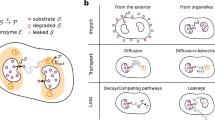
Abstract
Catalytic particles are spatially organized in a number of biological systems across different length scales, from enzyme complexes to metabolically coupled cells. Despite operating on different scales, these systems all feature localized reactions involving partially hindered diffusive transport, which is determined by the collective arrangement of the catalysts. Yet it remains largely unexplored how different arrangements affect the interplay between the reaction and transport dynamics, which ultimately determines the flux through the reaction pathway. Here we show that two fundamental trade-offs arise, the first between efficient inter-catalyst transport and the depletion of substrate, and the second between steric confinement of intermediate products and the accessibility of catalysts to substrate. We use a model reaction pathway to characterize the general design principles for the arrangement of catalysts that emerge from the interplay of these trade-offs. We find that the question of optimal catalyst arrangements generalizes the well-known Thomson problem of electrostatics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The numerical data generated for this study are available from the corresponding author upon reasonable request.
Code availability
The codes used to generate the numerical data that supports the findings of this study are available on GitHub at: https://github.com/gerland-group/flux_of_cat_arrangements
References
Agapakis, C. M., Boyle, P. M. & Silver, P. A. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 8, 527–535 (2012).
Reed, L. J. Multienzyme complexes. Acc. Chem. Res. 7, 40–46 (1974).
Kerfeld, C. A., Heinhorst, S. & Cannon, G. C. Bacterial microcompartments. Ann. Rev. Microbiol. 64, 391–408 (2010).
Schmitt, D. L. & An, S. Spatial organization of metabolic enzyme complexes in cells. Biochemistry 56, 3184–3196 (2017).
Fontes, C. M. & Gilbert, H. J. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Ann. Rev. Biochem. 79, 655–681 (2010).
An, S., Kumar, R., Sheets, E. D. & Benkovic, S. J. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 320, 103–106 (2008).
Yeates, T. O., Kerfeld, C. A., Heinhorst, S., Cannon, G. C. & Shively, J. M. Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nat. Rev. Microbiol. 6, 681–691 (2008).
Wheeldon, I. et al. Substrate channelling as an approach to cascade reactions. Nat. Chem. 8, 299–309 (2016).
Lee, H., DeLoache, W. C. & Dueber, J. E. Spatial organization of enzymes for metabolic engineering. Metab. Eng. 14, 242–251 (2012).
Küchler, A., Yoshimoto, M., Luginbühl, S., Mavelli, F. & Walde, P. Enzymatic reactions in confined environments. Nat. Nanotech. 11, 409–420 (2016).
Lin, J.-L., Palomec, L. & Wheeldon, I. Design and analysis of enhanced catalysis in scaffolded multienzyme cascade reactions. ACS Catal. 4, 505–511 (2014).
Xie, C. et al. Tandem catalysis for CO2 hydrogenation to C2-C4 hydrocarbons. Nano Lett. 17, 3798–3802 (2017).
Müller, J. & Niemeyer, C. M. DNA-directed assembly of artificial multienzyme complexes. Biochem. Biophys. Res. Commun. 377, 62–67 (2008).
Wilner, O. I. et al. Enzyme cascades activated on topologically programmed DNA scaffolds. Nat. Nanotech. 4, 249 (2009).
Fu, J., Liu, M., Liu, Y., Woodbury, N. W. & Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 134, 5516–5519 (2012).
Zhang, Y., Tsitkov, S. & Hess, H. Proximity does not contribute to activity enhancement in the glucose oxidase–horseradish peroxidase cascade. Nat. Commun. 7, 13982 (2016).
Wanders, R. J., Waterham, H. R. & Ferdinandusse, S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front. Cell Dev. Biol. 3, 83 (2015).
Linka, M. & Weber, A. P. Shuffling ammonia between mitochondria and plastids during photorespiration. Trends Plant Sci. 10, 461–465 (2005).
French, J. B. et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science 351, 733–737 (2016).
Ma, E. H. & Jones, R. G. TORCing up purine biosynthesis. Science 351, 670–671 (2016).
Boetius, A. et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626 (2000).
Müller, J. & Overmann, J. Close interspecies interactions between prokaryotes from sulfureous environments. Front. Microbiol. 2, 146 (2011).
Flores, E. & Herrero, A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8, 39–50 (2010).
Costa, E., Pérez, J. & Kreft, J.-U. Why is metabolic labour divided in nitrification? Trends Microbiol. 14, 213–219 (2006).
Buchner, A., Tostevin, F. & Gerland, U. Clustering and optimal arrangement of enzymes in reaction–diffusion systems. Phys. Rev. Lett. 110, 208104 (2013).
Buchner, A., Tostevin, F., Hinzpeter, F. & Gerland, U. Optimization of collective enzyme activity via spatial localization. J. Chem. Phys. 139, 135101 (2013).
Castellana, M. et al. Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat. Biotech. 32, 1011–1018 (2014).
Minton, A. P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 276, 10577–10580 (2001).
Berry, H. Monte Carlo simulations of enzyme reactions in two dimensions: fractal kinetics and spatial segregation. Biophys. J. 83, 1891–1901 (2002).
Schnell, S. & Turner, T. Reaction kinetics in intracellular environments with macromolecular crowding: simulations and rate laws. Prog. Biophys. Mol. Biol. 85, 235–260 (2004).
Hu, Z., Jiang, J. & Rajagopalan, R. Effects of macromolecular crowding on biochemical reaction equilibria: a molecular thermodynamic perspective. Biophys. J. 93, 1464–1473 (2007).
Dix, J. A. & Verkman, A. Crowding effects on diffusion in solutions and cells. Annu. Rev. Biophys. 37, 247–263 (2008).
Albe, K. R., Butler, M. H. & Wright, B. E. Cellular concentrations of enzymes and their substrates. J. Theor. Biol. 143, 163–195 (1990).
Samson, R. & Deutch, J. Exact solution for the diffusion controlled rate into a pair of reacting sinks. J. Chem. Phys. 67, 847 (1999).
Tsao, H.-K. Competitive diffusion into two reactive spheres of different reactivity and size. Phys. Rev. E 66, 011108 (2002).
Eun, C., Kekenes-Huskey, P. M. & McCammon, J. A. Influence of neighboring reactive particles on diffusion-limited reactions. J. Chem. Phys. 139, 044117 (2013).
Hirakawa, H., Haga, T. & Nagamune, T. Artificial protein complexes for biocatalysis. Top. Catal. 55, 1124–1137 (2012).
Shi, J. et al. Bioinspired construction of multi-enzyme catalytic systems. Chem. Soc. Rev. 47, 4295–4313 (2018).
Liu, M. et al. A three-enzyme pathway with an optimised geometric arrangement to facilitate substrate transfer. ChemBioChem 17, 1097–1101 (2016).
Thomson, J. J. On the structure of the atom: an investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the circumference of a circle; with application of the results to the theory of atomic structure. Philos. Mag. 7, 237–265 (1904).
Schwartz, R. E. The five-electron case of Thomson’s problem. Exp. Math. 22, 157–186 (2013).
Yudin, V. The minimum of potential energy of a system of point charges. Discrete Math. App. 3, 75–82 (1993).
Andreev, N. N. An extremal property of the icosahedron. East J. Approx. 2, 459–462 (1996).
Smale, S. Mathematical problems for the next century. Math. Intell. 20, 7–15 (1998).
Caspar, D. L. & Klug, A. in Cold Spring Harbor Symposia on Quantitative Biology, vol. 27, 1–24 (Cold Spring Harbor Laboratory Press, 1962).
Mackenzie, D. Proving the perfection of the honeycomb. Science 285, 1338–1339 (1999).
Maritan, A., Micheletti, C., Trovato, A. & Banavar, J. R. Optimal shapes of compact strings. Nature 406, 287–290 (2000).
Zandi, R., Reguera, D., Bruinsma, R. F., Gelbart, W. M. & Rudnick, J. Origin of icosahedral symmetry in viruses. Proc. Natl Acad. Sci. USA 101, 15556–15560 (2004).
Nisoli, C., Gabor, N. M., Lammert, P. E., Maynard, J. & Crespi, V. H. Annealing a magnetic cactus into phyllotaxis. Phys. Rev. E 81, 046107 (2010).
Sachdeva, G., Garg, A., Godding, D., Way, J. C. & Silver, P. A. In vivo co-localization of enzymes on RNA scaffolds increases metabolic production in a geometrically dependent manner. Nucleic Acids Res. 42, 9493–9503 (2014).
Grotzky, A., Nauser, T., Erdogan, H., Schlüter, A. D. & Walde, P. A fluorescently labeled dendronized polymer–enzyme conjugate carrying multiple copies of two different types of active enzymes. J. Am. Chem. Soc. 134, 11392–11395 (2012).
Zhang, Y. et al. Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and mammalian cells. J. Am. Chem. Soc. 128, 13030–13031 (2006).
Nakamura, S., Hayashi, S. & Koga, K. Effect of periodate oxidation on the structure and properties of glucose oxidase. Biochim. Biophys. Acta Enzymol. 445, 294–308 (1976).
Ban, Y. & Rizzolo, L. J. A culture model of development reveals multiple properties of RPE tight junctions. Mol. Vis. 3, 1 (1997).
Pappenheimer, J., Renkin, E. & Borrero, L. Filtration, diffusion and molecular sieving through peripheral capillary membranes: a contribution to the pore theory of capillary permeability. Am. J. Physiol. 167, 13–46 (1951).
Fogolari, F. et al. Studying interactions by molecular dynamics simulations at high concentration. BioMed Res. Int. 2012, 303190 (2012).
Agmon, N. & Szabo, A. Theory of reversible diffusion-influenced reactions. J. Chem. Phys. 92, 5270 (1990).
Vijaykumar, A., Bolhuis, P. G. & ten Wolde, P. R. The intrinsic rate constants in diffusion-influenced reactions. Faraday Discuss. 195, 421–441 (2016).
Vijaykumar, A., ten Wolde, P. R. & Bolhuis, P. G. The magnitude of the intrinsic rate constant: how deep can association reactions be in the diffusion limited regime? J. Chem. Phys. 147, 184108 (2017).
Martens-Habbena, W., Berube, P. M., Urakawa, H., José, R. & Stahl, D. A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461, 976–979 (2009).
Acknowledgements
We thank E. Frey, D. Nelson and A. Walczak for useful discussions, and B. Altaner and G. Giunta for comments on the initial manuscript. This work was supported by the German Research Foundation (DFG) through SFB 1032 (project ID 201269156, to U.G.) and the Excellence Cluster ORIGINS under Germany’s Excellence Strategy (EXC-2094-390783311, to U.G.). F.H. was supported by a DFG fellowship through the Graduate School of Quantitative Biosciences Munich.
Author information
Authors and Affiliations
Contributions
All authors designed the research. F.H. performed the research. F.H., F.T. and U.G. analysed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Physics thanks Pieter Rein ten Wolde and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary text with Supplementary Figs. 1–15.
Rights and permissions
About this article
Cite this article
Hinzpeter, F., Tostevin, F., Buchner, A. et al. Trade-offs and design principles in the spatial organization of catalytic particles. Nat. Phys. 18, 203–211 (2022). https://doi.org/10.1038/s41567-021-01444-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-021-01444-4
This article is cited by
-
Optimal spatial allocation of enzymes as an investment problem
Communications Physics (2022)