Abstract
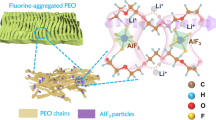
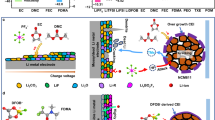
Solid polymers are promising electrolytes for Li-metal batteries, but they have limitations: they cannot simultaneously achieve high ionic conductivity, good mechanical strength and compatibility with high-voltage cathodes while suppressing Li dendrites. Here, we design a class of locally high-concentration solid polymer electrolytes based on polymer blends, which are termed Li-polymer in F diluter (LPIFD). The Li-polymer (polymer-in-salt) ensures continuous Li-ion conduction channels and contributes to the solid electrolyte interphase (SEI), and the F diluter (inert fluorinated polymer) adds mechanical strength. Studies reveal that a single-phase LPIFD, which is based on a miscible polymer blend, lacks phase boundaries and forms an organic-less and LiF-rich SEI, effectively suppressing lithium dendrites. The single-phase LPIFD delivers ionic conductivity of 3.0 × 10−4 S cm−1, and enables the Li anode to reach a high coulombic efficiency of 99.1% and a critical current density of 3.7 mA cm−2. Furthermore, the ability to form an F-rich cathode electrolyte interphase allows LiNi0.8Co0.1Mn0.1O2||Li cells to achieve a cycle life of 450 cycles at a high operating voltage of 4.5 V. This design will inspire efforts to commercialize polymer electrolytes for high-energy Li-metal batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials.
References
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Huang, W., Feng, X., Han, X., Zhang, W. & Jiang, F. Questions and answers relating to lithium-ion battery safety issues. Cell Rep. Phys. Sci. 2, 100285 (2021).
Bai, P., Li, J., Brushett, F. R. & Bazant, M. Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 9, 3221–3229 (2016).
Li, W. et al. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 6, 7436 (2015).
Ren, Y., Shen, Y., Lin, Y. & Nan, C.-W. Direct observation of lithium dendrites inside garnet-type lithium-ion solid electrolyte. Electrochem. Commun. 57, 27–30 (2015).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Cheng, X.-B., Zhang, R., Zhao, C.-Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Liu, S. et al. In situ solid electrolyte interphase from spray quenching on molten Li: a new way to construct high‐performance lithium‐metal anodes. Adv. Mater. 31, 1806470 (2019).
Paul, P. P. et al. A review of existing and emerging methods for lithium detection and characterization in Li‐ion and Li‐metal batteries. Adv. Energy Mater. 11, 2100372 (2021).
Park, R. J.-Y. et al. Semi-solid alkali metal electrodes enabling high critical current densities in solid electrolyte batteries. Nat. Energy 6, 314–322 (2021).
Fan, X. et al. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 4, eaau9245 (2018).
Ji, X. et al. Solid‐state electrolyte design for lithium dendrite suppression. Adv. Mater. 32, 2002741 (2020).
Ren, Y. X. et al. Rational design of spontaneous reactions for protecting porous lithium electrodes in lithium–sulfur batteries. Nat. Commun. 10, 3249 (2019).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Wang, J. et al. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 7, 12032 (2016).
Yamada, Y. & Yamada, A. Superconcentrated electrolytes for lithium batteries. J. Electrochem. Soc. 162, A2406 (2015).
Chen, J. et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 5, 386–397 (2020).
Fan, X. et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 4, 174–185 (2018).
Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Ren, X. et al. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 4, 1877–1892 (2018).
Yu, Z. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 5, 526–533 (2020).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396 (2005).
Monroe, C. & Newman, J. The effect of interfacial deformation on electrodeposition kinetics. J. Electrochem. Soc. 151, A880 (2004).
Krauskopf, T., Richter, F. H., Zeier, W. G. & Janek, J. Physicochemical concepts of the lithium metal anode in solid-state batteries. Chem. Rev. 120, 7745–7794 (2020).
Cao, D. et al. Lithium dendrite in all-solid-state batteries: growth mechanisms, suppression strategies, and characterizations. Matter 3, 57–94 (2020).
Lu, Y. et al. Critical current density in solid‐state lithium metal batteries: mechanism, influences, and strategies. Adv. Funct. Mater. 31, 2009925 (2021).
Wang, Y. et al. Solid-state rigid-rod polymer composite electrolytes with nanocrystalline lithium ion pathways. Nat. Mater. 20, 1255–1263 (2021).
Zhao, Q., Liu, X., Stalin, S., Khan, K. & Archer, L. A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 4, 365–373 (2019).
Cabañero Martínez, M. A. et al. Are polymer‐based electrolytes ready for high‐voltage lithium battery applications? An overview of degradation mechanisms and battery performance. Adv. Energy Mater. 12, 2201264 (2022).
Lin, R. et al. Characterization of the structure and chemistry of the solid–electrolyte interface by cryo-EM leads to high-performance solid-state Li-metal batteries. Nat. Nanotechnol. 17, 768–776 (2022).
Holoubek, J. et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 6, 303–313 (2021).
Gao, H., Grundish, N. S., Zhao, Y., Zhou, A. & Goodenough, J. B. Formation of stable interphase of polymer-in-salt electrolyte in all-solid-state lithium batteries. Energy Mater. Adv. 2021, 1932952 (2021).
Chen, F., Wang, X., Armand, M. & Forsyth, M. Cationic polymer-in-salt electrolytes for fast metal ion conduction and solid-state battery applications. Nat. Mater. 21, 1175–1182 (2022).
Kimura, K., Yajima, M. & Tominaga, Y. A highly-concentrated poly(ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature. Electrochem. Commun. 66, 46–48 (2016).
Fan, R. et al. Versatile strategy for realizing flexible room-temperature all-solid-state battery through a synergistic combination of salt affluent PEO and Li6.75La3Zr1.75Ta0.25O12 nanofibers. ACS Appl. Mater. Interfaces 12, 7222–7231 (2020).
Wang, X. et al. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries. ACS Appl. Mater. Interfaces 10, 24791–24798 (2018).
Wu, J. et al. Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries. Energy Environ. Sci. 14, 12–36 (2021).
Wang, X. et al. Ultra-stable all-solid-state sodium metal batteries enabled by perfluoropolyether-based electrolytes. Nat. Mater. 21, 1057–1065 (2022).
Zhao, Y. et al. A rational design of solid polymer electrolyte with high salt concentration for lithium battery. J. Power Sources 407, 23–30 (2018).
Cheng, X.-B., Zhao, C.-Z., Yao, Y.-X., Liu, H. & Zhang, Q. Recent advances in energy chemistry between solid-state electrolyte and safe lithium-metal anodes. Chem 5, 74–96 (2019).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Wu, B. et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 11, 1803–1810 (2018).
Wu, Q. et al. Phase regulation enabling dense polymer-based composite electrolytes for solid-state lithium metal batteries. Nat. Commun. 14, 6296 (2023).
Xi, J. et al. PVDF–PEO blends based microporous polymer electrolyte: effect of PEO on pore configurations and ionic conductivity. J. Power Sources 157, 501–506 (2006).
Chaput, S., Carrot, C., Castro, M. & Prochazka, F. Co-continuity interval in immiscible polymer blends by dynamic mechanical spectroscopy in the molten and solid state. Rheol. Acta 43, 417–426 (2004).
Liu, J. et al. Blending-based poly(vinylidene fluoride)/polymethyl methacrylate membrane for rechargeable lithium-ion batteries. Ionics 25, 5201–5211 (2019).
Cznotka, E., Jeschke, S., Schmohl, S., Johansson, P. & Wiemhöfer, H.-D. 3D laser scanning confocal microscopy of siloxane-based comb and double-comb polymers in PVDF-HFP thin films. J. Coat. Technol. Res. 13, 577–587 (2016).
Tang, L. et al. Polyfluorinated crosslinker-based solid polymer electrolytes for long-cycling 4.5 V lithium metal batteries. Nat. Commun. 14, 2301 (2023).
Zhang, X. et al. Self‐suppression of lithium dendrite in all‐solid‐state lithium metal batteries with poly(vinylidene difluoride)‐based solid electrolytes. Adv. Mater. 31, 1806082 (2019).
Liu, W. et al. Designing polymer‐in‐salt electrolyte and fully infiltrated 3D electrode for integrated solid‐state lithium batteries. Angew. Chem. 133, 13041–13050 (2021).
Zhang, W., Yi, Q., Li, S. & Sun, C. An ion-conductive Li7La3Zr2O12-based composite membrane for dendrite-free lithium metal batteries. J. Power Sources 450, 227710 (2020).
Guo, S. et al. PVDF-HFP/LiF composite interfacial film to enhance the stability of Li-metal anodes. ACS Appl. Energy Mater. 3, 7191–7199 (2020).
Borodin, O. Challenges with prediction of battery electrolyte electrochemical stability window and guiding the electrode–electrolyte stabilization. Curr. Opin. Electrochem. 13, 86–93 (2019).
Yang, Z., Zhang, W., Li, J. & Chen, J. Polyphosphazene membrane for desulfurization: selecting poly[bis(trifluoroethoxy) phosphazene] for pervaporative removal of thiophene. Sep. Purif. Technol. 93, 15–24 (2012).
Kaskhedikar, N. et al. Ionic conductivity of polymer electrolyte membranes based on polyphosphazene with oligo(propylene oxide) side chains. Solid State Ion. 177, 703–707 (2006).
Jankowsky, S., Hiller, M. M. & Wiemhöfer, H.-D. Preparation and electrochemical performance of polyphosphazene based salt-in-polymer electrolyte membranes for lithium ion batteries. J. Power Sources 253, 256–262 (2014).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Xu, S. et al. Decoupling of ion pairing and ion conduction in ultrahigh-concentration electrolytes enables wide-temperature solid-state batteries. Energy Environ. Sci. 15, 3379–3387 (2022).
Wang, J., Zhou, J., Hu, Y. & Regier, T. Chemical interaction and imaging of single Co3O4/graphene sheets studied by scanning transmission X-ray microscopy and X-ray absorption spectroscopy. Energy Environ. Sci. 6, 926–934 (2013).
Schmeißer, D. et al. Characterization of oxidic and organic materials with synchrotron radiation based XPS and XAS. Mater. Sci. 27, 141–157 (2009).
Widstrom, M. D. et al. Water domain enabled transport in polymer electrolytes for lithium-ion batteries. Macromolecules 54, 2882–2891 (2021).
Chen, L. et al. A 63 m superconcentrated aqueous electrolyte for high-energy Li-ion batteries. ACS Energy Lett. 5, 968–974 (2020).
Piao, N. et al. Countersolvent electrolytes for lithium‐metal batteries. Adv. Energy Mater. 10, 1903568 (2020).
Smith, J. W. et al. X-ray absorption spectroscopy of LiBF4 in propylene carbonate: a model lithium ion battery electrolyte. Phys. Chem. Chem. Phys. 16, 23568–23575 (2014).
Ong, M. T. et al. Lithium ion solvation and diffusion in bulk organic electrolytes from first-principles and classical reactive molecular dynamics. J. Phys. Chem. B 119, 1535–1545 (2015).
Wu, C. H. et al. Molecular-scale structure of electrode–electrolyte interfaces: the case of platinum in aqueous sulfuric acid. J. Am. Chem. Soc. 140, 16237–16244 (2018).
Yamane, H. et al. Critical absorbed dose of resinous adhesive material towards non-destructive chemical-state analysis using soft X-rays. J. Electron Spectros. Relat. Phenom. 232, 11–15 (2019).
Zhou, D. et al. In situ synthesis of a hierarchical all‐solid‐state electrolyte based on nitrile materials for high‐performance lithium‐ion batteries. Adv. Energy Mater. 5, 1500353 (2015).
Deng, T. et al. In situ formation of polymer-inorganic solid-electrolyte interphase for stable polymeric solid-state lithium-metal batteries. Chem 7, 3052–3068 (2021).
Wu, N. et al. In situ formation of Li3P layer enables fast Li+ conduction across Li/solid polymer electrolyte interface. Adv. Funct. Mater. 30, 2000831 (2020).
Fan, X. et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 4, 882–890 (2019).
Chen, J. et al. Electrolyte design for Li metal-free Li batteries. Mater. Today 39, 118–126 (2020).
Bhattacharyya, R. et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 9, 504–510 (2010).
Chandrashekar, S. et al. 7Li MRI of Li batteries reveals location of microstructural lithium. Nat. Mater. 11, 311–315 (2012).
Tsai, C.-L. et al. Li7La3Zr2O12 interface modification for Li dendrite prevention. ACS Appl. Mater. Interfaces 8, 10617–10626 (2016).
Liu, S. et al. An inorganic‐rich solid electrolyte interphase for advanced lithium‐metal batteries in carbonate electrolytes. Angew. Chem. Int. Ed. 60, 3661–3671 (2021).
Liu, S. et al. Salt‐in‐salt reinforced carbonate electrolyte for Li metal batteries. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202210522 (2022).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Fujigaya, T. et al. New photoresist materials for 157-nm lithography. Poly[vinylsulfonyl fluoride-co-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropyl)-styrene] partially protected with tert-butoxycarbonyl. Chem. Mater. 15, 1512–1517 (2003).
Gao, Y. et al. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019).
Lerotic, M., Jacobsen, C., Schäfer, T. & Vogt, S. Cluster analysis of soft X-ray spectromicroscopy data. Ultramicroscopy 100, 35–57 (2004).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Jorgensen, W. L. & Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 110, 1657–1666 (1988).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Abraham, M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Pronk, S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 (2013).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
van der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Berendsen, H. J. C., van der Spoel, D. & van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995).
Robertson, M. J., Tirado-Rives, J. & Jorgensen, W. L. Improved peptide and protein torsional energetics with the OPLS-AA force field. J. Chem. Theory Comput. 11, 3499–3509 (2015).
Dodda, L. S., Cabeza de Vaca, I., Tirado-Rives, J. & Jorgensen, W. L. LigParGen web server: an automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 45, W331–W336 (2017).
Singh, U. C. & Kollman, P. A. An approach to computing electrostatic charges for molecules. J. Comput. Chem. 5, 129–145 (1984).
Tomasi, J. et al. Gaussian 16 Rev. C. 01 (Gaussian Inc., 2016).
Twum, E. B., McCord, E. F., Lyons, D. F., Fox, P. A. & Rinaldi, P. L. Characterization of end groups and branching structures in copolymers of vinylidene fluoride with hexafluoropropylene using multidimensional NMR spectroscopy. Eur. Polym. J. 51, 136–150 (2014).
Apostolo, M., Arcella, V., Storti, G. & Morbidelli, M. Kinetics of the emulsion polymerization of vinylidene fluoride and hexafluoropropylene. Macromolecules 32, 989–1003 (1999).
Ameduri, B., Boutevin, B. & Kostov, G. Fluoroelastomers: synthesis, properties and applications. Prog. Polym. Sci. 26, 105–187 (2001).
Twum, E. B., McCord, E. F., Fox, P. A., Lyons, D. F. & Rinaldi, P. L. Characterization of backbone structures in poly(vinylidene fluoride-co-hexafluoropropylene) copolymers by multidimensional 19F NMR spectroscopy. Macromolecules 46, 4892–4908 (2013).
Gartner, T. E. III & Jayaraman, A. Modeling and simulations of polymers: a roadmap. Macromolecules 52, 755–786 (2019).
Hockney, R. W., Goel, S. P. & Eastwood, J. W. Quiet high-resolution computer models of a plasma. J. Comput. Phys. 14, 148–158 (1974).
Hess, B., Bekker, H., Berendsen, H. J. C. & Lincs, J. F. A linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Darden, T., & York, D. An N ⋅ log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Berendsen, H. J. C., van Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Brehm, M. & Kirchner, B. TRAVIS-a free analyzer and visualizer for Monte Carlo and molecular dynamics trajectories. J. Cheminform. 4, F1 (2011).
Brehm, M., Thomas, M., Gehrke, S. & Kirchner, B. TRAVIS—A free analyzer for trajectories from molecular simulation. J. Chem. Phys. 152, 164105 (2020).
Bannwarth, C., Ehlert, S. & Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 15, 1652–1671 (2019).
Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput Mol. Sci. 2, 73–78 (2012).
Roemelt, M., Maganas, D., DeBeer, S. & Neese, F. A combined DFT and restricted open-shell configuration interaction method including spin-orbit coupling: application to transition metal L-edge X-ray absorption spectroscopy. J. Chem. Phys. 138, 204101 (2013).
Maganas, D. et al. First principles calculations of the structure and V L-edge X-ray absorption spectra of V2O5 using local pair natural orbital coupled cluster theory and spin–orbit coupled configuration interaction approaches. Phys. Chem. Chem. Phys. 15, 7260–7276 (2013).
Axel, D. B. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
Schäfer, A., Horn, H. & Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 97, 2571–2577 (1992).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Kendall, R. A. & Früchtl, H. A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 97, 158–163 (1997).
Feyereisen, M., Fitzgerald, G. & Komornicki, A. Use of approximate integrals in ab initio theory. An application in MP2 energy calculations. Chem. Phys. Lett. 208, 359–363 (1993).
Pantazis, D. A., Chen, X.-Y., Landis, C. R. & Neese, F. All-electron scalar relativistic basis sets for third-row transition metal atoms. J. Chem. Theory Comput. 4, 908–919 (2008).
Acknowledgements
We acknowledge the advice on the manuscript from B. Dunn at the University of California, Los Angeles, and technical support from the Maryland NanoCenter. STXM was performed at the Canadian Light Source, a national research facility of the University of Saskatchewan. We thank the computational resources provided on Bebop, a high-performance computing cluster operated by the Laboratory Computing Resource Center at Argonne National Laboratory. This work was supported by the US Department of Energy (DOE: Grant No. DE-EE0008856 to C.W.; and Grant No. DE-AC05-76RL01830 to C.W.) and DOE, Office of Energy Efficiency and Renewable Energy (Grant No. DE-EE0009183 to C.W.). The XAS test was supported by DOE, Battery500 Consortium (Grant No. DE-SC0012704 to S.T., X.Y. and E.H.) and DOE, Office of Science User Facilities (Grant No. DE-SC0012704 to Brookhaven National Laboratory). We also thank the Analytical NMR Service & Research Center at the University of Maryland, College Park, for using 600 MHz solution and 500 MHz solid-state NMR spectrometers (supported by the National Science Foundation under Grant No. NSF-1726058).
Author information
Authors and Affiliations
Contributions
W.Z. and C.W. proposed the research. W.Z. conceived the idea, performed the electrochemical, SEM and TEM experiments, and wrote the manuscript. V.K. and N.K.D. performed the molecular dynamics simulations and DFT calculations. S.L. helped with the electrochemical experiments and the SEM. P.B. helped with the FTIR. J.Z. and J.W. performed the STXM. S.T., X.Y. and E.H. performed the XAS. Z.W. helped with the analysis of the calculation results. J.X., H.W. and X.Z. helped with editing the manuscript. H.Y. performed the Instron mechanical test. B.L. and A.L. performed the XPS and analysed the results. F.C. performed the NMR spectroscopy. S.R. drew the schematic, edited the manuscript and supervised the analysis of the polymer properties. A.N. supervised the simulations and calculations, and C.W. supervised the study and the manuscript writing. All authors discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Grazia Accardo, Kan Hatakeyama-Sato and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–8, Figs. 1–63, Table 1 and refs. 1–19.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Koverga, V., Liu, S. et al. Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries. Nat Energy 9, 386–400 (2024). https://doi.org/10.1038/s41560-023-01443-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01443-0