic potential for disease prognosis and response to therapy to support cardiologists in successful treatment guidance, dosing regimens and therapeutic selection. Adapted with permission from ref. 1, © 2020, American Chemical Society.
Heart valves
Nanomaterials offer multiple potential strategies to improve the safety profile and mechanical strength and durability of implanted heart valves. By effectively sterically blocking the valve surface from biomolecular interactions and simultaneously delivering sustained-release therapeutics, nanomaterials can reduce calcification and immunogenicity while increasing valve durability35,36. For example, biomimetic nanomaterials fabricated out of erythrocyte membranes loaded with immunosuppressive (rapamycin) and anti-inflammatory (atorvastatin calcium) agents were chemically linked to a conventional glutaraldehyde-treated valve surface35. Despite some advantages, glutaraldehyde-treated surfaces are toxic and immunogenic and contribute to valve degeneration37. The nano-coatings shielded the valve surface, endowing the implant with endothelialization, anti-coagulant and anti-calcification effects shown in a rat model over 4 months in vivo35. A different group fashioned a nanofiber network, designed to mimic the mechanical and chemical properties of true valvular extracellular matrix (ECM), into the shape of a valve. Jet spinning technology integrated polymeric gelatin-based nanofibers with ECM to fabricate human-sized valves within minutes with high biocompatibility and effective functionality in tests with sheep38. Other nanotechnologies have furthered the scope of tissue-engineered valves by ensuring appropriate porosities and textures via development of nanoscale or microscale porous structures with nanoscale-dimensional fidelity, controlling the topography to deliver growth factors or genes to specific cells. Moreover, because native heart valves exhibit anisotropic mechanical properties, anisotropic nanostructures and microstructures can be fabricated to support the multi-dimensional biomimetic anisotropy necessary to ensure proper in vivo function39,40.
The vasculature
As the body’s nutrient-delivery and waste-cleanup system, the vasculature is critical to the function of every tissue. Comprising blood and lymphatic vessels both small (peripheral) and large, at widely varying rates of shear and bifurcations, blood vessel diseases vary widely including atherosclerosis, peripheral artery disease and aneurysms. Intravascular (systemic) injection is the primary strategy used to introduce nanomaterials to treat vascular disease, which is appropriate given that the target (blood vessels) comprises the system into which they are injected. Nanomaterials are also used to improve intravascular and intervascular biomaterials such as nanoengineered stents, prostheses and grafts. The most lethal of blood vessel disorders, atherosclerosis, is a highly inflammatory chronic condition in which plaque builds up over time and may rupture, leading to vascular blockage. We focus primarily on atherosclerosis and hypertension as the main culprits underlying CVD-associated death and separate our discussion into sections on therapeutic nanomaterials and diagnostic (imaging) nanomaterials. We further subdivide discussion of nanomaterials into those that selectively target the immune system for therapeutic immunomodulation (nanoimmunotherapy) and for visualization of immune cell trafficking, activation and response to therapy (nanoimmunoimaging) in the context of vascular disease (Fig. 3).
Localized delivery via nano-enabled surfaces and structures
For some disease states, systemic delivery is inappropriate, for instance, when sufficient amounts of drug cannot be localized. When constant concentrations of therapy are required over longer time periods, localized nanodelivery may be favored. For example, restenosis avoidance and thrombosis resolution may necessitate local catheterization. Representative of this delivery paradigm, endovascular micro-infusion of rapamycin-containing albumin nanomaterials reduced stenosis in a porcine model41. In other cases, stents and engineered vascular grafts may incorporate nanomaterials for their ability to consistently and locally release drugs and growth factors42. In particular, current synthetic vascular grafts are thrombogenic, immunogenic and prone to infection and do not correspond to the native tissue’s mechanical properties. To overcome these issues, vascular grafts (needed, for example, in bypass operations to replace a blocked vessel) are often tissue engineered from primarily electrospun polymer nanofibers to mimic the topology, structure, mechanical properties and biocompatibility of true vasculature43,44,45. By integrating 3D printing and nanofibers, tissue-engineered patient-specific vascular grafts display similar mechanics and elastin and collagen content as those of native vessels in a sheep model with near-complete resorption after 6 months44.
Drug-eluting stents have sharply reduced rates of clinical restenosis, yet they increase late thrombosis risk and tissue hyperplastic reactions persist in certain patient subsets46,47. The release characteristics from drug-eluting stents may be undesirable, preventing successful use of some drugs such as the water-soluble chemotherapy imatinib47. In these situations, polymeric nanomaterials such as poly(lactic-co-glycolic acid) (PLGA) are useful for encapsulation for sustained stent elution because, unlike drug-loaded polymeric stent coatings, nanomaterials enable efficient loading and slower release with longer stent-site retention of imatinib and other drugs including the anti-proliferative sirolimus, thereby reducing restenosis47,48. On the other hand, bionanomaterials such as exosomes (small extracellular vesicles) may themselves promote an anti-inflammatory, pro-angiogenic microenvironment conducive to re-endothelialization and reduced stenosis. For example, mesenchymal stem cell-derived exosomes, which display healing properties in myocardial infarction46,49, can be eluted from stents to treat ischemia–reperfusion injury50. Finally, creative strategies can be deployed to maintain the advantages of nanoparticle-coated sustained drug release stents over the long term: for example, by using magnetizable stent meshes, systemically injected magnetic nanomaterials can be repeatedly captured by stents for multiple localized treatments each time drug is depleted51.
Systemic injection
Nanomaterials are more commonly systemically injected to specifically treat, image or simultaneously treat and image CVD. Nanotherapies may treat lipid dysfunction, vascular inflammation, endothelial dysfunction and many other facets of CVD. Imaging strategies apply nanomaterials as either the signal source or amplifier to visualize molecular uptake and expression, cellular localization and trafficking and other proxies of CVD.
Nanotherapy
Intravenously injected vascular nanotherapies are generally designed to target sites of atherosclerosis, thrombosis or intimal hyperplasia: these vascular diseases form an interconnected arc from atherosclerotic plaque development to plaque-related thrombosis to aftermath blockage, that is, restenosis (thrombosis and intimal hyperplasia may also have other causes). Nanotherapies used to treat these related yet independent CVDs can be separated into two general categories: nanomaterials that encapsulate currently used therapeutic molecules to increase payload delivery and/or to restrict delivery to specific cell subsets or tissues (targeted delivery) to decrease adverse effects and those that enable completely unprecedented CVD therapies (for example, encapsulating otherwise undeliverable drugs or transducing intrinsic or applied energy to physically modulate tissues).
For all CVD therapies, it is important to limit exposure to off-target sites. Nanotherapies modularly designed to attain this objective often achieve not only reduced side effects but also improved therapeutic outcomes52. Major classes of non-immune-mediated nanotherapies include those for modulation of lipid and glycolytic metabolism and prevention of plaque neoangiogenesis. Liposomes, for example, altered lipid metabolism by delivering small interfering RNA (siRNA) to silence apolipoprotein B and proprotein convertase subtilisin–kexin type 9 (PCSK9) expression, reducing low-density lipoprotein levels in small and large animal models53,54. Polysaccharide (sugar) nanoparticles activated macrophage metabolism and normalized vasculature by delivering a glycolysis inhibitor, thereby decreasing atherosclerotic plaque inflammation in murine models1,55. Other non-immune-mediated approaches involve polymeric nanodelivery of the biochemical machinery to drive anti-oxidant responses56.
Restricting off-target effects is also important for commonly used statins and anti-thrombotic agents. Liposomal, perfluorocarbon, iron oxide and biomimetic nanomaterials have encapsulated anti-thrombotic enzymes (for example, urokinase, streptokinase and tissue plasminogen activator) or thrombin inhibitors to directly act upon and reduce thrombus size with diminished adverse effects. Other nanomaterials, by contrast, act on the immune system to indirectly support thrombus depletion (nanoimmunotherapy). To reduce the likelihood of restenosis, nanotherapies may prevent neointimal growth by delivering statins, chemotherapy (for example, paclitaxel), immunosuppressives (for example, rapamycin), anti-inflammatories (for example, NADPH oxidase 2 (NOX2) siRNA) and chemical or small-molecule mediators (for example, nitric oxide)41,51,57,58,59,60. Encapsulation of statins and atheroprotective ganglioside GM3 into high-density lipoprotein (HDL)-based nanomaterials prevented plaque formation in murine models by reducing intraplaque inflammation and lipid accumulation61,62. To further reduce off-target effects without ligand targeting, some strategies weave plaque-specific molecular responsiveness into the nanomaterials: examples include reactive oxygen species (ROS, implicated in atherogenesis)-triggerable micelles and simvastatin release upon cholesterol binding by cyclodextrin nanoparticles63,64.
Nanomaterials’ capacity to be tunably engineered to respond to external energy and dissipate it as heat provides an innovative therapeutic platform largely unavailable to small molecules and biologics. Optical approaches are most common, applying photothermal energy to alleviate CVD by ablation or thermal signaling. For instance, near-infrared-absorbing copper sulfide nanomaterials photothermally activated transient receptor potential cation channel subfamily V member 1 (TRPV1) signaling on vascular smooth muscle cells, opening Ca2+ channels to activate autophagy and cholesterol efflux, thereby reducing lipid accumulation, foam cell development and atherosclerotic plaque in a murine model65. A multi-target nanotherapeutic employed a stabilin 2-targeted nanozyme strategy by releasing anti-inflammatory cyclooxygenase 2 (COX-2) inhibitors using optical energy and scavenging multiple ROS by structurally engineering palladium-based nanomaterials to display enzyme-like properties (nanozymes). This combination nanomaterial reduced inflammation, led to M2-like macrophage polarization and prevented plaque progression66. Optical ablation therapies, on the other hand, can thermally destroy inflammatory macrophages to alleviate vascular inflammation and reduce restenosis, with or without combination drug therapy67,68.
Finally, mRNA-based nanovaccines have rapidly become an exciting platform given clinical COVID-19 nanovaccine successes. Intriguingly, an mRNA-based nanovaccine directed toward the endothelium suggests that this dynamic field has parallel tremendous potential in CVD by diminishing neointimal formation and restenosis in murine models69.
Cardiovascular nanoimmunotherapy
It has become increasingly clear that the immune system is strongly implicated in most vascular diseases; in many cases, immunity drives disease pathophysiology, making it a compelling clinical target. While cancer immunotherapy is a rapidly maturing field, CVD immunotherapy remains in its early stages; indeed, the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study trial initially proved the clinical efficacy of CVD immunotherapy70,71. However, despite successes, there are key risks: systemically targeting innate immunity can induce profound effects on organism defenses, risking infection and sepsis. Nanomaterials are exciting in large part for their ability to minimize systemic effects by targeting only the cell subsets or tissues of interest. Restrictive cell selectivity can reduce injected drug loads and decrease adverse effects, all while increasing the drug’s efficacy.
Many nanotherapies are designed to target highly phagocytic macrophages and their precursor inflammatory monocytes (iMos), because they natively take up nanomaterials, are key drivers of inflammation and often of disease pathogenesis72, can launch antigen-specific immune responses and regulate trained immunity (a stimulated hyper-responsive functional state)73 and naturally traffic to inflammatory regions such as atherosclerotic plaques and thus are plentiful there74,75,76,77 (Fig. 3). Nanomaterials can deliver therapeutics to inhibit monocyte and macrophage proliferation and trafficking, inhibit their activation by helper T cells, polarize their state, drive cholesterol efflux or increase apoptosis, supporting anti-inflammatory resolution of atherosclerotic plaque and other CVDs76,78,79,80,81,82,83. Such studies rely upon cellular phagocytosis or myeloid targeting ligands for nanomaterial selectivity to temper the adverse effect landscape. Boosting selectivity is the principal strategy to minimize adverse effects. In vivo immune cell screens of nanomaterial libraries can thus be applied to enhance immune cell selectivity84. In other cases, nanomaterials are intrinsically selective. For instance, i.v. injected short single-walled carbon nanotubes (SWNTs) showed exquisite selectivity to iMos, without the targeting ligands required by most nanomaterials, with ~100% uptake by target cells and <3% uptake by other immune cell types85. By delivering a SHP1 tyrosine phosphatase inhibitor within iMos or macrophages, this SWNT Trojan horse nanotherapy re-stimulated intraplaque macrophage efferocytosis, thereby clearing apoptotic plaque debris, reducing plaque and necrotic core size and driving an anti-inflammatory microenvironment in mouse models86,87 (Fig. 3). Remarkably, compared with anti-CD47 antibody treatment, a targeted therapy in its own right, SWNT immune cell selectivity eliminated all side effects including anemia, reticulocytosis and splenomegaly86. Yet increased selectivity for nanomaterials to other CVD-related immune cell subsets remains a glaring need to improve the safety and toxicity profiles of CVD immunotherapy and boost the likelihood of translational success. An alternative to i.v. injected cell-targeted nanomaterials is the collection of primary immune cells (for example, neutrophils or monocytes), ex vivo nanomaterial internalization or attachment for drug delivery, and re-injection. Such strategies can be used to affix nanomaterials on neutrophils for locomotive support, enabling them to release thrombolytic neutrophil extracellular traps to help clear thrombi and inhibit rethrombosis88.
Atherosclerotic overexpression of molecules such as collagen IV (Col-IV) can also provide nanomaterial targeting opportunities. Col-IV peptide-targeted polymeric nanomaterials can deliver either a proresolving peptide that acts upon myeloid cells or interleukin 10 (IL-10) to successfully stabilize advanced plaques in murine models89,90. While such emerging strategies are translationally exciting, cautionary clinical tales include prednisolone-containing liposomes that minimized plaque inflammation much better than drug alone91 yet failed phase I–II clinical trials. This likely was due to lack of detailed understanding of drug dosing and frequency in large animal models92, entreating prudence in constructing preclinical endpoints.
Nanoimaging
Preclinical CVD nanoimaging, particularly atherosclerosis, employs magnetic, nuclear, acoustic and optical nanomaterials and combinations thereof5 (Fig. 3). These nanomaterials produce signal contrast matched to their cognate imaging modality, for example, magnetic iron oxide nanomaterials for MRI and magnetic particle imaging (MPI) and gold nanomaterials for computed tomography (CT). Prudent choice of the appropriate modality for in vivo CVD imaging requires consideration of (1) depth-of-penetration and spatial-resolution requirements, (2) potential nanomaterial toxicities (for example, mass of material injected) and modalities (for example, ionizing radiation or invasiveness of optical approaches such as intravascular catheterization) for clinical imaging and (3) the match between the information required by cardiologists to make clinical decisions and the data offered by the nanoimaging strategy.
Atherosclerosis is often imaged using contrast-producing nanomaterials targeted to key intraplaque molecular characteristics, including apoptosis, atherosclerosis-related lipids and ECM proteins, endothelial activation, neoangiogenesis, calcium deposition and, perhaps most prominently, immune-related phenomena such as inflammation, immune cell trafficking and activation and myeloid and/or macrophage phagocytosis5,93,94,95,96,97,98,99.
A notable advantage of cancer imaging compared with CVD imaging is the myriad unique molecular signatures and mutational burdens that are specific to or highly overexpressed by malignant versus normal cells, promoting the use of targeting. The differences in CVD pathogenesis versus normal tissues comparatively limits the possible molecular targets for nanomaterials because far fewer identified different molecular pathways are implicated. Thus, disadvantageously, in CVD, it is more challenging to identify unique, specific targets valuable for imaging. Indeed, a primary imaging target remains inflammatory immune archetypes, for example, of dangerous atherosclerotic plaques or myocarditis, including inflammatory or activated cells and molecules. By contrast, a key advantage of CVD imaging is that, once an effective imaging target is found, high prevalence is far more likely across diverse populations than it is for cancer, potentially facilitating an outsized impact.
The magnetic imaging modalities MRI and MPI are often used for CVD diagnostics due to their advantages in depth of penetration, fairly high spatial resolution and lack of invasiveness and ionizing radiation5. Magnetic nanomaterials, primarily small iron oxides or gadolinium-containing agents, have advanced atherosclerosis MRI based on the phagocytic activity of intraplaque macrophages, high levels of dead or dying cells and vascularization95,96,97,100. For example, conjugating these magnetic nanomaterials to biomolecules that bind apoptotic cells (for example, annexin V) or newly growing endothelium (for example, RGD peptide) in plaques can yield intraplaque signal dropout on T2*-weighted MRI sequences97,99. Myocardial inflammation can also be visualized by magnetic resonance using the phagocytic tendency of infiltrating macrophages to accumulate iron oxides101. MPI is a new magnetic imaging strategy developed in 2005 that, unlike MRI, requires magnetic nanomaterials to produce signal. It provides advantages over MRI, offering real-time high-resolution quantitative functional information, near-infinite contrast, potential multicolor imaging and even local rates of drug delivery102,103. MPI identified abdominal aortic aneurysm inflammation in a murine model using macrophage-phagocytosed iron oxides104 and has potential for CVD-related in vivo cell tracking and drug-delivery assessment.
A major form of nanoimmunoimaging involves identification of vascular inflammation by nanomaterial labeling of inflammatory cells that traffic to and reside within plaques105,106,107,108. The optical absorption and efficient heat-generation properties of nanomaterials enable production of potent photoacoustic (‘light in, sound out’) signal5. SWNTs, the iMo selectivity of which also enables them to specifically deliver pro-efferocytic drugs85,86, can exploit excellent photoacoustic properties to image monocyte trafficking to inflammatory carotid plaques with minimal off-target signal105 (Fig. 3). Such strategies are intriguing for non-invasive plaque detection in externally facing vasculature such as carotids or as part of integrated intravascular ultrasound–photoacoustic approaches109. Whole-body-imaging approaches 19F-MRI (imaging 19-fluorine-labeled nuclei) and PET using 89Zr-19F-HDL tracers can visualize pro-inflammatory myeloid cell dynamics from splenic and liver reservoirs into inflammatory hotspots such as atherosclerotic plaque110,111. Other nanoimmunoimaging techniques involve detection of trafficking molecules expressed on the surface of atherosclerosis-homing immune cells (for example, macrophages and T cells). Polymeric comb-like nanomaterials decorated with anti-C–C motif chemokine receptor 5 (CCR5) peptides and 64Cu for PET–CT nuclear imaging, for example, enabled long circulation times and optimized biodistributions to sensitively detect atherosclerotic plaque inflammation107.
‘Smart’ nanomaterials, which can be stimulated to produce imaging signals after encounter of the nanomaterial with a molecular or physicochemical trigger, comprise an ideal framework to amplify contrast by facilitating ‘signal on’ or modified (for example, a different color in optical approaches) only upon interaction with CVD biomarkers and ‘signal off’ otherwise. Smart nanoimaging approaches often employ enzymatic cleavage and temperature112,113. These strategies also apply thrombin as a molecular switch to detect thrombosis by MRI114.
Vascular calcification, long associated with established atherosclerotic plaques and CVD-related mortality, can be visualized with or without nanomaterial support by nuclear, magnetic and optical modalities115,116,117. To better characterize early plaque calcification, a bisphosphonate-functionalized magnetic nanomaterial targeted the calcium salt hydroxyapatite in a multimodal PET–MRI strategy to assess murine plaque progression116.
Collectively, while single molecular targets often provide valuable information, convergence of data from multiple biomarkers is preferable to make accurate diagnoses. Multicolor (spectral) CT is one such option: using gold HDL nanoparticles, iodine and calcium phosphate, multiple CVD biomarkers were simultaneously identified in mice via macrophage accumulation of gold HDL and vascular calcium deposit imaging118. However, CT requires large masses of material to produce signal for each ‘color’, decreasing its clinical viability as a multicolor imaging option. Despite few CVD-related studies to date, MPI is a multicolor imaging alternative119 for future preclinical development that does not require high material masses and is non-ionizing and non-invasive.
Ex vivo diagnostics
Imaging-based diagnostics serve as the benchmark, allowing not only detection of disease but also millimeter-scale spatial localization and below. However, imaging strategies are potentially time consuming, expensive and logistically challenging for CVD screening. By contrast, ex vivo diagnostics enable rapid, inexpensive alerts leading to advanced imaging follow-up if prescribed.
Detection of endogenous biomarkers in clinical samples including blood, saliva and urine is often hampered by background signal, requiring more sensitive and specific strategies. Nanomaterials offer advantages in detecting CVD-related molecular and cellular biomarkers, including C-reactive protein (CRP) and troponins, by amplifying signal and boosting sensitivity using magnetic and other physicochemical properties. CRP signal can be enhanced using nanomaterials that modulate electrical (for example, field-effect transistors), electrochemical120, optical (for example, surface plasmons)121,122,123 and chemiluminescent124 properties125. CRP is an outstanding biomarker of inflammation, and, though often used as a clinical predictor for CVD126,127, its non-CVD-specific nature suggests the need for improved individual biomarkers and/or robust multiplexed biomarker assays. Responding to this need, microfluidic nanogold-based strategies parallelized to produce results within 1 min yielded sensitive simultaneous multiplexed measurements of myoglobin, d-dimer and CRP to confirm myocardial infarction diagnosis128. Other optical approaches apply easily multiplexable plasmonics (for example, surface-enhanced Raman scattering, SERS129) to sensitively measure hypertension-related blood autoantibodies and microalbuminuria in urine, indicating vascular or endothelial dysfunction130,131. Electrochemical approaches have employed quantum dots and metallic nanomaterials to detect cardiac troponin, myoglobin, creatine kinase and CRP using voltammetry, impedance spectroscopy and amperometry through direct electron transfer to sense analytes132,133. The simplicity and cost effectiveness of electrochemical sensors plus their accuracy, speed and ultrasensitivity have elevated their profile for prediction of patient myocardial infarctions.
The COVID-19 pandemic engendered funding and interest in ex vivo diagnostic research and tools, exponentially expanding the sensitivity, specificity and accuracy of tests that can be applied to CVD. Recent tests, for example, apply magnetic SERS and biobarcoded particles to rapidly and sensitively detect proteins and nucleic acids down to attomolar levels134,135. With appropriate biomarkers, access to such detection limits could identify new patient populations at early risk of CVD.
Integrating ex vivo diagnostics with in vivo imaging
In an era beset by big data, exponentially increasing complexity and multimodal information, frameworks for improving clinical decision making are critical. Multiple types of tests (for example, ‘liquid biopsies’, tissue sampling and in vivo imaging) must be presciently and coherently linked together136, and this wealth of data must converge on one-to-few actionable values, a seemingly obvious application of AI and computational simulations137,138,139. In current clinical practice, radiology, pathology and liquid biopsy evaluations are typically siloed, leading to improper integration136 and potentially inferior treatment decisions. Thus, the value of the multiple tests pursued as parts of the screening, diagnostic, patient-stratification, therapeutic dosage-optimization and/or response-to-therapy evaluation processes may not be fully captured. Instead, AI might be used to ‘learn’ which constellation(s) of ex vivo and in vivo biomarkers inform a particular disease state, which therapy or combination of therapies is likeliest to succeed for a particular phenotype and how to interpret results of therapeutic-response testing, including across diverse patient subpopulations. Nanomaterials’ capabilities to boost multiplexing to simultaneously quantify >1 biomarkers for ex vivo diagnostics (for example, biobarcoded strategies) and for in vivo multicolor molecular imaging (for example, spectral CT and MPI) and to improve sensitivity and accuracy can thus efficiently increase information density with little increase in time or cost. However, not only must nanotechnologies continue to be developed, but so too must more CVD-related biomarkers be validated with understanding of their interconnections and links to diagnostic and/or prognostic clinical data. This approach can also enable researchers to choose optimal biomarker sets to test, in a standardized manner, using emerging nanodiagnostic approaches. Thus, the advent of innovative nanomaterial applications and CVD biomarkers will support the integration of ex vivo diagnostics with current and emerging in vivo imaging strategies to provide a more comprehensive, nuanced and precise picture of human CV health.
Limitations and opportunities
Nanomaterials have remarkable potential to support CVD across the full spectrum of pathophysiologic processes and presentations (Table 1), yet, as with all technologies, careful consideration must be given to their limitations. Indeed, as nanomaterials are taken from the laboratory to the clinic, key translational challenges arise. All materials have limitations in fabrication and quality control, and, when drugs are introduced, issues also emerge related to nanopharmaceutical scale-up, batch-to-batch reproducibility and clinical trial design140. Other nanoformulation-related concerns include material- and size-based toxicity and safety issues, long-term biocompatibility, circulation half-life, reproducibility and serum stability, any of which could derail a pharmaceutical program. While immune reactivity to nanodevices remains incompletely resolved, physicochemical nanoengineering to tune the surface properties can optimize the protein corona, that is, the material’s ‘biological identity’ displayed to the immune system141. Essential to fulfilling CVD nanomedicine’s lofty clinical expectations is a balance between nanomaterial safety concerns, synthetic simplicity integrated with efficient, inexpensive scale-up and sufficient complementarity between nanomaterial and clinical needs that produces superior therapeutic and diagnostic properties (Fig. 4), including precise, localized delivery, efficacy and accuracy5.
CVD nanomedicine’s many opportunities depend upon the creativity and innovation both of the researchers developing the nanomaterials and of those matching the nanomaterial properties with the proper therapeutic and/or diagnostic application and route of administration. Perhaps the most exciting opportunities lie in harnessing the power of the immune system for nanotherapeutic and nanoimaging applications in terms of improved localized delivery and inventive strategies that indirectly control pathogenesis or visualize molecular-to-cellular-scale pathogenic processes. In the longer term, we envision highly multiplexed diagnostic applications that would integrate into synergistically potent combination therapies leveraging AI frameworks to link biomarker detection and imaging with individual patients’ combined personal data (including age, sex, environment, ethnicity, co-morbidities) to recommend an optimized drug and/or nanodrug combination treatment plan to each patient’s cardiologist.
Conclusions
Nanotechnology is far more than progressive reduction in size of existing technology: it is a platform by which to create new opportunities across the breadth of medicine. Such miniaturization has propelled achievements in imaging anatomic structures and functional biological states in health and disease like never before. Innovations now allow targeting active moieties on cellular and molecular levels to achieve directed therapy and using specific, smart therapeutics, materials and devices such that interactions with the individual are programmed rather than simply tolerated. Thus, the opportunity is afforded to specifically and intelligently modulate immune response and immune reactivity of implants on the shoulders of the incredible progress in fundamental immunological knowledge142 and to gain real-time, iterative feedback from implants and injections of the local environmental state. Integrated with other fields’ advancements such as AI and systems biology, the full spectrum of health can be enhanced by nanotechnology, from health monitoring and disease detection to interconnected programs in therapy and in vivo prognostic and response-to-therapy imaging.
Nowhere is this more evident, important and impactful than in cardiovascular medicine, in which the future will allow for the first time physiologic rather than pharmacologic therapeutics and patient-specific and environmentally specific interventions and will reveal a new appreciation for biology on the cellular-to-subcellular scale.
References
Kanthi, Y., de la Zerda, A. & Smith, B. R. Nanotherapeutic shots through the heart of plaque. ACS Nano 14, 1236–1242 (2020).
Koene, R. J., Prizment, A. E., Blaes, A. & Konety, S. H. Shared risk factors in cardiovascular disease and cancer. Circulation 133, 1104–1114 (2016).
Lenoir, T. & Herron, P. The NCI and the takeoff of nanomedicine. J. Nanomedicine Biotherapeutic Discov. 5, 135 (2015).
Von Eschenbach, A. C. NCI sets goal of eliminating suffering and death due to cancer by 2015. J. Natl Med. Assoc. 95, 637–639 (2003).
Smith, B. R. & Gambhir, S. S. Nanomaterials for in vivo imaging. Chem. Rev. 117, 901–986 (2017). This paper comprehensively reviews the variety of nanomaterials applied in biomedical imaging, including imaging of inflammatory diseases such as CVD, with special focus on imaging within living individuals preclinically and clinically.
Ashtari, K. et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 144, 162–179 (2019).
Malki, M., Fleischer, S., Shapira, A. & Dvir, T. Gold nanorod-based engineered cardiac patch for suture-free engraftment by near IR. Nano Lett. 18, 4069–4073 (2018).
Zhu, X., Vo, C., Taylor, M. & Smith, B. R. Non-spherical micro- and nanoparticles in nanomedicine. Mater. Horizons 6, 1094–1121 (2019).
Park, S. M., Aalipour, A., Vermesh, O., Yu, J. H. & Gambhir, S. S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2, 17014 (2017).
Jiang, W., Rutherford, D., Vuong, T. & Liu, H. Nanomaterials for treating cardiovascular diseases: a review. Bioact. Mater. 2, 185–198 (2017).
Fung, G., Luo, H., Qiu, Y., Yang, D. & McManus, B. Myocarditis. Circ. Res. 118, 496–514 (2016).
Oster, M. E. et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. J. Am. Med. Assoc. 327, 331–340 (2022).
Mele, D., Flamigni, F., Rapezzi, C. & Ferrari, R. Myocarditis in COVID-19 patients: current problems. Intern. Emerg. Med. 16, 1123–1129 (2021).
Solazzo, M., O’Brien, F. J., Nicolosi, V. & Monaghan, M. G. The rationale and emergence of electroconductive biomaterial scaffolds in cardiac tissue engineering. APL Bioeng. 3, 041501 (2019).
Mahmoudi, M. et al. Multiscale technologies for treatment of ischemic cardiomyopathy. Nat. Nanotechnol. 12, 845–855 (2017).
Sadek, H. & Olson, E. N. Toward the goal of human heart regeneration. Cell Stem Cell 26, 7–16 (2020).
Huang, K. et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 12, 9683 (2020).
R Amin, D. et al. Nanomaterials for cardiac tissue engineering. Molecules 25, 5189 (2020).
Feiner, R. et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 15, 679–685 (2016). This study developed a cardiac patch integrated with cardiac cells, electronics for cell recording and electrical synchronization for cell contraction that is also capable of drug release to help regulate cardiac function.
Brazhkina, O. & Davis, M. E. 3D bioprinting in cardiovascular nanomedicine. Nanomedicine 16, 1347–1350 (2021).
Vong, L. B. et al. Novel angiogenesis therapeutics by redox injectable hydrogel—regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 167, 143–152 (2018).
Qi, Q. et al. Spatiotemporal delivery of nanoformulated liraglutide for cardiac regeneration after myocardial infarction. Int. J. Nanomedicine 12, 4835 (2017).
Oduk, Y. et al. VEGF nanoparticles repair the heart after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 314, H278–H284 (2018).
Somasuntharam, I. et al. Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction. Biomaterials 34, 7790–7798 (2013).
Yang, J. et al. High-throughput screening identifies microRNAs that target Nox2 and improve function after acute myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 312, H1002–H1012 (2017).
Ferreira, M. P. A. et al. Drug-loaded multifunctional nanoparticles targeted to the endocardial layer of the injured heart modulate hypertrophic signaling. Small 13, 1701276 (2017).
Nakano, Y. et al. Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia–reperfusion injury by antagonizing monocyte-mediated inflammation. Sci. Rep. 6, 29601 (2016).
Nagaoka, K. et al. A new therapeutic modality for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin induces cardioprotection from ischemia–reperfusion injury via activation of PI3K/Akt pathway and anti-inflammation in a rat model. PLoS ONE 10, e0132451 (2015).
Saludas, L. et al. Extracellular vesicle-based therapeutics for heart repair. Nanomaterials 11, 570 (2021).
Tang, J. et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat. Biomed. Eng. 2, 17–26 (2018). This paper describes the use of platelet-derived nanovesicles to enhance the delivery and binding of CSCs by fusing the stem cells with platelet nanovesicles. Thus, the fused cells express platelet surface markers that are associated with platelet adhesion to injury sites, thereby enhancing cellular retention and myocardial repair.
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Bejerano, T., Etzion, S., Elyagon, S., Etzion, Y. & Cohen, S. Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett. 18, 5885–5891 (2018).
Somasuntharam, I. et al. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials 83, 12–22 (2016).
Niu, J., Azfer, A., Rogers, L. M., Wang, X. & Kolattukudy, P. E. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 73, 549–559 (2007).
Hu, C., Luo, R. & Wang, Y. Heart valves cross-linked with erythrocyte membrane drug-loaded nanoparticles as a biomimetic strategy for anti-coagulation, anti-inflammation, anti-calcification, and endothelialization. ACS Appl. Mater. Interfaces 12, 41113–41126 (2020).
Li, Y. et al. Biofunctionalization of decellularized porcine aortic valve with OPG-loaded PCL nanoparticles for anti-calcification. RSC Adv. 9, 11882–11893 (2019).
Lopez-Moya, M. et al. Optimizing glutaraldehyde-fixed tissue heart valves with chondroitin sulfate hydrogel for endothelialization and shielding against deterioration. Biomacromolecules 19, 1234–1244 (2018).
Capulli, A. K. et al. JetValve: rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials 133, 229–241 (2017).
Hasan, A. et al. Micro and nanotechnologies in heart valve tissue engineering. Biomaterials 103, 278–292 (2016).
Vellayappan, M. V. et al. Tangible nanocomposites with diverse properties for heart valve application. Sci. Technol. Adv. Mater. 16, 033504 (2015).
Gasper, W. J. et al. Adventitial nab-rapamycin injection reduces porcine femoral artery luminal stenosis induced by balloon angioplasty via inhibition of medial proliferation and adventitial inflammation. Circ. Cardiovasc. Interv. 6, 701–709 (2013).
Yin, R. X., Yang, D. Z. & Wu, J. Z. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics 4, 175–200 (2014).
Tu, C., Das, S., Baker, A. B., Zoldan, J. & Suggs, L. J. Nanoscale strategies: treatment for peripheral vascular disease and critical limb ischemia. ACS Nano 9, 3436–3452 (2015).
Fukunishi, T. et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J. Thorac. Cardiovasc. Surg. 153, 924–932 (2017).
Rocco, K. A., Maxfield, M. W., Best, C. A., Dean, E. W. & Breuer, C. K. In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng. Part B Rev. 20, 628–640 (2014).
Stine, S. J., Popowski, K. D., Su, T. & Cheng, K. Exosome and biomimetic nanoparticle therapies for cardiac regenerative medicine. Curr. Stem Cell Res. Ther. 15, 674–684 (2020).
Masuda, S. et al. Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. J. Atheroscler. Thromb. 18, 1043–1053 (2011).
Sane, M. et al. Bivalirudin and sirolimus co-eluting coronary stent: potential strategy for the prevention of stent thrombosis and restenosis. Int. J. Pharm. 600, 120403 (2021).
Vandergriff, A. et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 8, 1869–1878 (2018).
Hu, S. et al. Exosome-eluting stents for vascular healing after ischaemic injury. Nat. Biomed. Eng. 5, 1174–1188 (2021). This paper describes the development of stents that release mesenchymal stem cell-derived exosomes to enhance vascular healing in rats with renal ischemia–reperfusion injury, promoting endothelial cell tube formation and proliferation and impairing the migration of smooth muscle cells.
Chorny, M. et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl Acad. Sci. USA 107, 8346–8351 (2010).
Singh, A. P., Biswas, A., Shukla, A. & Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 4, 33 (2019).
Frank-Kamenetsky, M. et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl Acad. Sci. USA 105, 11915–11920 (2008).
Tadin-Strapps, M. et al. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J. Lipid Res. 52, 1084–1097 (2011).
Beldman, T. J. et al. Nanoparticle-aided characterization of arterial endothelial architecture during atherosclerosis progression and metabolic therapy. ACS Nano 13, 13759–13774 (2019).
Park, S. et al. Therapeutic use of H2O2-responsive anti-oxidant polymer nanoparticles for doxorubicin-induced cardiomyopathy. Biomaterials 35, 5944–5953 (2014).
Tsukie, N. et al. Pitavastatin-incorporated nanoparticle-eluting stents attenuate in-stent stenosis without delayed endothelial healing effects in a porcine coronary artery model. J. Atheroscler. Thromb. 20, 32–45 (2013).
Chan, J. M. et al. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid–polymeric nanoparticles. Proc. Natl Acad. Sci. USA 108, 19347–19352 (2011).
Bahnson, E. S. M. et al. Targeted nitric oxide delivery by supramolecular nanofibers for the prevention of restenosis after arterial injury. Antioxid. Redox Signal. 24, 401–418 (2016).
Li, J. M. et al. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther. 17, 1279–1287 (2010).
Duivenvoorden, R. et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun. 5, 3065 (2014). This study developed an HDL-based nanoparticle loaded with statins to reduce atherosclerotic plaque inflammation.
Rong, T. et al. Enhanced anti-atherosclerotic efficacy of pH-responsively releasable ganglioside GM3 delivered by reconstituted high-density lipoprotein. Int. J. Mol. Sci. 22, 13624 (2021).
Shen, M. et al. Shear stress and ROS-responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C Mater. Biol. Appl. 126, 112164 (2021).
Kim, H. et al. Affinity-driven design of cargo-switching nanoparticles to leverage a cholesterol-rich microenvironment for atherosclerosis therapy. ACS Nano 14, 6519–6531 (2020).
Gao, W. et al. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat. Commun. 9, 231 (2018).
Xu, M. et al. Enhanced macrophage polarization induced by COX-2 inhibitor-loaded Pd octahedral nanozymes for treatment of atherosclerosis. Chin. Chem. Lett. 34, 107585 (2022).
Huang, Y. et al. Platelet-derived nanomotor coated balloon for atherosclerosis combination therapy. J. Mater. Chem. B 8, 5765–5775 (2020).
Peng, X. et al. AgFeS2 nanoparticles as a novel photothermal platform for effective artery stenosis therapy. Nanoscale 12, 11288–11296 (2020).
Lockhart, J. H. et al. Self-assembled miRNA-switch nanoparticles target denuded regions and prevent restenosis. Mol. Ther. 29, 1744–1757 (2021).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Baylis, R. A., Gomez, D., Mallat, Z., Pasterkamp, G. & Owens, G. K. The CANTOS trial: one important step for clinical cardiology but a giant leap for vascular biology. Arterioscler. Thromb. Vasc. Biol. 37, e174–e177 (2017).
Nahrendorf, M. Myeloid cell contributions to cardiovascular health and disease. Nat. Med. 24, 711–720 (2018).
van Leent, M. M. T. et al. Regulating trained immunity with nanomedicine. Nat. Rev. Mater. 7, 465–481 (2022).
Smith, B. R. Nanotherapeutics for cardiovascular disease. Nat. Rev. Cardiol. 18, 617–618 (2021).
Chen, W. et al. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2021, 228–249 (2021).
Tang, J. et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 1, e1400223 (2015).
Lameijer, M. A., Tang, J., Nahrendorf, M., Beelen, R. H. J. & Mulder, W. J. M. Monocytes and macrophages as nanomedicinal targets for improved diagnosis and treatment of disease. Expert Rev. Mol. Diagn. 13, 567–580 (2013).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011). This work describes a monocyte-targeted nanoparticle that delivers siRNA to knockdown surface homing proteins and decrease iMo trafficking, thereby suppressing inflammation for the treatment of atherosclerotic plaques and other inflammatory diseases.
Sager, H. B. et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 8, 342ra80 (2016).
Majmudar, M. D. et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 112, 755–761 (2013).
Lameijer, M. et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng. 2, 279–292 (2018).
Seijkens, T. T. P. et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J. Am. Coll. Cardiol. 71, 527–542 (2018).
Guo, Y. et al. Synthetic high-density lipoprotein-mediated targeted delivery of liver X receptors agonist promotes atherosclerosis regression. EBioMedicine 28, 225–233 (2018).
Tang, J. et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl Acad. Sci. USA 113, E6731–E6740 (2016).
Smith, B. R. et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 9, 481–487 (2014).
Flores, A. M. et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 15, 154–161 (2020). This study reports an immune cell-selective nanoparticle to treat atherosclerosis by stimulating intraplaque macrophage efferocytosis, demonstrating the elimination of off-target side effects due to the high iMo selectivity of the nanoparticles.
Zhang, Y. et al. Macrophage-targeted single walled carbon nanotubes stimulate phagocytosis via pH-dependent drug release. Nano Res. 14, 762–769 (2021).
Zheng, J., Qi, R., Dai, C., Li, G. & Sang, M. Enzyme catalysis biomotor engineering of neutrophils for nanodrug delivery and cell-based thrombolytic therapy. ACS Nano 16, 2330–2344 (2022).
Kamaly, N. et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano 10, 5280–5292 (2016).
Fredman, G. et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 7, 275ra20 (2015).
Lobatto, M. E. et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol. Pharm. 7, 2020–2029 (2010).
Flores, A. M. et al. Nanoparticle therapy for vascular diseases. Arterioscler. Thromb. Vasc. Biol. 39, 635–646 (2019).
Alaarg, A. et al. Applying nanomedicine in maladaptive inflammation and angiogenesis. Adv. Drug Deliv. Rev. 119, 143–158 (2017).
Tarkin, J. M. et al. Imaging atherosclerosis. Circ. Res. 118, 750–769 (2016).
Chin, D. D., Chowdhuri, S. & Chung, E. J. Calcium-binding nanoparticles for vascular disease. Regen. Eng. Transl. Med. 5, 74–85 (2019).
Zhang, L. et al. An atherosclerotic plaque-targeted single-chain antibody for MR/NIR-II imaging of atherosclerosis and anti-atherosclerosis therapy. J. Nanobiotechnology 19, 296 (2021).
Smith, B. R. et al. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed. Microdevices 9, 719–727 (2007).
Evans, R. J. et al. Targeted molecular iron oxide contrast agents for imaging atherosclerotic plaque. Nanotheranostics 4, 184–194 (2020).
Kim, M. et al. Comparison of in vivo targeting ability between cRGD and collagen-targeting peptide conjugated nano-carriers for atherosclerosis. J. Control. Release 269, 337–346 (2018).
Kim, C. W. et al. In vivo MRI detection of intraplaque macrophages with biocompatible silica-coated iron oxide nanoparticles in murine atherosclerosis. J. Appl. Biomater. Funct. Mater. 19, 22808000211014751 (2021).
Moon, H. et al. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation 125, 2603–2612 (2012).
Tay, Z. W. et al. Magnetic particle imaging: an emerging modality with prospects in diagnosis, targeting and therapy of cancer. Cancers 13, 5285 (2021).
Zhu, X., Li, J., Peng, P., Hosseini Nassab, N. & Smith, B. R. Quantitative drug release monitoring in tumors of living subjects by magnetic particle imaging nanocomposite. Nano Lett. 19, 6725–6733 (2019).
Mangarova, D. B. et al. Ex vivo magnetic particle imaging of vascular inflammation in abdominal aortic aneurysm in a murine model. Sci. Rep. 10, 12410 (2020).
Gifani, M. et al. Ultraselective carbon nanotubes for photoacoustic imaging of inflamed atherosclerotic plaques. Adv. Funct. Mater. 31, 2101005 (2021). These results demonstrate that highly immune cell-selective nanoparticles can exploit native cell trafficking to specifically image inflamed atherosclerotic plaques by photoacoustic tomography.
Xie, Z. et al. In vivo assessment of inflammation in carotid atherosclerosis by noninvasive photoacoustic imaging. Theranostics 10, 4694–4704 (2020).
Luehmann, H. P. et al. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J. Nucl. Med. 55, 629–634 (2014).
Wang, P. et al. Nano-immunoimaging. Nanoscale Horiz. 5, 628–653 (2020).
Bourantas, C. V. et al. Hybrid intravascular imaging: recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur. Heart J. 38, 400–412 (2017).
Senders, M. L. et al. Probing myeloid cell dynamics in ischemic heart disease by nanotracer hot spot imaging. Nat. Nanotechnol. 15, 398–405 (2020).
Leeper, N. J., Park, S. & Smith, B. R. High-density lipoprotein nanoparticle imaging in atherosclerotic vascular disease. JACC Basic Transl. Sci. 2, 98–100 (2017).
Syed, M. B. J. et al. Emerging techniques in atherosclerosis imaging. Br. J. Radiol. 92, 20180309 (2019).
Yu, S. S. et al. Enzymatic- and temperature-sensitive controlled release of ultrasmall superparamagnetic iron oxides (USPIOs). J. Nanobiotechnology 9, 7 (2011).
Ta, H. T. et al. Activatable magnetic resonance nanosensor as a potential imaging agent for detecting and discriminating thrombosis. Nanoscale 10, 15103–15115 (2018).
Wang, Y., Osborne, M. T., Tung, B., Li, M. & Li, Y. Imaging cardiovascular calcification. J. Am. Heart Assoc. 7, e008564 (2018).
Pellico, J. et al. HAP-multitag, a PET and positive MRI contrast nanotracer for the longitudinal characterization of vascular calcifications in atherosclerosis. ACS Appl. Mater. Interfaces 13, 45279–45290 (2021).
Chin, D. D. et al. Hydroxyapatite-binding micelles for the detection of vascular calcification in atherosclerosis. J. Mater. Chem. B 7, 6449–6457 (2019).
Cormode, D. P. et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology 256, 774–782 (2010).
Muslu, Y., Utkur, M., Demirel, O. B. & Saritas, E. U. Calibration-free relaxation-based multi-color magnetic particle imaging. IEEE Trans. Med. Imaging 37, 1920–1931 (2018).
Vilian, A. T. E. et al. Efficient electron-mediated electrochemical biosensor of gold wire for the rapid detection of C-reactive protein: a predictive strategy for heart failure. Biosens. Bioelectron. 142, 111549 (2019).
Lee, S. H. et al. A photothermal biosensor for detection of C-reactive protein in human saliva. Sens. Actuators B Chem. 246, 471–476 (2017).
Vashist, S. K., Schneider, E. M. & Luong, J. H. T. Surface plasmon resonance-based immunoassay for human C-reactive protein. Analyst 140, 4445–4452 (2015).
Aray, A. et al. SPR-based plastic optical fibre biosensor for the detection of C-reactive protein in serum. J. Biophotonics 9, 1077–1084 (2016).
Xing, Y. et al. The improved sensitive detection of C-reactive protein based on the chemiluminescence immunoassay by employing monodispersed PAA-Au/Fe3O4 nanoparticles and zwitterionic glycerophosphoryl choline. J. Mater. Chem. B 5, 3919–3926 (2017).
Wu, B. et al. A simple label-free aptamer-based method for C-reactive protein detection. Anal. Methods 8, 4177–4180 (2016).
Adukauskiene, D. et al. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medicina 52, 1–10 (2016).
Sproston, N. R. & Ashworth, J. J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754 (2018).
Byzova, N. A., Vengerov, Y. Y., Voloshchuk, S. G., Zherdev, A. V. & Dzantiev, B. B. Development of a lateral flow highway: ultra-rapid multitracking immunosensor for cardiac markers. Sensors 19, 5494 (2019). This study developed a nanoparticle-based method to rapidly and reproducibly test levels of multiple cardiovascular blood biomarkers.
Zavaleta, C. L. et al. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl Acad. Sci. USA 106, 13511–13516 (2009).
Huang, Z. et al. Sensitive polydopamine bi-functionalized SERS immunoassay for microalbuminuria detection. Biosens. Bioelectron. 142, 111542 (2019).
Li, X. et al. Autoantibody profiling on a plasmonic nano-gold chip for the early detection of hypertensive heart disease. Proc. Natl Acad. Sci. USA 114, 7089–7094 (2017).
Mansuriya, B. D. & Altintas, Z. Enzyme-free electrochemical nano-immunosensor based on graphene quantum dots and gold nanoparticles for cardiac biomarker determination. Nanomaterials 11, 578 (2021).
Tabish, T. A., Hayat, H., Abbas, A. & Narayan, R. J. Graphene quantum dots-based electrochemical biosensing platform for early detection of acute myocardial infarction. Biosensors 12, 77 (2022).
Jang, A. S., Praveen Kumar, P. P. & Lim, D. K. Attomolar sensitive magnetic microparticles and a surface-enhanced Raman scattering-based assay for detecting SARS-CoV-2 nucleic acid targets. ACS Appl. Mater. Interfaces 14, 138–149 (2022).
Shipp, G. Ultrasensitive measurement of protein and nucleic acid biomarkers for earlier disease detection and more effective therapies. Biotechnol. Healthc. 3, 35–40 (2006).
Weissleder, R., Schwaiger, M. C., Gambhir, S. S. & Hricak, H. Imaging approaches to optimize molecular therapies. Sci. Transl. Med. 8, 355ps16 (2016).
Johnson, K. B. et al. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 14, 86–93 (2021).
Shah, P. et al. Artificial intelligence and machine learning in clinical development: a translational perspective. NPJ Digit. Med. 2, 69 (2019).
Shah, P. N. et al. Extravasation of Brownian spheroidal nanoparticles through vascular pores. Biophys. J. 115, 1103–1115 (2018).
Iafisco, M., Alogna, A., Miragoli, M. & Catalucci, D. Cardiovascular nanomedicine: the route ahead. Nanomedicine 14, 2391–2394 (2019).
Barbero, F. et al. Formation of the protein corona: the interface between nanoparticles and the immune system. Semin. Immunol. 34, 52–60 (2017).
Immune discovery aplenty at twenty. Nat. Rev. Immunol. 21, 613 (2021).
Hergenreider, E. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 14, 249–256 (2012).
Kharlamov, A. N. et al. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 13, 345–363 (2017). This first-in-human clinical nanoparticle study showed that silica gold nanoparticles used to treat human atherosclerotic plaques by photothermal therapy resulted in better safety and fewer adverse cardiovascular events and revascularization than stents.
Binderup, T. et al. Imaging-assisted nanoimmunotherapy for atherosclerosis in multiple species. Sci. Transl. Med. 11, eaaw7736 (2019).
Yao, J. et al. Low-intensity focused ultrasound-responsive ferrite-encapsulated nanoparticles for atherosclerotic plaque neovascularization theranostics. Adv. Sci. 8, e2100850 (2021).
Ma, B. et al. Biomimetic-coated nanoplatform with lipid-specific imaging and ROS responsiveness for atherosclerosis-targeted theranostics. ACS Appl. Mater. Interfaces 13, 35410–35421 (2021).
Wang, K. et al. Highly bright AIE nanoparticles by regulating the substituent of rhodanine for precise early detection of atherosclerosis and drug screening. Adv. Mater. 34, 2106994 (2022).
Zapotoczny, S., Szczubialka, K. & Nowakowska, M. Nanoparticles in endothelial theranostics. Pharm. Rep. 67, 751–755 (2015).
Keliher, E. et al. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat. Commun. 8, 14064 (2017).
Adir, O. et al. Integrating artificial intelligence and nanotechnology for precision cancer medicine. Adv. Mater. 32, e1901989 (2020).
Hu, R. et al. Living macrophage-delivered tetrapod PdH nanoenzyme for targeted atherosclerosis management by ROS scavenging, hydrogen anti-inflammation, and autophagy activation. ACS Nano 16, 15959–15976 (2022).
Wang, B. et al. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Optics Lett. 37, 1244–1246 (2012).
Wensink, G. E. et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 5, 30 (2021).
Wang, W. et al. Endogenous stimuli-activatable nanomedicine for immune theranostics for cancer. Adv. Funct. Mater. 31, 2100386 (2021).
Acknowledgements
We gratefully acknowledge our funding sources, including the American Heart Association Transformational Project Award 18TPA34230113 and the NIH (R01 CA244491 to B.R.S. and R01HL161069-02 to E.R.E.). Correspondence and requests should be addressed to B.R.S. at smit2901@msu.edu.
Author information
Authors and Affiliations
Contributions
B.R.S. wrote the draft and made figures; E.R.E. made key edits and additions.
Corresponding author
Ethics declarations
Competing interests
E.R.E. receives research support from Abiomed and Shockwave; is a consultant to BEV, Cascade, Cipherome, Marusan Pharmaceutics, Peregrine and Tekla; serves on the boards of Butterfly, Colossal and Xenter; and is a founder of Autus, BioDevek and PanTher Therapeutics. B.R.S. declares no competing interests.
Peer review information
Nature Cardiovascular Research thanks Ke Cheng, Kanyi Pu and Willem Mulder for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smith, B.R., Edelman, E.R. Nanomedicines for cardiovascular disease. Nat Cardiovasc Res 2, 351–367 (2023). https://doi.org/10.1038/s44161-023-00232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-023-00232-y
This article is cited by
-
Nanotechnology-based theranostic and prophylactic approaches against SARS-CoV-2
Immunologic Research (2024)
-
Thylakoid engineered M2 macrophage for sonodynamic effect promoted cell therapy of early atherosclerosis
Nano Research (2024)
-
Selenopeptide nanomedicine ameliorates atherosclerosis by reducing monocyte adhesions and inflammations
Nano Research (2024)
-
Nanomaterial-based contrast agents for common disease imaging
Science China Materials (2024)
-
Atherosclerotic plaque development in mice is enhanced by myeloid ZEB1 downregulation
Nature Communications (2023)