Abstract
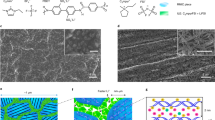
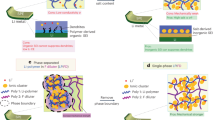
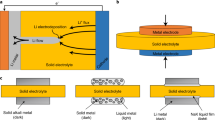
Alternative solid electrolytes are the next key step in advancing lithium batteries with better thermal and chemical stability. A soft solid electrolyte, (Adpn)2LiPF6 (Adpn, adiponitrile), is synthesized and characterized that exhibits high thermal and electrochemical stability and good ionic conductivity, overcoming several limitations of conventional organic and ceramic materials. The surface of the electrolyte possesses a liquid nano-layer of Adpn that links grains for a facile ionic conduction without high pressure/temperature treatments. Further, the material can quickly self-heal if fractured and provides liquid-like conduction paths via the grain boundaries. A substantially high ion conductivity (~10−4 S cm–1) and lithium-ion transference number (0.54) are obtained due to weak interactions between ‘hard’ (charge dense) Li+ ions and the ‘soft’ (electronically polarizable) –C≡N group of Adpn. Molecular simulations predict that Li+ ions migrate at the co-crystal grain boundaries with a (preferentially) lower activation energy Ea and within the interstitial regions between the co-crystals with higher Ea values, where the bulk conductivity is a smaller but extant contribution. These co-crystals establish a special concept of crystal design to increase the thermal stability of LiPF6 by separating ions in the Adpn solvent matrix, and also exhibit a unique mechanism of ion conduction via low-resistance grain boundaries, which contrasts with ceramics or gel electrolytes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Additional details of the simulation and DFT, as well as crystallographic tables, are provided in the Supplementary Information. The X-ray crystallographic coordinates for the structure reported in this study have been deposited at the Cambridge Crystallographic Data Centre under deposition number 1986269. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
References
Gao, Z. et al. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 30, 1705702 (2018).
Lau, J. et al. Sulfide solid electrolytes for lithium battery applications. Adv. Energy Mater. 8, 1800933 (2018).
Gadjourova, Z., Marero, D. M., Andersen, K. H., Andreev, Y. G. & Bruce, P. G. Structures of the polymer electrolyte complexes PEO6:LiXF6 (X = P, Sb), determined from neutron powder diffraction data. Chem. Mater. 13, 1282–1285 (2001).
MacGlashan, G. S., Andreev, Y. G. & Bruce, P. G. Structure of the polymer electrolyte poly(ethylene oxide)6:LiAsF6. Nature 398, 792–794 (1999).
Gadjourova, Z., Andreev, Y. G., Tunstall, D. P. & Bruce, P. G. Ionic conductivity in crystalline polymer electrolytes. Nature 412, 520–523 (2001).
Zhang, C. H., Ainsworth, D., Andreev, Y. G. & Bruce, P. G. Ionic conductivity in the solid glyme complexes [CH3O(CH2CH2O)nCH3]:LiAsF6 (n = 3,4). J. Am. Chem. Soc. 129, 8700–8701 (2007).
Stoeva, Z., Martin-Litas, I., Staunton, E., Andreev, Y. G. & Bruce, P. G. Ionic conductivity in the crystalline polymer electrolytes PEO6:LiXF6, X = P, As, Sb. J. Am. Chem. Soc. 125, 4619–4626 (2003).
Staunton, E., Andreev, Y. G. & Bruce, P. G. Factors influencing the conductivity of crystalline polymer electrolytes. Faraday Discuss. 134, 143–156 (2007).
Pearson, R. G. Absolute electronegativity and hardness: application to inorganic chemistry. Inorg. Chem. 27, 734–740 (1988).
Tanaka, K. et al. High Li-ion conductivity in Li{N(SO2F)2}(NCCH2CH2CN)2 molecular crystal. Nano Lett. 20, 8200–8204 (2020).
Chinnam, P. R., Clymer, R. N., Jalil, A. A., Wunder, S. L. & Zdilla, M. J. Bulk-phase ion conduction in cocrystalline LiCl·N,N-dimethylformamide: a new paradigm for solid electrolytes based upon the Pearson hard–soft acid–base concept. Chem. Mater. 27, 5479–5482 (2015).
Chinnam, P. R. et al. A self-binding, melt-castable, crystalline organic electrolyte for sodium ion conduction. Angew. Chem. Int. Ed. 55, 15254–15257 (2016).
Prakash, P. et al. Solvate sponge crystals of (DMF)3NaClO4: reversible pressure/temperature controlled juicing in a melt/press-castable sodium-ion conductor. Chem. Sci. 12, 5574–5581 (2021).
Prakash, P. et al. Mechanism of ion conduction and dynamics in tris(N,N-dimethylformamide) perchloratosodium solid electrolytes. J. Phys. Chem. C 126, 4744–4750 (2022).
Fall, B. et al. Crystal structure and ionic conductivity of the soft solid crystal: isoquinoline3•(LiCl)2. Ionics 24, 343–349 (2018).
Fall, B. et al. Experimental and theoretical investigation of the ion conduction mechanism of tris(adiponitrile)perchloratosodium, a self-binding, melt-castable crystalline sodium electrolyte. Chem. Mater. 31, 8850–8863 (2019).
Abouimrane, A., Whitfield, P. S., Niketic, S. & Davidson, I. J. Investigation of Li salt doped succinonitrile as potential solid electrolytes for lithium batteries. J. Power Sources 174, 883–888 (2007).
Alarco, P.-J., Abu-Lebdeh, Y., Abouimrane, A. & Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 3, 476–481 (2004).
Yang, J. et al. Safety-enhanced polymer electrolytes for sodium batteries: recent progress and perspectives. ACS Appl. Mater. Interfaces 11, 17109–17127 (2019).
Hu, P. et al. Progress in nitrile-based polymer electrolytes for high performance lithium batteries. J. Mater. Chem. A 4, 10070–10083 (2016).
Forsyth, M., Sun, J. & Macfarlane, D. R. Novel polymer-in-salt electrolytes based on polyacrylonitrile (PAN)-lithium triflate salt mixtures. Solid State Ion. 112, 161–163 (1998).
Yoon, H.-K., Chung, W.-S. & Jo, N.-J. Study on ionic transport mechanism and interactions between salt and polymer chain in PAN based solid polymer electrolytes containing LiCF3SO3. Electrochim. Acta 50, 289–293 (2004).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Li, W., Song, B. & Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017).
Wang, X. et al. Adiponitrile as lithium-ion battery electrolyte additive: a positive and peculiar effect on high-voltage systems. ACS Appl. Energy Mater. 1, 5347–5354 (2018).
Lee, S. H., Hwang, J., Park, S., Park, G. & Sun, Y. Adiponitrile (C6H8N2): a new bi‐functional additive for high‐performance Li‐metal batteries. Adv. Funct. Mater. 29, 1902496 (2019).
Di Censo, D., Exnar, I. & Graetzel, M. Non-corrosive electrolyte compositions containing perfluoroalkylsulfonyl imides for high power Li-ion batteries. Electrochem. Commun. 7, 1000–1006 (2005).
Abu-Lebdeh, Y. & Davidson, I. High-voltage electrolytes based on adiponitrile for Li-ion batteries. J. Electrochem. Soc. 156, A60 (2009).
Yamada, Y. et al. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries. J. Am. Chem. Soc. 136, 5039–5046 (2014).
Kerner, M., Plylahan, N., Scheers, J. & Johansson, P. Thermal stability and decomposition of lithium bis(fluorosulfonyl)imide (LiFSI) salts. RSC Adv. 6, 23327–23334 (2016).
Murata, K., Asakawa, H., Nagashima, K., Furukawa, Y. & Sazaki, G. Thermodynamic origin of surface melting on ice crystals. Proc. Natl Acad. Sci. USA 113, E6741–E6748 (2016).
Prakash, P. et al. Unravelling the structural and dynamical complexity of the equilibrium liquid grain-binding layer in highly conductive organic crystalline electrolytes. J. Mater. Chem. A 6, 4394–4404 (2018).
Wohde, F., Balabajew, M. & Roling, B. Li+ transference numbers in liquid electrolytes obtained by very-low-frequency impedance spectroscopy at variable electrode distances. J. Electrochem. Soc. 163, A714–A721 (2016).
Ue, M., Takeda, M., Takehara, M. & Mori, S. Electrochemical properties of quaternary ammonium salts for electrochemical capacitors. J. Electrochem. Soc. 144, 2684–2688 (1997).
Cheng, X.-B. & Zhang, Q. Dendrite-free lithium metal anodes: stable solid electrolyte interphases for high-efficiency batteries. J. Mater. Chem. A 3, 7207–7209 (2015).
Kerner, M. et al. Towards more thermally stable Li-ion battery electrolytes with salts and solvents sharing nitrile functionality. J. Power Sources 332, 204–212 (2016).
Bruce, P. G. & Vincent, C. A. Steady state current flow in solid binary electrolyte cells. J. Electroanal. Chem. 225, 1–17 (1987).
Evans, J., Vincent, C. A. & Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28, 2324–2328 (1987).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Breneman, C. M. & Wiberg, K. B. Determining atom centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 11, 361–373 (1990).
Frisch, M. J. et al. Gaussian 09, Revision E.01 (Gaussian, 2009).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Humphrey, W., Dalke, A. & Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 14, 33–38 (1996).
Giannozzi, P. et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 29, 465901 (2017).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
We gratefully acknowledge the support of this work by the National Science Foundation under award no. DMR-2138432 (L.A.S., S.L.W., M.J.Z.) and by the Department of Science and Technology’s Nanomission SR/NM/TP-13/2016, Scientific and Engineering Research Board (SERB) DST CRG/2018/001536 and IUSSTF/JC-031/2017 (A.V.). We acknowledge the National Supercomputing Mission ‘PARAM Brahma’ at Indian Institute of Science Education and Research, Pune, which is implemented by Centre for Development of Advanced Computing (C-DAC) and supported by the Ministry of Electronics and Information Technology and the Department of Science and Technology of the Government of India; Temple University’s Energy Frontier Research Center cluster, which is supported by the Center for Complex Materials, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences, under award no. DESC0012575; and Temple University’s High-Performance Computing clusters, which were supported in part by the National Science Foundation through major research instrumentation grant no. CNS-1625061 and by the US Army Research Laboratory under contract no. W911NF-16-2-018 for computational resources. P.P. acknowledges the foreign Fulbright Program from United States Department of State for a visiting research fellowship. For SEM work, we acknowledge the Temple University College of Engineering Nano Instrumentation Center at Temple University founded on a US Department of Defense, Defense University Research Instrumentation Program (DURIP) award no. N0014-12-1-0777 from the Office of Naval Research.
Author information
Authors and Affiliations
Contributions
P.P. assisted with the optimization of the synthetic protocol and the electrochemical data collection and interpretation, and led the computational efforts on the MD simulations and DFT calculations. B.F. discovered and optimized the synthesis of the electrolyte, obtained electrochemical and physical data and assisted in their interpretation, and constructed and tested the lithium metal electrochemical cells. J.A. collected and interpreted the Raman data and constructed and tested the LTO | NMC full-cells. L.A.S. prepared samples and performed the pressing experiments shown in Supplementary Fig. 3. S.C. prepared the cathode composite and assisted with cell assembly and electrochemical data interpretation. P.R.C. designed the electrochemical experimental testing, devised the experimental strategy and assisted with data interpretation. D.A.D. obtained the SEM images and energy-dispersive X-ray spectroscopy data and assisted with interpretation. A.V. supervised the computational efforts. S.L.W. supervised the electrochemistry and characterization efforts. M.J.Z. supervised the synthetic and X-ray characterization efforts. P.P., A.V., S.L.W. and M.J.Z wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Yuki Yamada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 TGA data of (Adpn)2LiPF6, LiPF6 and Adpn.
LiF remains at 800 oC for LiPF6 and (Adpn)2LiPF6. For Sample 1, a typical TGA pan (open) with N2 purge was used. For Sample 2, a punch-holed Tzero aluminium pan (used in DSC) was used to avoid excessive removal of Adpn from crystals due to N2 purge. The sample 2 shows a Td at 200 oC, higher than the Tm = 180 oC from DSC (Fig. 2a).
Extended Data Fig. 2 Snapshots of (Adpn)2LiPF6 cocrystals after an equilibration of 10 ns (every temperature) using model V.
(a) 300 K, (b) 400 K and (c) 500 K. Li–N(Adpn) coordinated networks are shown as yellow tetrahedrons and PF6− anions are shown as red octahedrons. Adpn solvent is shown as line representations. (d) Non-bonded interaction energy vs. temperature during the simulated heating of (Adpn)2LiPF6 cocrystals in model V. The liquid-like surface-layer is shown in a cyan ring in (c).
Extended Data Fig. 3 SEM SE images of (Adpn)6LiPF6 at different magnifications taken at RT.
(a, b, c) pressed pallets; (d, e) pressed pallets quenched in liquid N2; (f) pressed pellet that has been quenched in liquid N2 so that some grain boundaries broken; (g, h, i) powders, fusion between the grains can be observed by comparison between powders and powders pressed to make pellets; even in powder, there can be connection between the grains (i); in the pressed pellet the large grains contain smaller crystallites and there are needle-like structures between the grains; (j, k, l) samples synthesized in glass fiber.
Extended Data Fig. 4 SEM SE images of (Adpn)6LiPF6 after cycling.
Post-mortem (30 days to failure) SEM images after Li plating/stripping of Li0/(Adpn)2LiPF6/Li0 showing places where the (Adpn)2LiPF6 cocrystal still adhered to the Li0, and the SEI layer at three magnifications (a-c); (d) edge of Li0 metal showing thin SEI layer.
Extended Data Fig. 5 SEM EDX spectra of (Adpn)6LiPF6 post-mortem.
(a) and (b) are EDX spectra taken at positions 2 and 4, respectively, in Extended Data Fig. 4a, which show places where the (Adpn)2LiPF6 cocrystal still adhered to the Li0, as indicated by strong C, F, N and P signals; (c) – (g) are EDX spectra taken at positions 5, 6, 7, 8 and 9 in Extended Data Fig. 4b-c, which show SEI layer dominated by strong O and C signals; (h) Atom % for EDX spectra.
Extended Data Fig. 6 Additional electrochemistry data.
(a) Representative constant phase-element (CPE) circuit; (b) Li+ ion transference number (tLi+) measured after stabilization of the impedance; t+Li = 0.54; a zoomed-in view of Fig. 4d, Li plating, 2 h charge/discharge cycles at (c) J = 0.05 mA/cm2 for 60 cycles, (d) J = 0.1 mA/cm2 for 60 cycles; (e) Discharge capacity as a function of C rate for a Li0/(Adpn)2LiPF6/LiFePO4 half-cell #1 at RT, 2 h charge/2 h discharge cycles. The cell did not recover after C/2; (f) Discharge capacity as a function of C rate for a Li0/(Adpn)2LiPF6/LiFePO4 half-cell #2 at 10 °C, 2 h charge/2 h discharge cycles. The cell recovered after 1 C. At high charge/discharge rates (1 C), the capacity drops to zero since at 10 °C the grain boundary region is close to the freezing point of the “free” Adpn in the (Adpn)2LiPF6 cocrystals, possibly preventing growth of Li dendrites through the grain boundaries; (g) Capacity and Coulombic efficiency of LTO/(ADN)2LiPF6/NMC622 cells at 25 °C at C/10 rate as a function of cycle number, 2 h charge/2 h discharge where two cycles of preconditioning at C/40 rate were performed; (h) Impedance spectra before and after the full cell cycling at RT, two semicircles represent interfacial resistance at the Li anode and LiFePO4 cathode. Impedance increases after the cycling; (i) Impedance spectra (Nyquist plots) over time for NMC-622/(Adpn)2LiPF6−glass fiber/NMC-622 (1 MHz – 0.01 Hz), a zoomed in view is shown inset.
Extended Data Fig. 7 Electronic structure interpretation of redox stability of cocrystals.
(a) Density of states (DOS) for (Adpn)2LiPF6. Solid lines show p-orbital densities for optimized structure of (Adpn)2LiPF6: [Adpn2LiPF6]opt. Dotted lines show the effect of one electron addition; after addition of the electron, the structure was optimized and then this ‘excess’ electron was removed to calculate DOS from SCF (without optimizing the structure): [{(Adpn2LiPF6)−1opt}-e-]scf; Differential Electron density map (ρe-[Adpn2LiPF6]opt-ρe-[{(Adpn2LiPF6)−1opt}-e-]scf) showing the effect of addition of an electron to a unit cell of (Adpn)2LiPF6 - (b) in the plane perpendicular to c-crystallographic direction and (c) in the plane perpendicular to b-crystallographic direction. Close to Li+•••CN: blue lobes are larger compared to red, suggesting greater change in the electron density close to C≡N compared to P—F, on the addition of electron.
Extended Data Fig. 8 Average mean squared displacement of constituent Li+ ions, PF6−, ions and Adpn molecules in model V2g,sol.
(a) 300 K, (b) 325 K, and (c) 350 K. The calculated diffusion coefficients are provided in Supplementary Table S2.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Tables 1–6 and Discussion.
MD simulation of eight grains (model V8g) of Adpn2LiPF6 exhibiting the formation of grain boundaries.
Mechanism of Li+ ion conduction in the b crystallographic direction of Adpn2LiPF6 crystals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prakash, P., Fall, B., Aguirre, J. et al. A soft co-crystalline solid electrolyte for lithium-ion batteries. Nat. Mater. 22, 627–635 (2023). https://doi.org/10.1038/s41563-023-01508-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01508-1
This article is cited by
-
A solid-state lithium-ion battery with micron-sized silicon anode operating free from external pressure
Nature Communications (2024)