Abstract
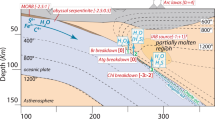
High-pressure dehydration of serpentinite during subduction generates fluids that flux and melt the overlying mantle wedge, forming primary arc basalts. These basalts are substantially more oxidized than their mid-ocean ridge counterparts. At the slab surface of current subduction zones, these deserpentinization fluids are intrinsically oxidized, but, owing to the low sulfur content of subducted serpentinite, they only result in a low mantle wedge oxidation rate, which cannot account for the oxidized source of arc basalts. Here we show that infiltration of sediment-derived fluids modulates and can drastically change the oxidation capacity of deserpentinization slab fluids. The modulation of the deserpentinization oxidation capacity mostly depends on the stability and abundance of dissolved oxidized aqueous species of redox-sensitive elements—notably sulfate—and not solely on the oxidation state of the sediment. Infiltration of CH4-bearing fluids derived from graphite-bearing sediment reduces the intrinsically high oxidant capacity of deserpentinization fluids, explaining the relatively low fO2 observed in natural metaperidotite. Infiltration of sulfate-CO2-bearing, sediment-derived fluids—prevalent in modern subduction zones—generates deserpentinization fluids with a high oxidation capacity in cold and hot subduction zones, resulting in a global mantle wedge oxidation rate of 3.5 km3 yr−1. Such slab fluids will oxidize the mantle wedge at a rate similar to that of arc-basalt generation and thus account for the oxidized nature of arc volcanism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
A spreadsheet file (xlsx) is provided containing all chemical analyses used to construct Fig. 1 and Extended Data Fig. 4, including the duplicate and new analyses from CdA from this work, and also a Supplementary file (csv). The worldwide subduction zone database83 was used to compute the pressure and temperature conditions for the slab surface deserpentinization84. These pressure and temperature conditions were used to compute the intrinsic fluid chemistry and the fluid composition for high- and low-reducing capacity sediments (graphite and GLOSS, respectively) that were used for infiltration at the same serpentinite dehydration pressure and temperature conditions. The main species are given for three cases: intrinsic (_intr), and for infiltration of 12 mol kg−1 for the cases of graphite-bearing and GLOSS sediment-derived fluids (_graph and _gloss). Fluid bulk compositions are given in mol per formula unit of fluid, and species concentrations are given in units of mol kg−1. This database can be generated for other degrees of infiltration using the Jupyter notebook available at https://github.com/bertopadron/Redox.git. Source data are provided with this paper.
Code availability
All computations were produced using Perple_X v6.9.0 version (Methods).
References
Evans, K. A. The redox budget of subduction zones. Earth Sci. Rev. 113, 11–32 (2012).
Lécuyer, C. & Ricard, Y. Long-term fluxes and budget of ferric iron: implication for the redox states of the Earth’s mantle and atmosphere. Earth Planet. Sci. Lett. 165, 197–211 (1999).
Li, J. L. et al. Uncovering and quantifying the subduction zone sulfur cycle from the slab perspective. Nat. Commun. 11, 514 (2020).
Mayhew, L. E. & Ellison, E. T. A synthesis and meta-analysis of the Fe chemistry of serpentinites and serpentine minerals. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 378, 20180420 (2020).
Andreani, M., Muñoz, M., Marcaillou, C. & Delacour, A. μXANES study of iron redox state in serpentine during oceanic serpentinization. Lithos 178, 70–83 (2013).
Debret, B. & Sverjensky, D. A. Highly oxidising fluids generated during serpentinite breakdown in subduction zones. Sci. Rep. 7, 10351 (2017).
Evans, K. A. & Powell, R. The effect of subduction on the sulphur, carbon and redox budget of lithospheric mantle. J. Metamorph. Geol. 33, 649–670 (2015).
Evans, K. A., Reddy, S. M., Tomkins, A. G., Crossley, R. J. & Frost, B. R. Effects of geodynamic setting on the redox state of fluids released by subducted mantle lithosphere. Lithos 278–281, 26–42 (2017).
Bénard, A. et al. Oxidising agents in sub-arc mantle melts link slab devolatilisation and arc magmas. Nat. Commun. 9, 3500 (2018).
O’Neill, H. S. C. et al. Ferric iron in the upper mantle and in transition zone assemblages: implications for relative oxygen fugacities in the mantle. In Evolution of the Earth and Planets (eds Takahashi, E. et al.) 73–88 (American Geophysical Union, 2013); https://doi.org/10.1029/GM074p0073
Debret, B. et al. Redox transfer at subduction zones: insights from Fe isotopes in the Mariana forearc. Geochem. Perspect. Lett. 12, 46–51 (2020).
Iacovino, K., Guild, M. R. & Till, C. B. Aqueous fluids are effective oxidizing agents of the mantle in subduction zones. Contrib. Mineral. Petrol. 175, 36 (2020).
Maurice, J. et al. The intrinsic nature of antigorite breakdown at 3 GPa: experimental constraints on redox conditions of serpentinite dehydration in subduction zones. Contrib. Mineral. Petrol. 175, 94 (2020).
Merkulova, M. V. et al. Experimental insight into redox transfer by iron- and sulfur-bearing serpentinite dehydration in subduction zones. Earth Planet. Sci. Lett. 479, 133–143 (2017).
Evans, K. A. & Frost, B. R. Deserpentinization in subduction zones as a source of oxidation in arcs: a reality check. J. Petrol. 62, egab016 (2021).
Duan, W., Li, X., Schertl, H. & Willner, A. P. C–O–H–S fluids released by oceanic serpentinite in subduction zones: implications for arc-magma oxidation. Earth Planet. Sci. Lett. 594, 117709 (2022).
Bretscher, A., Hermann, J. & Pettke, T. The influence of oceanic oxidation on serpentinite dehydration during subduction. Earth Planet. Sci. Lett. 499, 173–184 (2018).
Vieira Duarte, J. F., Piccoli, F., Pettke, T. & Hermann, J. Textural and geochemical evidence for magnetite production upon antigorite breakdown during subduction. J. Petrol. https://doi.org/10.1093/petrology/egab053 (2021).
Piccoli, F. et al. Subducting serpentinites release reduced, not oxidized, aqueous fluids. Sci. Rep. 9, 19573 (2019).
Ague, J. J. et al. Slab-derived devolatilization fluids oxidized by subducted metasedimentary rocks. Nat. Geosci. 15, 320–326 (2022).
Frost, R. & Ballhaus, C. Comment on ‘Constraints on the origin of the oxidation state of mantle overlying subduction zones: an example from Simcoe, Washington, USA’ by A. D. Brandon and D. S. Draper. Geochim. Cosmochim. Acta 62, 329–331 (1998).
Rielli, A. et al. Evidence of sub-arc mantle oxidation by sulphur and carbon. Geochem. Perspect. Lett. 3, 124–132 (2017).
Colin, A. et al. In situ determination of sulfur speciation and partitioning in aqueous fluid-silicate melt systems. Geochem. Perspect. Lett. 14, 31–35 (2020).
Lazar, C. Using silica activity to model redox-dependent fluid compositions in serpentinites from 100 to 700 °C and from 1 to 20 kbar. J. Petrol. 61, egaa101 (2020).
Debret, B. et al. Redox state of iron during high-pressure serpentinite dehydration. Contrib. Mineral. Petrol. 169, 36 (2015).
Padrón-Navarta, J. A., López Sánchez-Vizcaíno, V., Gómez-Pugnaire, M. T. & Garrido, C. J. Metamorphic record of high-pressure dehydration of antigorite serpentinite to chlorite harzburgite in a subduction setting (Cerro del Almirez, Nevado-Filábride complex, Southern Spain). J. Petrol. 52, 2047–2078 (2011).
Alt, J. C. et al. Recycling of water, carbon and sulfur during subduction of serpentinites: a stable isotope study of Cerro del Almirez, Spain. Earth Planet. Sci. Lett. 327–328, 50–60 (2012).
Padrón-Navarta, J. A., Hermann, J., Garrido, C. J., López Sánchez-Vizcaíno, V. & Gómez-Pugnaire, M. T. An experimental investigation of antigorite dehydration in natural silica-enriched serpentinite. Contrib. Mineral. Petrol. 159, 25–42 (2010).
Bromiley, G. D. & Pawley, A. R. The stability of antigorite in the systems MgO-SiO2-H2O (MAH) and MgO-Al2O3-SiO2-H2O (MASH): the effects of Al3+ substitution on high-pressure stability. Am. Mineral. 88, 99–108 (2003).
Evans, B. W. & Trommsdorff, V. Petrogenesis of garnet lherzolite, Cima di Gagnone, Lepontine Alps. Earth Planet. Sci. Lett. 40, 333–348 (1978).
Truckenbrodt, J., Ziegenbein, D. & Johannes, W. Redox conditions in piston-cylinder apparatus: the different behavior of boron nitride and unfired pyrophyllite assemblies. Am. Mineral. 82, 337–344 (1997).
Marchesi, C., Garrido, C. J., Padrón-Navarta, J. A., López Sánchez-Vizcaíno, V. & Gómez-Pugnaire, M. T. Element mobility from seafloor serpentinization to high-pressure dehydration of antigorite in subducted serpentinite: insights from the Cerro del Almirez ultramafic massif (southern Spain). Lithos 178, 128–142 (2013).
Scambelluri, M., Pettke, T. & Cannaò, E. Fluid-related inclusions in Alpine high-pressure peridotite reveal trace element recycling during subduction-zone dehydration of serpentinized mantle (Cima di Gagnone, Swiss Alps). Earth Planet. Sci. Lett. 429, 45–59 (2015).
Cannaò, E., Agostini, S., Scambelluri, M., Tonarini, S. & Godard, M. B, Sr and Pb isotope geochemistry of high-pressure Alpine metaperidotites monitors fluid-mediated element recycling during serpentinite dehydration in subduction mélange (Cima di Gagnone, Swiss Central Alps). Geochim. Cosmochim. Acta 163, 80–100 (2015).
Garrido, C. J. et al. Enrichment of HFSE in chlorite-harzburgite produced by high-pressure dehydration of antigorite-serpentinite: implications for subduction magmatism. Geochem. Geophys. Geosyst. https://doi.org/10.1029/2004GC000791 (2005).
Bebout, G. E. & Penniston-Dorland, S. C. Fluid and mass transfer at subduction interfaces—the field metamorphic record. Lithos 240–243, 228–258 (2016).
Angiboust, S., Wolf, S., Burov, E., Agard, P. & Yamato, P. Effect of fluid circulation on subduction interface tectonic processes: insights from thermo-mechanical numerical modelling. Earth Planet. Sci. Lett. 357–358, 238–248 (2012).
Brounce, M., Cottrell, E. & Kelley, K. A. The redox budget of the Mariana subduction zone. Earth Planet. Sci. Lett. 528, 115859 (2019).
Clift, P. D. A revised budget for Cenozoic sedimentary carbon subduction. Rev. Geophys. 55, 97–125 (2017).
Plank, T. & Langmuir, C. H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 145, 325–394 (1998).
Plank, T. & Langmuir, C. H. Tracing trace elements from sediment input to volcanic output at subduction zones. Nature 362, 739–743 (1993).
Galvez, M. E. & Jaccard, S. L. Reducing capacity of rocks by high temperature chalcometric titration. Chem. Geol. 564, 120016 (2020).
Galvez, M. E., Fischer, W. W., Jaccard, S. L. & Eglinton, T. I. Materials and pathways of the organic carbon cycle through time. Nat. Geosci. 13, 535–546 (2020).
Augier, R. et al. Exhumation constraints for the lower Nevado-Filabride Complex (Betic Cordillera, SE Spain): a Raman thermometry and Tweequ multiequilibrium thermobarometry approach. Bull. Soc. Géol. Fr. 176, 403–416 (2005).
Kuhn, B. K., Reusser, E., Powell, R. & Günther, D. Metamorphic evolution of calc-schists in the Central Alps, Switzerland. Schweiz. Mineral. Petrogr. Mitteilungen 85, 175–190 (2005).
Green, D. H., Hibberson, W. O., Kovacs, I. & Rosenthal, A. Water and its influence on the lithosphere-asthenosphere boundary. Nature 467, 448–451 (2010).
Malaspina, N., Poli, S. & Fumagalli, P. The oxidation state of metasomatized mantle wedge: insights from C–O–H-bearing garnet peridotite. J. Petrol. 50, 1533–1552 (2009).
Stolper, E. M., Shorttle, O., Antoshechkina, P. M. & Asimow, P. D. The effects of solid-solid phase equilibria on the oxygen fugacity of the upper mantle. Am. Mineral. 105, 1445–1471 (2020).
Van Keken, P. E., Hacker, B. R., Syracuse, E. M. & Abers, G. A. Subduction factory: 4. Depth-dependent flux of H2O from subducting slabs worldwide. J. Geophys. Res. Solid Earth 116, B01401 (2011).
Jicha, B. R. & Jagoutz, O. Magma production rates for intraoceanic arcs. Elements 11, 105–112 (2015).
Tumiati, S., Godard, G., Martin, S., Malaspina, N. & Poli, S. Ultra-oxidized rocks in subduction mélanges? Decoupling between oxygen fugacity and oxygen availability in a Mn-rich metasomatic environment. Lithos 226, 116–130 (2015).
Skora, S., Freymuth, H., Blundy, J., Elliott, T. & Guillong, M. An experimental study of the behaviour of cerium/molybdenum ratios during subduction: implications for tracing the slab component in the Lesser Antilles and Mariana Arc. Geochim. Cosmochim. Acta 212, 133–155 (2017).
Freymuth, H., Elliott, T., van Soest, M. & Skora, S. Tracing subducted black shales in the Lesser Antilles arc using molybdenum isotope ratios. Geology 44, 987–990 (2016).
Clément, M., Padrόn-Navarta, J. A. & Tommasi, A. Interplay between fluid extraction mechanisms and antigorite dehydration reactions (Val Malenco, Italian Alps). J. Petrol. 60, 1935–1962 (2019).
Lafay, R., Baumgartner, L. P. & Delacour, A. Preservation of mantle heterogeneities and serpentinization signature during antigorite dehydration: the example of the Bergell contact aureole. J. Metamorph. Geol. https://doi.org/10.1111/JMG.12699 (2022).
Debret, B. et al. Evolution of Fe redox state in serpentine during subduction. Earth Planet. Sci. Lett. 400, 206–218 (2014).
Klein, F. et al. Magnetite in seafloor serpentinite—some like it hot. Geology 42, 135–138 (2014).
Klein, F., Marschall, H. R., Bowring, S. A., Humphris, S. E. & Horning, G. Mid-ocean ridge serpentinite in the Puerto Rico Trench: from seafloor spreading to subduction. J. Petrol. 58, 1729–1754 (2017).
Paulick, H. et al. Geochemistry of abyssal peridotites (Mid-Atlantic Ridge, 15° 20′ N, ODP Leg 209): implications for fluid/rock interaction in slow spreading environments. Chem. Geol. 234, 179–210 (2006).
Evans, K. A. Redox decoupling and redox budgets: conceptual tools for the study of earth systems. Geology 34, 489–492 (2006).
Jennings, E. S. & Holland, T. J. B. B. A simple thermodynamic model for melting of peridotite in the system NCFMASOCr. J. Petrol. 56, 869–892 (2015).
Evans, K. A., Powell, R. & Holland, T. J. B. Internally consistent data for sulphur-bearing phases and application to the construction of pseudosections for mafic greenschist facies rocks in Na2O–CaO–K2O–FeO–MgO–Al2O3–SiO2–CO2–O–S–H2O. J. Metamorph. Geol. 28, 667–687 (2010).
Holland, T. & Powell, R. Thermodynamics of order-disorder in minerals. 2. Symmetric formalism applied to solid solutions. Am. Mineral. 81, 1425–1437 (1996).
White, R. W., Powell, R. & Holland, T. J. B. Progress relating to calculation of partial melting equilibria for metapelites. J. Metamorph. Geol. 25, 511–527 (2007).
Connolly, J. A. D. Multivariable phase-diagrams—an algorithm based on generalized thermodynamics. Am. J. Sci. 290, 666–718 (1990).
Connolly, J. A. D. The geodynamic equation of state: what and how. Geochem. Geophys. Geosyst. https://doi.org/10.1029/2009GC002540 (2009).
Connolly, J. A. D. Computation of phase equilibria by linear programming: a tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet. Sci. Lett. 236, 524–541 (2005).
Connolly, J. A. D. & Galvez, M. E. Electrolytic fluid speciation by Gibbs energy minimization and implications for subduction zone mass transfer. Earth Planet. Sci. Lett. 501, 90–102 (2018).
Holland, T. J. B. & Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. https://doi.org/10.1111/j.1525-1314.2010.00923.x (2011).
Holland, T., Baker, J. & Powell, R. Mixing properties and activity-composition relationships of chlorites in the system MgO-FeO-Al2O3-SiO2-H2O. Eur. J. Mineral 10, 395–406 (1998).
Holland, T. J. B., Powell, R., Sciences, E. & Cb, C. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 16, 309–343 (1998).
Dale, J., Holland, T. & Powell, R. Hornblende-garnet-plagioclase thermobarometry: a natural assemblage calibration of the thermodynamics of hornblende. Contrib. Mineral. Petrol. V140, 353–362 (2000).
Padrón-Navarta, J. A. et al. Tschermak’s substitution in antigorite and consequences for phase relations and water liberation in high-grade serpentinites. Lithos 178, 186–196 (2013).
Chatterjee, N. D. & Froese, E. A thermodynamic study of the pseudobinary join muscovite-paragonite in the system KAlSi3O8–NaAlSi3O8–Al2O3–SiO2–H2O. Am. Mineral. 60, 985–993 (1975).
Fuhrman, M. L. & Lindsley, D. H. Ternary-feldspar modeling and thermometry. Am. Mineral. 73, 201–215 (1988).
White, R. W., Powell, R. & Johnson, T. E. The effect of Mn on mineral stability in metapelites revisited: new a–x relations for manganese-bearing minerals. J. Metamorph. Geol. 32, 809–828 (2014).
Holland, T. J. B., Green, E. C. R. & Powell, R. Melting of peridotites through to granites: a simple thermodynamic model in the system KNCFMASHTOCr. J. Petrol. 59, 881–900 (2018).
Laborda-López, C. et al. High-P metamorphism of rodingites during serpentinite dehydration (Cerro del Almirez, Southern Spain): implications for the redox state in subduction zones. J. Metamorph. Geol. 36, 1141–1173 (2018).
Pitzer, K. S. & Sterner, S. M. Equations of state valid continuously from zero to extreme pressures with H2O and CO2 as examples. Int. J. Thermophys. 16, 511–518 (1995).
Connolly, J. A. D. & Cesare, B. C–O–H–S fluid composition and oxygen fugacity in graphitic metapelites. J. Metamorph. Geol. 11, 379–388 (1993).
Galvez, M. E., Connolly, J. A. D. & Manning, C. E. Implications for metal and volatile cycles from the pH of subduction zone fluids. Nature 539, 420–424 (2016).
Huang, F. & Sverjensky, D. A. Extended Deep Earth Water Model for predicting major element mantle metasomatism. Geochim. Cosmochim. Acta 254, 192–230 (2019).
Syracuse, E. M., van Keken, P. E. & Abers, G. A. The global range of subduction zone thermal models. Phys. Earth Planet. Inter. 183, 73–90 (2010).
Menzel, M. D., Garrido, C. J. & López Sánchez-Vizcaíno, V. Fluid-mediated carbon release from serpentinite-hosted carbonates during dehydration of antigorite-serpentinite in subduction zones. Earth Planet. Sci. Lett. 531, 115964 (2020).
Salters, V. J. M. & Stracke, A. Composition of the depleted mantle. Geochem. Geophys. Geosyst. https://doi.org/10.1029/2003GC000597 (2004).
Canil, D. et al. Ferric iron in peridotites and mantle oxidation states. Earth Planet. Sci. Lett. 123, 205–220 (1994).
Ding, S. & Dasgupta, R. The fate of sulfide during decompression melting of peridotite—implications for sulfur inventory of the MORB-source depleted upper mantle. Earth Planet. Sci. Lett. 459, 183–195 (2017).
Elson, P. et al. SciTools/cartopy: v0.20.2. Zenodo https://doi.org/10.5281/ZENODO.5842769 (2022).
Eberhard, L. Serpentinite Phase Relations—An Experimental Study on Redox Conditions and Fluid Migration in Subduction Zones. PhD thesis, Univ. Bayreuth (2020).
Cannaò, E. & Malaspina, N. From oceanic to continental subduction: implications for the geochemical and redox evolution of the supra-subduction mantle. Geosphere 14, 2311–2336 (2018).
Acknowledgements
This work is part of the DESTINE grant PID2019-105192GB-I00, funded by MICIN/AEI/10.13039/501100011033, and ‘EFRD a way making Europe’. J.A.P.-N. acknowledges Ramón y Cajal fellowship RYC2018-024363-I, funded by MICIN/AEI/10.13039/501100011033 and the ESF program ‘FSE invierte en tu futuro’. M.D.M. acknowledges a postdoctoral fellowship (Postdoc_21_00791) funded by the Junta de Andalucía (Consejeria de Conocimiento y Universidades), and co-funded by EFRD and ESF. This research is part of the Junta de Andalucía research groups RNM-131 and RNM-374.
Author information
Authors and Affiliations
Contributions
J.A.P.-N. conceived the project, processed the data, acquired funding and wrote the original manuscript. V.L.S.-V. contributed to the conceptualization, performed the computations, organized the raw data and contributed to the writing of the manuscript. M.D.M. computed the global deserpentinization conditions and assisted with computations. M.T.G.-P. contributed to the writing of the manuscript. C.J.G. contributed to the conceptualization, funding and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Antoine Benard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rebecca Neely, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The stable mineral assemblage for a high-pressure serpentinite.
X(O2)-P/T (along a thermal gradient, see Methods) pseudosection7 for a representative Ca-poor high-pressure serpentinite from CdA (sample Al98-05a35) with sulphur and carbon content from ref. 27 (these values were confirmed by new, duplicate analyses) and ferric iron from this work (see Supplementary Table 1). The vertical line represents the intrinsic deserpentinization for a fixed O2 content of the system (Path I), corresponding to the bulk O2 for sample Al98-05a (15.602 mol/kg is used instead of the measured 15.672 mol/kg for better agreement with the observed sequence of mineral assemblages at CdA; it likely reflects the amount of ferric iron in antigorite, not accounted for in the available solid solution models89). The horizontal path (IIa) shows schematically the evolution if the system is externally infiltrated by fluids equilibrated with metasedimentary rocks with a high reducing capacity (graphite-bearing metapelite). The quantitative evolution along path IIa is shown in Fig. 3b in the main text (see also Supplementary Figure 6 for the evolution of the speciation in the fluid). Path IIb corresponds to the prograde evolution after the graphite metapelite-infiltrated deserpentinization potentially followed by CdG.
Extended Data Fig. 2 Redox-sensitive elements in the solid phases for a high-pressure serpentinite.
The absolute concentration of redox-sensitive components in the solids (expressed as mol/kg of rock Al98-05a) for the pseudosection shown in Supplementary Figure 1 (see also Fig. 3a in the main text for the contouring of oxygen fugacity relative to the buffer FMQ). All panels were computed from the absolute amounts of mineral phases containing oxygen-sensitive components and their concentration in pure and solid solution endmembers from WERAMI outputs. Computations used the back-calculated method for fluid speciation in PerpleX, except for panel S6+ which was computed using the lagged speciation method that allows mass balance constraints in the region below the complete serpentinite dehydration. The last panel shows the redox budget of the rock referred to the whole mantle reference redox state (Methods).
Extended Data Fig. 3 Prograde evolution for the intrinsic deserpentinization model.
Evolution of key parameters along intrinsic deserpentinization (intrinsic path I in Fig. 3, red vertical line). (a) XMg in antigorite and their dehydrated product olivine and orthopyroxene; (b) H2O content hosted in the solid phases; (c) and (d) bulk sulphur and carbon contents retained in the solid phases; (e) evolution of the oxygen fugacity relative to the FMQ buffer. The blue region corresponds to the temperature conditions of dehydration in Cerro del Almirez (CdA). Note that none of the observables (XMg, S and C content, see Fig. 3b-e in the main text) agrees with the model predictions along with the intrinsic deserpentinization model.
Extended Data Fig. 4 Magnetite content as a function of the state of hydration.
Global compilation of magnetite content in serpentinite and metaperidotite (Chl-harzburgite) against the water content (measured for CdA, this work and ref. 27) or loss of ignition (L.O.I.) as a proxy for water content for samples from the literature (see Methods). The observed decrease in magnetite content relative to common magnetite-bearing serpentinite is reproduced by deserpentinization infiltrated with highly reducing fluids equilibrated with graphite-bearing metapelite. The decrease in magnetite for the intrinsic deserpentinization model is coeval with the precipitation of haematite (dashed red line) which is not observed in natural samples.
Extended Data Fig. 5 Variation in oxygen fugacity for the intrinsic model.
Intrinsic deserpentinization oxygen fugacity conditions (a) relative to the FMQ buffer, Δlog10fO2[FMQ] and in absolute values (b) for a representative metaserpentinite (sample Al98-05a, see Supplementary Table 1) in a pressure-temperature space. Yellow dots are pressure-temperature deserpentinization conditions at the slab surface for a worldwide compilation of subduction zones83,84, geographically located in (c). (d) Difference between the slab surface intrinsic deserpentinization and the mantle wedge oxygen fugacity (expressed as Δlog10fO2[FMQ]) along the 1000 °C isotherm for the DMM (solid line) and ultradepleted mantle (red dashed line), see also Extended Data Fig. 7, right panels.
Extended Data Fig. 6 Changes in the fluid composition during the infiltration of an external fluid with high reducing potential into a reacting serpentinite.
a. Fluid speciation evolution during the infiltration of a partially dehydrated serpentinite with a fluid equilibrated with a graphite-bearing metapelite at 650 °C and 1.7 GPa. The solvent species H2S and CO2 are expressed as mole fraction, whereas the solute species are expressed as molality (mol/kg). The main oxidizing species (HSO4−) is represented on a linear scale whereas other less abundant species are on a logarithmic scale. b. Modal (vol.%) pyrite abundance in the metaperidotite induced by graphite metapelite fluid infiltration.
Extended Data Fig. 7 Oxygen fugacity conditions of the mantle wedge prior to its interaction with deserpentinization fluids.
Effect of peridotite mantle wedge depletion on the Δlog10fO2[FMQ] evolution during fluxing by different types of deserpentinization slab fluids. The left panel shows the results for (a) a depleted MORB mantle wedge source85 (DMM) and (b) an ultradepleted mantle wedge source86, both for a hot (Central Cascadia) and a cold (Tonga) subduction zone. The mantle wedge Δlog10fO2[FMQ] evolution of the mantle wedge fluxed by fluids sourced from intrinsic deserpentinization and sediment-infiltrated deserpentinization fluids produced by the infiltration of 12 mol/kg of fluids equilibrated with GLOSS and graphite-bearing metapelite. Right panels are the contours of the initial Δlog10fO2[FMQ] of the mantle wedge for a DMM (upper panel) and ultradepleted source (lower panel) before fluxing with slab fluids; shown as yellow dots are the initial Δlog10fO2[FMQ] conditions at the 1000 °C of the mantle wedge for a worldwide compilation of dehydration conditions in hot to cold subduction zones. Central Cascadia (hot subduction) and Tonga (cold subduction) correspond, respectively, to a minimum (2.4 GPa) and maximum pressure (3.3 GPa) for the dehydration of serpentinization at the slab surface. Note that the initial Δlog10fO2[FMQ] conditions depend on the thermal regime of the subduction zone and the depletion of the mantle wedge source, but have a subsidiary effect on the Δlog10fO2[FMQ] evolution of the mantle wedge during fluxing of different types of deserpentinization fluids.
Extended Data Fig. 8 Mantle wedge oxidation capacity of deserpentinization fluids modulated by graphite-bearing metasediments derived fluids.
(a) Modification at the slab surface of the Δlog10fO2[FMQ] and the concentration of HSO4− —relative to the intrinsic deserpentinization fluid (ID)— during infiltration of fluids equilibrated with metasedimentary rocks with a high reducing capacity (graphite-bearing metapelite) for a worldwide compilation of subduction zones83,84 (colour-coded for the pressure at which the serpentinite dehydrates at the slab surface, Source Data). (b) The capacity of these modified, serpentinite-derived fluids (empty dots in a) to oxidize the mantle wedge on top of the slab at near wet-solidus conditions is computed for the hottest (Central Cascadia) and coldest (Tonga) subduction zones. A minimum value range of Δlog10fO2[FMQ] inferred for oxidized IAB source and recorded by high-pressure metasomatized mantle atop of the slab47,90 is given as a horizontal blue-shaded range. Sediment (graphite-bearing)-serpentinite derived fluids have a variable capacity to oxidize the mantle wedge for hot and cold subduction zones, a variable potential that is directly related to the contrasting solubility of HSO4− for the two extreme thermal cases. The metasomatized mantle wedge has an initially depleted composition85. Squares and stars on the red and blue lines indicate the condition range limits at which pyrrhotite (Po), or anhydrite (anh) are the stable minerals hosting S in the rocks. For an ultradepleted MORB mantle, see Extended Data Fig. 7. For interaction with sediments with low reducing capacity (GLOSS), see Fig. 4 in the main text.
Supplementary information
Supplementary Information
Supplementary discussion including two figures.
Supplementary Tables
Bulk rock composition used for modelling and fluid composition and speciation and after interaction with a sediment with high reducing capacity.
Supplementary Data
Composition of deserpentinization fluids for all subduction segments for the intrinsic and modulated models.
Supplementary Code
Jupyter notebook and associated data.
Source data
Source Data Fig. 1
Data used to construct Fig. 1.
Source Data Extended Data Fig. 4
Data used to construct Extended Data Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Padrón-Navarta, J.A., López Sánchez-Vizcaíno, V., Menzel, M.D. et al. Mantle wedge oxidation from deserpentinization modulated by sediment-derived fluids. Nat. Geosci. 16, 268–275 (2023). https://doi.org/10.1038/s41561-023-01127-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-023-01127-0
This article is cited by
-
Sub-arc mantle fugacity shifted by sediment recycling across the Great Oxidation Event
Nature Geoscience (2023)
-
Compositional changes in garnet: trace element transfer during eclogite-facies metamorphism
Contributions to Mineralogy and Petrology (2023)