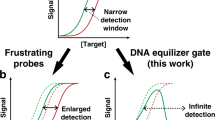
Abstract
Detecting genetic mutations such as single nucleotide polymorphisms (SNPs) is necessary to prescribe effective cancer therapies, perform genetic analyses and distinguish similar viral strains. Traditionally, SNP sensing uses short oligonucleotide probes that differentially bind the SNP and wild-type targets. However, DNA hybridization-based techniques require precise tuning of the probe’s binding affinity to manage the inherent trade-off between specificity and sensitivity. As conventional hybridization offers limited control over binding affinity, here we generate heteromultivalent DNA-functionalized particles and demonstrate optimized hybridization specificity for targets containing one or two mutations. By investigating the role of oligo lengths, spacer lengths and binding orientation, we reveal that heteromultivalent hybridization enables fine-tuned specificity for a single SNP and dramatic enhancements in specificity for two non-proximal SNPs empowered by highly cooperative binding. Capitalizing on these abilities, we demonstrate straightforward discrimination between heterozygous cis and trans mutations and between different strains of the SARS-CoV-2 virus. Our findings indicate that heteromultivalent hybridization offers substantial improvements over conventional monovalent hybridization-based methods.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data files containing all individual replicate data from main text figures and all individual replicate data from Extended Data figures are provided with this manuscript. All P values from the performed statistical analyses are provided in the corresponding figure captions. Source data are provided with this paper.
References
Gunderson, K. L., Steemers, F. J., Lee, G., Mendoza, L. G. & Chee, M. S. A genome-wide scalable SNP genotyping assay using microarray technology. Nat. Genet. 37, 549–554 (2005).
Koltai, H. & Weingarten-Baror, C. Specificity of DNA microarray hybridization: characterization, effectors and approaches for data correction. Nucleic Acids Res. 36, 2395–2405 (2008).
Tyagi, S., Bratu, D. P. & Kramer, F. R. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 16, 49–53 (1998).
Tulpan, D. et al. Thermodynamically based DNA strand design. Nucleic Acids Res. 33, 4951–4964 (2005).
Chen, X. et al. Thermodynamics and kinetics guided probe design for uniformly sensitive and specific DNA hybridization without optimization. Nat. Commun. 10, 4675 (2019).
Zhang, D. Y., Chen, S. X. & Yin, P. Optimizing the specificity of nucleic acid hybridization. Nat. Chem. 4, 208–214 (2012).
Wang, J. S. & Zhang, D. Y. Simulation-guided DNA probe design for consistently ultraspecific hybridization. Nat. Chem. 7, 545–553 (2015).
Tyagi, S. & Kramer, F. R. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14, 303–308 (1996).
Suzuki, S., Ono, N., Furusawa, C., Kashiwagi, A. & Yomo, T. Experimental optimization of probe length to increase the sequence specificity of high-density oligonucleotide microarrays. BMC Genomics 8, 373 (2007).
Kolpashchikov, D. M. Binary probes for nucleic acid analysis. Chem. Rev. 110, 4709–4723 (2010).
Taton, T. A., Mirkin, C. A. & Letsinger, R. L. Scanometric DNA array detection with nanoparticle probes. Science 289, 1757–1760 (2000).
Alhasan, A. H. et al. Scanometric microRNA array profiling of prostate cancer markers using spherical nucleic acid-gold nanoparticle conjugates. Anal. Chem. 84, 4153–4160 (2012).
Diehl, F. et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl Acad. Sci. USA 102, 16368–16373 (2005).
Schena, M., Shalon, D., Davis, R. W. & Brown, P. O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270, 467–470 (1995).
Curk, T. et al. Computational design of probes to detect bacterial genomes by multivalent binding. Proc. Natl Acad. Sci. USA 117, 8719–8726 (2020).
Estirado, E. M., Aleman Garcia, M. A., Schill, J. & Brunsveld, L. Multivalent ultrasensitive interfacing of supramolecular 1D nanoplatforms. J. Am. Chem. Soc. 141, 18030–18037 (2019).
Deal, B. R. et al. Engineering DNA-functionalized nanostructures to bind nucleic acid targets heteromultivalently with enhanced avidity. J. Am. Chem. Soc. 142, 9653–9660 (2020).
Regan, J. F. et al. A rapid molecular approach for chromosomal phasing. PLoS ONE 10, e0118270 (2015).
Zheng, G. X. et al. Haplotyping germline and cancer genomes with high-throughput linked-read sequencing. Nat. Biotechnol. 34, 303–311 (2016).
Song, T. et al. Fast and compact DNA logic circuits based on single-stranded gates using strand-displacing polymerase. Nat. Nanotechnol. 14, 1075–1081 (2019).
Schueder, F. et al. Super-resolution spatial proximity detection with proximity-PAINT. Angew. Chem. Int. Ed. 60, 716–720 (2021).
Chandrasekaran, A. R. et al. Cellular microRNA detection with miRacles: microRNA-activated conditional looping of engineered switches. Sci. Adv. 5, eaau9443 (2019).
Krishnamurthy, V. M., Semetey, V., Bracher, P. J., Shen, N. & Whitesides, G. M. Dependence of effective molarity on linker length for an intramolecular protein-ligand system. J. Am. Chem. Soc. 129, 1312–1320 (2007).
Huskens, J. et al. A model for describing the thermodynamics of multivalent host-guest interactions at interfaces. J. Am. Chem. Soc. 126, 6784–6797 (2004).
Sorensen, C. S. & Kjaergaard, M. Effective concentrations enforced by intrinsically disordered linkers are governed by polymer physics. Proc. Natl Acad. Sci. USA 116, 23124–23131 (2019).
Kane, R. S. Thermodynamics of multivalent interactions: influence of the linker. Langmuir 26, 8636–8640 (2010).
Wu, T., Xiao, X., Zhang, Z. & Zhao, M. Enzyme-mediated single-nucleotide variation detection at room temperature with high discrimination factor. Chem. Sci. 6, 1206–1211 (2015).
Chen, N. & Schrijver, I. Allelic discrimination of cis-trans relationships by digital polymerase chain reaction: GJB2 (p.V27I/p.E114G) and CFTR (p.R117H/5T). Genet. Med. 13, 1025–1031 (2011).
Fan, T. W., Yu, H. L. L. & Hsing, I. M. Conditional displacement hybridization assay for multiple SNP phasing. Anal. Chem. 89, 9961–9966 (2017).
Yu, H. A. et al. Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J. Thorac. Oncol. 10, 431–437 (2015).
Mammen, M., Choi, S. K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37, 2754–2794 (1998).
Yakovchuk, P., Protozanova, E. & Frank-Kamenetskii, M. D. Base-stacking and base-pairing contributions into thermal stability of the DNA double helix. Nucleic Acids Res. 34, 564–574 (2006).
Lane, M. J. et al. The thermodynamic advantage of DNA oligonucleotide ‘stacking hybridization’ reactions: energetics of a DNA nick. Nucleic Acids Res. 25, 611–617 (1997).
Maldonado-Rodriguez, R., Espinosa-Lara, M., Loyola-Abitia, P., Beattie, W. G. & Beattie, K. L. Mutation detection by stacking hybridization on genosensor arrays. Mol. Biotechnol. 11, 13–25 (1999).
Walter, A. E. et al. Coaxial stacking of helixes enhances binding of oligoribonucleotides and improves predictions of RNA folding. Proc. Natl Acad. Sci. USA 91, 9218–9222 (1994).
Munoz-Maldonado, C., Zimmer, Y. & Medova, M. A comparative analysis of individual RAS mutations in cancer biology. Front. Oncol. 9, 1088 (2019).
Lee Yu, H. L., Fan, T. W. & Hsing, I. M. Oligonucleotide hybridization with magnetic separation assay for multiple SNP phasing. Anal. Chim. Acta X 5, 100050 (2020).
Zhuang, X., Yu, H. L. L. & Hsing, I. M. Toehold probe-based interrogation for haplotype phasing of long nucleic acid strands. Anal. Methods 12, 4185–4190 (2020).
Chang, W. et al. Molecular AND logic gate for multiple single-nucleotide mutations detection based on CRISPR/Cas9n system-trigged signal amplification. Anal. Chim. Acta 1112, 46–53 (2020).
Gao, Y., Wolf, L. K. & Georgiadis, R. M. Secondary structure effects on DNA hybridization kinetics: a solution versus surface comparison. Nucleic Acids Res. 34, 3370–3377 (2006).
Bazrafshan, A. et al. DNA gold nanoparticle motors demonstrate processive motion with bursts of speed up to 50 nm per second. ACS Nano 15, 8427–8438 (2021).
Yehl, K. et al. Catalytic deoxyribozyme-modified nanoparticles for RNAi-independent gene regulation. ACS Nano 6, 9150–9157 (2012).
Karadeema, R. J., Stancescu, M., Steidl, T. P., Bertot, S. C. & Kolpashchikov, D. M. The owl sensor: a ‘fragile’ DNA nanostructure for the analysis of single nucleotide variations. Nanoscale 10, 10116–10122 (2018).
Zhou, L. et al. Sequence-selective purification of biological RNAs using DNA nanoswitches. Cell. Rep. Methods 1, 100126 (2021).
Bossert, D. et al. A hydrofluoric acid-free method to dissolve and quantify silica nanoparticles in aqueous and solid matrices. Sci. Rep. 9, 7938 (2019).
Hail, M., Elliott, B. & Anderson, K. High throughput analysis of oligonucleotides using automated electrospray ionization mass spectrometry. Am. Biotechnol. Lab. 12, 12–14 (2004).
Acknowledgements
Heat-inactivated SARS-CoV-2 and human corona (229E, OC43) virus samples (original/Washington strain) at known concentrations were provided by the NIH RADx-Radical Data Coordination Center (DCC) at the University of California San Diego and BEI Resources. The following reagent was obtained from UCSD: SARS-related coronavirus 2, isolate hCoV-19/USA/CA-SEARCH-59467/2021 (lineage BA.1; omicron variant), contributed by A. Carlin and the UCSC CALM and EXCITE laboratories. We acknowledge support from grant no. NSF MSN 2004126. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
B.R.D., R.M., S.N., H.O. and Y.D. planned and carried out the experiments. J.T.K. designed and derived the mathematical model. B.R.D., R.M. and K.S. conceived the original idea. B.R.D. analysed the data and took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Tine Curk, Jessica Rouge and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Modelling the specificity of heteroMV particles for single mutant targets.
a, Scheme showing the two-step reversible binding pathway of an n = 2 heteroMV particle binding either a SNP-containing target or a WT target and corresponding equations used to model the binding affinity to each target. b, c, Heatmap showing the predicted arbitrary signals when binding the SNP target (b) or WT target (c) as the monovalent binding affinities of the S and the T oligo are varied. d, Predicted discrimination factors for an n = 2 heteroMV particle as the affinity of T (Keq,T only) is increased (different color dots) causing the total affinity for the SNP target (Keq,S+T,SNP) to increase (x-axis) for each discrete value of Keq,S only chosen (same color dots). The curves were generated by fitting the predicted values to a gaussian distribution in GraphPad. e, The maximum DF value predicted from the curve in (d) for each discrete value of Keq,T only. f, Heatmap showing the predicted discrimination factor when the monovalent binding affinities of the S and the T oligo are varied. Black boxes indicate the n = 1 and n = 2 combination with the highest discrimination factors. g, Heatmap showing the predicted cooperativity factor when the monovalent binding affinities of the S and the T oligo are varied. Black box indicates the n = 2 combination with the highest cooperativity factor.
Extended Data Fig. 2 Modelling the specificity of homoMV particles for single mutant targets.
a, Scheme showing binding pathway of a homoMV particle binding either a SNP-containing target or a WT target and modification of binding affinity equation with MM factor. b, Predicted arbitrary signals when a homoMV particle with different affinities binds the SNP target or the WT target. c, Predicted discrimination factors for a homoMV particle with different affinities. Red dots correspond to discrimination factors for six values of Keq chosen to mimic a series of oligos of length x, x + 1, …, x + 5 nt. The black dashed curve was generated by fitting the predicted values to a gaussian distribution in GraphPad.
Extended Data Fig. 3 Modelling the specificity of heteroMV particles for double mutant targets.
a, Scheme showing the two-step reversible binding pathway of an n = 2 heteroMV particle binding either a double mutant target or a double WT target and corresponding equations used to model the binding affinity to each target. b, Heatmap showing the predicted cis/trans discrimination factor when the monovalent binding affinities of the S1 and the S2 oligo are varied. Black box indicates the n = 2 combination with the highest predicted cis/trans discrimination factor. c, Heatmap showing the predicted double mutant discrimination factor when the monovalent binding affinities of the S1 and the S2 oligo are varied. Black box indicates the n = 2 combination with the highest predicted double mutant discrimination factor.
Extended Data Fig. 4 Characterization of DNA-functionalized silica particles.
a, Bright-field microscopy images of 5-µm silica beads incubated in 0 M or 0.1 M KOH overnight. Similar results were obtained from three independent experiments. b, Oligreen fluorescence intensity after incubation of 0 or 100 nM of a 20 nt oligo in 0 or 0.1 M KOH for ~6 hours. Following incubation, each sample was split into two tubes, and then Oligreen was added directly to the first tube and added following P2 gel filtration to the second tube. The plot shows that the presence of KOH in solution inhibits Oligreen fluorescence and that removing KOH using a P2 gel before adding Oligreen enables strong Oligreen fluorescence, though some DNA may be lost during filtration. c, Flow cytometry plot showing side scatter vs forward scatter area of DNA-coated 5-µm silica beads after incubation in 0.1 M KOH for 0, 1, 4, or 8 hours. The plot shows that over time, the bead size is reduced, and the bead structure is damaged following KOH incubation, suggesting that the DNA has been released from the bead surface. d, Oligreen fluorescence intensity following incubation of beads in 0.1 M KOH for 0, 1, 4, 8, or 24 hours, followed by P2 gel filtration. The plot indicates that all of the DNA has been released from the beads after ~8 hours. e, Scheme showing the finalized assay for quantifying the density of the DNA on the silica beads using the Oligreen reagent. f, Standard curves of Oligreen fluorescence intensity vs [DNA] from different concentrations of 4 different oligo mixtures. g, Table showing the # of beads, the measured [DNA], DNA/µm2 on the bead surface, and the calculated mean distance ± standard error of the mean between each oligo on the bead surface from the n = 8 distinct samples. h, Approximately-to-scale illustration of a 6R-10S bead binding the no spacer G12C target based on the DNA density measurements and literature values for single stranded and double stranded DNA lengths.
Extended Data Fig. 6 Impact of spacer length and type on binding of 8T, 8S, and 8T-8S beads.
a, Representative histograms for the 8T-8S beads binding the WT or G12C version of each of the different spacer length and spacer type targets. b, Representative histograms for the 8T, 8S, and 8T-8S beads binding the G12C version of each of the different spacer length and spacer type targets. Surprisingly, 8T and 8S only beads also showed increased binding to the internal spacer-containing targets, potentially due to weak binding between S’ and T as well as T’ and S. c,d, A simplified hypothetical illustration showing the 8T (c) and 8S (d) beads binding multivalently to an internal spacer-containing target. e,f,) Scheme showing the possible base pairs formed for the 8T (e) and 8S (f) beads binding multivalently to the target. g–j, Measured median fluorescence intensity values for the 8T (g,i) and 8S (h,j) beads binding the G12C (g,h) or WT (i,j) version of each of the different spacer length and spacer type targets. Error bars represent standard error of the mean from n = 3 distinct samples.
Extended Data Fig. 7 Impact of linker orientation on n = 1 bead binding and representative histograms for n = 2 beads binding WT targets.
a, Scheme illustrating the possible binding interaction of the 5′ 8T, 3′ 8T, 5′ 8S, and 3′ 8S beads binding the no spacer G12C target monovalently. b, Measured median fluorescence intensity values for the 5′ 8T, 3′ 8T, 5′ 8S, and 3′ 8S beads binding the no spacer G12C target. As expected, the oligo’s anchoring terminus did not have a notable effect on n = 1 beads binding the G12C no spacer target. c,d, Representative histograms (c) and measured median fluorescence intensity values (d) for 8T-8S beads with head-to-tail, head-to-head, or tail-to-tail orientation binding the WT target with no spacer, short spacer, or long spacer (P values for no spacer: head-to-tail vs. head-to-head = 0.0126, head-to-tail vs. tail-to-tail = 0.2359, head-to-head vs. tail-to-tail = 0.0185; P values for short spacer: head-to-tail vs. head-to-head = 0.0131, head-to-tail vs. tail-to-tail = 0.2934, head-to-head vs. tail-to-tail = 0.0997; P values for long spacer: head-to-tail vs. head-to-head = 0.1217, head-to-tail vs. tail-to-tail = 0.1463, head-to-head vs. tail-to-tail = 0.0469). e, Representative histograms for 8T-8S beads with head-to-tail, head-to-head, or tail-to-tail orientation binding the G12C and WT no spacer targets. f, Measured discrimination factors for 8T-8S beads with head-to-tail, head-to-head, or tail-to-tail orientation binding the no spacer, short spacer, or long spacer targets (P values for no spacer: head-to-tail vs. head-to-head = 0.082, head-to-tail vs. tail-to-tail = 0.2045, head-to-head vs. tail-to-tail = 0.012; P values for short spacer: head-to-tail vs. head-to-head = 0.0715, head-to-tail vs. tail-to-tail = 0.4181, head-to-head vs. tail-to-tail = 0.3223; P values for long spacer: head-to-tail vs. head-to-head = 0.0441, head-to-tail vs. tail-to-tail = 0.236, head-to-head vs. tail-to-tail = 0.1265). Error bars represent standard error of the mean from n = 3 distinct samples. Values were compared using paired one-way ANOVA with multiple comparisons follow-up tests (nsP > 0.05, *P < 0.05).
Extended Data Fig. 8 Representative histograms and results for all bead combinations binding the SNP1/SNP2, WT1/SNP2, SNP1/WT2, and WT1/WT2 targets.
a,b, Scheme showing the sequences, anchor location, and spacer length for each bead combination with the head-to-tail orientation (a) or head-to-head orientation (b) and corresponding representative histograms for each bead combination binding the SNP1/SNP2, WT1/SNP2, SNP1/WT2, and WT1/WT2 targets. c,d, Measured discrimination factors for SNP1, SNP2, or SNP1 + SNP2 for each bead combination with the head-to-tail orientation (c) or head-to-head orientation (d). Error bars represent standard error of the mean from n = 3 distinct samples. Interestingly, both beads containing the 8S2 oligo had higher DFcis/trans values when binding in the head-to-head orientation (Fig. 5g,h). Alternatively, beads containing the 9S2 oligo bound the SNP1/SNP2 and WT1/SNP2 targets similarly, resulting in poor specificity for SNP1, and had similar DFcis/trans values in both orientations. This suggests that the 9S2 oligo’s affinity for the target is too high resulting in a total binding affinity that is too strong to be appreciably impacted by a mismatch in the S1′ region. These results offer further evidence that the head-to-head orientation can yield higher binding, particularly when the two immobilized oligos are binding cooperatively.
Extended Data Fig. 9 Representative histograms and results for all bead combinations binding the model SARS-CoV-2 targets.
a–d, Scheme showing the sequences, anchor location, and spacer length, as well as corresponding representative histograms for the 8S1-8S2 (a), 8S1-9S2 (b), 9S1-8S2 (c), and 9S1-9S2 (d) beads binding the original, alpha, and omicron strain targets. e, Measured median fluorescence intensity values for each bead combination binding the three targets. f, Measured discrimination factors for the omicron target versus the original or alpha targets for each bead combination. Error bars represent standard error of the mean from n = 3 distinct samples.
Extended Data Fig. 10 Design, characterization, and binding measurements for the 88 nt synthetic and SARS-CoV-2 virus extracted targets.
a, Scheme showing the 88 nt original and omicron target sequences, as well as the mutations unique to the omicron target and the primer locations. The 9S1-9S2 n = 2 bead is complementary to the Q498R and Y505H mutations (yellow) while the n = 1 bead is complementary to Q498R, N501Y, and Y505H. Therefore, the n = 2 bead binds the original target with two mismatches, whereas the n = 1 bead binds with three mutations. Primer sequences were designed to not overlap with any mutations, including the Q493R and G496S mutations. b, PAGE gel comparing synthetic 88 nt original and omicron targets to virus extracted original and omicron targets. The left image (DNA) shows ethidium bromide fluorescence, middle image (Atto647N) shows Atto647N fluorescence, and the right image (Merged) shows the two channels merged. c,d, Representative flow cytometry histograms for the n = 1 and 9S1-9S2 n = 2 beads binding 10 nM virus extracted original and omicron targets (c) and 10 nM synthetic 88 nt original and omicron targets (d). Moreover, to assess the impact of target concentration on heteroMV binding specificity, synthetic versions of the same targets were tested at concentrations ranging from 1 to 100 nM. e–i, Measured median fluorescence intensity values for the n = 1 and 9S1-9S2 n = 2 beads binding 1 nM (e), 5 nM (f), 10 nM (g), 25 nM (h), and 100 nM (i) of the synthetic 88 nt original and omicron targets. j, Measured discrimination factors corresponding to the data shown in e-i (P vales: 1 nM = 0.3583, 5 nM = 0.0907, 10 nM = 0.1717, 25 nM = 0.1071, 100 nM = 0.0224). n = 2 beads yielded increased average DF values at each concentration tested, showing that heteroMV beads can be used to improve specificity in a range of target concentrations. Error bars represent standard error of the mean from n = 3 distinct samples. Values were compared using two-sided paired student t tests (nsP > 0.05, *P < 0.05).
Supplementary information
Supplementary Information
Materials, Supplementary Figs. 1–6 and Tables 1–4.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig./Table 6
Statistical source data.
Source Data Extended Data Fig./Table 7
Statistical source data.
Source Data Extended Data Fig./Table 8
Statistical source data.
Source Data Extended Data Fig./Table 9
Statistical source data.
Source Data Extended Data Fig./Table 10
Statistical source data
Source Data Extended Data Fig./Table 10
Unmodified gel images.
Source Data Extended Data Fig./Table 10
Unmodified gel images.
Source Data Extended Data Fig./Table 10
Unmodified gel images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deal, B.R., Ma, R., Narum, S. et al. Heteromultivalency enables enhanced detection of nucleic acid mutations. Nat. Chem. 16, 229–238 (2024). https://doi.org/10.1038/s41557-023-01345-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01345-4