Abstract
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly emergent tick-borne bunyavirus first discovered in 2009 in China. SFTSV is a growing public health problem that may become more prominent owing to multiple competent tick-vectors and the expansion of human populations in areas where the vectors are found. Although tick-vectors of SFTSV are found in a wide geographic area, SFTS cases have only been reported from China, South Korea, Vietnam, and Japan. Patients with SFTS often present with high fever, leukopenia, and thrombocytopenia, and in some cases, symptoms can progress to severe outcomes, including hemorrhagic disease. Reported SFTSV case fatality rates range from ~5 to >30% depending on the region surveyed, with more severe disease reported in older individuals. Currently, treatment options for this viral infection remain mostly supportive as there are no licensed vaccines available and research is in the discovery stage. Animal models for SFTSV appear to recapitulate many facets of human disease, although none of the models mirror all clinical manifestations. There are insufficient data available on basic immunologic responses, the immune correlate(s) of protection, and the determinants of severe disease by SFTSV and related viruses. Many aspects of SFTSV virology and epidemiology are not fully understood, including a detailed understanding of the annual numbers of cases and the vertebrate host of the virus, so additional research on this disease is essential towards the development of vaccines and therapeutics.
Similar content being viewed by others
Introduction
Severe fever with thrombocytopenia syndrome virus (SFTSV) was first isolated from a patient in China during 20091. DH82 canine macrophage-like cells were infected with the virus and cytopathic effects (CPE), manifested as changes in cell morphology from round to elongated, were observed by light microscopy. After further characterization by electron microscopy and nucleotide sequencing, the virus was shown to be a member of the Bunyaviridae family and Phlebovirus genus1. It is currently classified as a member of the order Bunyavirales, family Phenuiviridae, genus Banyangvirus, and a member of the Bhanja virus serocomplex2. Early phylogenetic analysis showed that, although SFTSV had been shown to infect ticks, it was distantly related to viruses from both the Phlebotomus fever group as well as the Uukuneimi (UUKV) group, indicating that it was part of a new group of phleboviruses3,4. As more novel phleboviruses continued to emerge, it was determined that SFTSV is closely related to Malsoor (MV) and Heartland viruses (HRTV), isolated in India and the United States, respectively, and is more distantly related to Palma virus (PALV), originally isolated from ticks in Portugal, and Bhanja virus (BHAV), originally isolated from a goat in India5,6,7,8. Since its discovery, SFTSV has been isolated from several species of ticks, including Haemaphysalis longicornis, Rhipicephalus microplus, and Ixodes nipponensis, with one study confirming the ability of H. longicornis to act as a vector for SFTSV4,9,10,11. Based upon recent phylogenetic analyses, it has also been proposed that person-to-person transmission is likely12,13.
SFTS was first reported in the Hubei and Henan Provinces of central China in 20091,14. Between 2010 and 2011, it was confirmed that the new disease was caused by a previously unidentified Bunyavirus, now known as SFTSV3,14,15. SFTSV infection in humans has an incubation period of ~7–14 days followed by a clinical disease that typically presents as severe fever with thrombocytopenia and leukocytopenia with symptoms persisting ~7–13 days. Patients often also experience lymphadenopathy, gastrointestinal symptoms (nausea, vomiting, diarrhea), central nervous system symptoms (apathy, lethargy, tremors, convulsions, coma), and/or hemorrhagic symptoms (ecchymosis, disseminated intravascular coagulation, pulmonary, and/or gastrointestinal bleeding)14,15,16. In addition, patients often experience multiorgan dysfunction (MOD) in which two or more organ systems are compromised15,16,17. Although damage from MOD may be reversible, it can progress to multiorgan failure (MOF), which is typically associated with fatality in SFTS patients15,16,17. Although MOD is a relatively common outcome of SFTSV infection, there are reports of mild infections that do not require extensive intervention18. In addition, there are also reports of unusual disease outcomes, including one study of encephalopathy induced by SFTSV that did not result in a fatality, as well as a report of SFTSV-induced reversible myocardial dysfunction19,20. SFTSV infections are most often confirmed using RT-PCR, and although plasma exchange and ribavirin therapy have been used as treatments, their success as therapeutics for SFTS is controversial, as not all treated patients recovered and no results from clinical trials of candidate therapies have been reported to date14,21,22. In addition, candidate vaccines are still in the discovery phase.
Molecular virology and phylogenetics
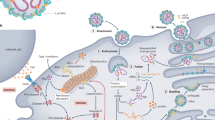
Like other members of the bunyaviruses, SFTSV has a spherical virion that is covered by an envelope, comprised of a lipid bilayer with glycoprotein spikes, that packages the three RNA segments; large (L) and medium (M) are negative-sense RNA and the small (S) RNA is ambisense1,23,24,25. The L segment, with 6368 nucleotides, encodes the RNA dependent RNA polymerase (RdRp) used by the virus in replication1,23. The M segment, 3378 nucleotides, has one open reading frame that encodes a precursor protein that is cleaved in the endoplasmic reticulum to yield two glycoproteins, Gn and Gc1, which are embedded in the envelope surface and are involved in virus entry26,27. The Gc protein is a class II fusion protein and has been shown to be involved in the fusion of SFTS viral and host membranes26,28,29. The Gn protein has been shown to be vital to the incorporation of Gc into the envelope surface and also encodes neutralizing epitopes27,29. The S segment is 1746 nucleotides30,31 and encodes the nucleocapsid protein (N) and the nonstructural protein (NSs)1. Although it has not been confirmed for SFTSV, the glycoproteins of bunyaviruses are suspected of interacting with the N as they have been co-immunoprecipitated with the N in studies using UUKV32. The NSs protein is involved in the formation of inclusion bodies that comprise an immune evasion strategy where intracellular vesicles are formed through the interaction of the NSs protein with perilipin A, adipose differentiation-related protein, and synaptogyrin-233. These vesicles sequester TBK1, IKKϵ, and IRF3, which in turn dampens the interferon (IFN) response to the virus34,35. These vesicles colocalize with multiple early endosomal markers and autophagy markers, suggesting a role of these pathways in SFTSV pathogenesis34. The NSs protein is also involved in inhibition of the IFN response by interfering with the JAK/STAT signaling pathway36,37. Deletion of the N-terminal 25 amino acids of NSs negated its ability to suppress the IFN response to the virus38.
Cell culture and virus entry
SFTSV is capable of infecting a variety of cell lines and the DC-specific intracellular molecule 3-grabbing receptor (DC-SIGN) has been shown to be a receptor for SFTSV through interaction with SFTSV glycoproteins, Gc and Gn39. The ability of SFTSV to infect cell lines from multiple species, such as hamster, monkey, mouse, and dog, suggests that receptors for the glycoproteins are conserved39. DC-SIGNR, liver and lymph node sinusoidal endothelial cell C-type lectin (LSECtin), and glucosylceramide synthase have been shown to also be important for viral entry into cells39,40,41. Gn was shown to bind non-muscle myosin heavy chain IIA (NMMHC-IIA) and overexpression of NMMHC-IIA was found to increase infection in HeLa cells, indicating that NMMHC-IIA is another potential component in SFTSV entry42. It was also found that SFTSV infectivity is irreversibly reduced when the pH is lower than 6.0, indicating that pH may influence the conformation of SFTSV glycoproteins41.
Phylogenetic studies
As stated above, SFTSV was found to be most closely related to MV and HRTV, and more distantly related to other phleboviruses, including Rift Valley fever virus (RVFV)4,5,6. The SFTSV RdRp was found to have 46.3% and 73.4% amino-acid similarity with that of RVFV and HRTV, respectively5,43. The N of SFTSV and RVFV are only partially homologous, with 41% similarity, whereas the N of HRTV is 62.8% homologous to SFTSV. SFTSV and HRTV glycoproteins share 62.6% sequence homology43,44,45. However, there are ten conserved cysteine residues in the Gn proteins of RVFV, SFTSV, and other related phleboviruses that are involved in the dimerization of Gn46. The NSs proteins of SFTSV and HRTV are 63.5% similar while the NSs of SFTSV and RVFV are only 11–16% similar43. Maximum likelihood phylogeny for each viral segment further revealed that SFTSV clusters into six genotypes: A, B, C, D, E, and F, whereas earlier analyses also using maximum likelihood divided the strains into five genotypes31,47,48,49. Strains in the F genotype are mostly from China but one analysis included a strain from South Korea31. It is also important to note that strains are reported in different genotypes between analyses4,31. All genotypes have been found in China, all genotypes but C have been isolated in South Korea, and genotypes B, C, and E have been isolated in Japan31,50. It has been shown that the L and M segments of the virus are capable of homologous recombination and/or reassortment in instances of coinfection, thereby causing antigenic shift48,49,51. The three segments of the virus have different rates of evolution with the S segment having the highest rate of evolution and the L segment having the lowest50.
Owing to the relatively recent discovery of SFTSV, the evolution of the virus has not been fully elucidated. Determination of the phylogenetic history and evolutionary trends of SFTSV would provide information to aid in the development of diagnostics to allow differentiation among related viruses and specific identification of SFTSV in clinical samples.
Epidemiology
Since its initial discovery, SFTSV has been reported in 23 provinces across China and confirmed human infections were recorded in 18 of these provinces14. Several Chinese provinces have had a higher disease burden than others, including Henan, Shandong, Anhui, and Hubei, and cases in these provinces frequently originate from rural, hilly regions14,52. Outside of China, SFTSV infections have also been confirmed in Japan, Vietnam, and South Korea, plus evidence in Taiwan and Pakistan. The first reported cases of SFTS in Japan and South Korea occurred in febrile patients hospitalized in 2012 who were confirmed as SFTSV-positive in 201310,14,53. Recently, confirmed cases of SFTSV were reported in Vietnam54. Serological surveys in Pakistan suggest that a low percentage of the population have neutralizing antibodies indicative of SFTSV infection and seroconversion55. Taiwan has also detected serological evidence of SFTSV as well as viral RNA in some of the surveyed ruminants56. As SFTSV is tick-borne, the typical season for SFTSV infection is from early spring through late autumn14,57,58 and there are a number of environmental predictors associated with ticks, including cattle density, forest coverage, rainfall, relative humidity, sunshine hours, and temperature elevation52. Cumulative data from 2010 to 2016 in China demonstrate that the highest reporting for SFTS cases was between May and July14. Reports in South Korea from 2013 to 2015 were highest between July–October, and reports in Japan from 2013 to 2014 were highest between April and August57,58. Serological studies throughout China demonstrate that infected individuals undergo seroconversion during the acute and convalescent course of the disease but reports of SFTSV seropositivity amongst healthy populations vary and overall seroprevalence data indicate that only a small portion of the general population has seroconverted (on average ~4.3%)14,15,59. Although the age of patients diagnosed has ranged widely, from children to the elderly, adults are more commonly infected and older age is associated with worsening disease outcome14,58,59. Specifically, most studies report that the majority of SFTS cases occur in patients older than 50 and case fatality rates (CFRs) are higher in the elderly14,52,60.
Reports of CFRs from SFTSV infection range between ~5 and >30% depending on the region surveyed and the amount of reporting in that area14,57,58. Owing to the variation in the dates and locations included in published reports of SFTSV incidence, it is unclear how many cases of SFTSV have been diagnosed since its discovery. Between 2011 and 2016, the Chinese Centers for Disease Control and Prevention reported between ~500 and 2500 SFTS cases annually resulting in ~50–100 deaths each year (cumulative CFR from 2010–2016 of 5.3%)14. South Korea and Japan tend to report fewer cases and a higher CFR than what is observed in China, however, this may be a result of differences in reporting and surveillance in different regions. South Korea typically reports fewer than 100 cases per year, however, the CFR in South Korea is high (32.6% CFR during 2013–2015)57. A retrospective analysis following the first case of SFTS in Japan identified 11 confirmed SFTS patients and six deaths in 2012, and through the end of 2015 a total of 161 cases of SFTS had been reported in Japan53,58. Several groups have noted the significant disparity in CFRs between China and other endemic countries. These differences are, in part, attributed to a lack of clinical reporting61. Several other factors may also play a role in these disparities.
There have been several isolated, putative infections in Greece, the United Arab Emirates, and the United States between 2009 and 2012, in which SFTSV was considered a possible etiological agent, however, laboratory tests have subsequently indicated that other closely related viruses likely caused these infections62,63,64. In particular, the tick-borne HRTV was identified in the United States in 201262.
A small study comparing fatal and non-fatal cases of SFTS in one region of China identified several differences between clinical symptoms and laboratory biomarkers that correlated with disease outcome15. During the first 1–7 days after disease onset, there was no significant difference in viral load, fever, or gastrointestinal symptoms between fatal and non-fatal cases15. During days 7–13 post onset, surviving patients had a decrease in viral load, whereas fatal cases continued to have sustained or increased viremia15. Both non-fatal and fatal cases were found to develop central nervous system symptoms and hemorrhagic symptoms, but the rate and severity of these manifestations were higher for fatal cases15. Both fatal and non-fatal cases experienced apathy, lethargy, and tremors, but convulsions and coma were more strongly correlated with a fatal disease outcome15. Similarly, although both fatal and non-fatal cases could manifest MOD, very few surviving patients progressed to the more severe MOF and all fatal cases were associated with MOF. Laboratory tests found that patients who developed fatal MOD/MOF maintained low platelet counts and high levels of the enzymes aspartate aminotransaminase (AST), creatine kinase (CK), creatine kinase-MB (CK-MB), and lactate dehydrogenase (LDH) beyond 13 days post onset, whereas recovering patients typically were experiencing a return to normal platelet and enzyme levels by day 1315.
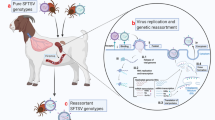
There is ongoing speculation about possible arthropod vectors and reservoir hosts, as studies continue to investigate the transmission cycle of SFTSV. Based on investigation of SFTSV RNA, multiple tick species are implicated as possible vectors while viral RNA has not been detected in mosquitoes, midges, or sandflies14,65. Haemophysalis longicornis ticks often have a high prevalence compared with other tick populations in China, South Korea, and Japan14. In China, in addition to H. longicornis, SFTSV RNA has also been detected in H. concinna and Rhipicephalus microplus ticks4,65. In South Korea, SFTSV RNA has been identified in H. longicornis, H. flava, Ixodes nipponensis, and Amblyomma testudinarium ticks10,66. In Japan, multiple tick species have been tested for SFTSV but none have yet been positive for infectious virus or viral RNA67. Prevalence rates of SFTSV in tick populations range from 0.6% to 23.5% based on tick species, geographic area, and sampling size4,10,65,66. In addition, the various tick species studied are not geographically restricted to the SFTSV endemic regions only. For example, H. longicornis is often implicated as a likely vector and can be found outside of China, South Korea, and Japan in countries including Australia, New Zealand, Fiji, New Caledonia, and Russia68. Recently, H. longicornis was introduced into the United States through agricultural trade and has been found in nine states69,70. Although some patients diagnosed with SFTSV report recent tick exposure, not every patient has a known tick-bite history as the apparent infection route3,14. Therefore, as regions that are endemic for SFTS do not necessarily have a high prevalence of SFTSV in tick populations, studies are in progress to investigate alternative routes of virus transmission.
As it is likely that ticks acquire SFTSV during feeding on an infected reservoir animal, studies across endemic regions have also tested various animal species for serological markers of infection. Multiple wild and domestic animals surveyed in SFTSV endemic regions of China have been found to carry viral RNA and/or SFTSV-specific antibodies, including sheep, cattle, dogs, pigs, chickens, goats, hedgehogs, geese, and rodents3,14,65,71,72,73. In South Korea, SFTSV RNA and/or antibodies have been detected in Korean water deer, wild boar, feral cats, lizards, and snakes, and in Japan virus-specific antibodies have been detected in cattle and wild boar66,74,75,76,77. Although SFTSV markers have been identified in several different animal species, there have been only a few reports of disease associated with SFTSV infection. In Japan, disease caused by SFTSV infection has been observed in domestic cats and captive cheetahs78. SFTSV-associated disease in a domestic canine has also been reported, with the companion animal suffering from fever, leukopenia, and thrombocytopenia prior to recovery79. One group proposed that ticks and migratory birds are responsible for the movement of SFTSV across geographic regions owing to the overlap of bird migratory patterns with the geographic distribution of H. longicornis ticks, but the potential role of birds has not been confirmed72. Although there is a notable degree of uncertainty about the natural transmission cycle of SFTSV, multiple reports have demonstrated that the virus can be transmitted person-to-person via contact with contaminated blood13,80. The majority of patients who are diagnosed with SFTS are farmers who work in fields and possibly have frequent tick exposure, indicating that agricultural work is a major risk factor for SFTS in endemic areas14,57,58,59.
Diagnostics
Case definition
Certain laboratory markers, such as elevated AST levels, coupled with relevant patient history, such as time of year, age, and location, can indicate if SFTSV infection is likely; however, the criteria for definitive SFTS diagnosis in China require that SFTSV or viral RNA be detected in patient serum, or patient be positive for anti-SFTSV IgM during active infection, or seroconversion indicated by a fourfold increase in virus-specific IgG in serum drawn during convalescence compared with IgG levels during active infection81,82,83. In Japan, the interim diagnostic criteria for SFTSV infection require a fever >38 °C, gastrointestinal symptoms, leukopenia with <4 × 109 leukocytes/L, thrombocytopenia with <100 × 109 platelets/L, increased lactate, alanine aminotransferase, and AST levels, as well as admission to a hospital owing to symptoms, or death84.
Viral RNA detection
Initially, viral RNA was detected in patient sera during active infection by RT-PCR. In 2012, a quantitative real-time RT-PCR assay was developed with primers targeting the L, M, and S segments that were 99% specific to SFTSV and was capable of diagnosing SFTSV in 98.6% of cases85. Another study developed an RT-PCR protocol that was capable of detecting SFTSV, dengue, Hantaan, and Seoul viruses with 99% specificity and 100% diagnosis rate86. This two-tube system reduced the cost of running four individual reactions by 40% and provided a quick diagnosis method for multiple viruses with overlapping geographic distributions and similar early clinical presentation86. Several other studies have also successfully developed RT-PCR assays to detect SFTSV RNA with all studies achieving a 10 viral RNA copies/μl lower limit of detection87,88,89. Reverse transcription loop-mediated isothermal amplification assays (RT-LAMP) and RT cross-priming amplification (RT-CPA) have also been used to successfully detect SFTSV RNA90,91,92,93. Multiple reports on the development of RT-LAMP assays to detect SFTSV have shown that those are effective tools for rapid diagnosis with high specificity and sensitivity for SFTSV and the ability to detect multiple strains90,94. The RT-CPA assay had a specificity of 94.1% and a sensitivity of 100% compared with real-time RT-PCR and culturing virus from viral samples isolated from 89 suspected SFTSV-infected donors92. Both RT-LAMP and RT-CPA assays require ~2 hours to obtain results90,91,92.
Serological assays
According to the SFTS diagnostic guidelines for China, the disease can be diagnosed by the presence of IgM antibodies in sera during acute illness or IgG antibodies in sera in recovering patients. One study developed an assay for the detection of total antibodies against SFTSV that had no cross-reactivity to hantavirus or dengue samples93. The study used a recombinant SFTSV N conjugated to horseradish peroxidase in a double-antigen sandwich enzyme-linked immunosorbent assay (ELISA) for detection of total serum antibodies against SFTSV and was also shown to be effective for testing sera from a variety of species including goats, hedgehogs, pigs, cattle, and chickens with a specificity of 100%95. Another study developed an immunochromatographic assay to detect IgG and IgM antibodies by conjugating recombinant SFTSV N (based on strain HB29) and streptavidin with colloidal gold93. The Colloidal Gold test was found to be SFTSV specific and as accurate for detecting anti-SFTSV antibodies as indirect immunofluorescence antibody tests previously used for laboratory confirmation of SFTSV infection, with sensitivity to IgG of 1:512 and IgM of 1:12893. An indirect ELISA has also been developed that has comparable performance for detection of anti-SFTSV IgG to that of the reference sandwich ELISA kit detailed above, with a specificity and sensitivity of 100%. The same assay can also detect serum IgM with 100% specificity but only 90.59% sensitivity compared with the sandwich ELISA method96. Neutralization assays are also a highly regarded method for detecting the presence of SFTSV neutralizing antibodies in serum.
Although great progress has been made with the development of serological and RT-PCR-based diagnostics, there is a lack of standardized reference reagents and a standardized diagnostic methodology that would improve consistency and comparability of surveillance methods and facilitate evaluation of candidate vaccines.
Immune response
Innate immune response
In vitro and in vivo analyses have begun to elucidate the innate immune responses resulting from SFTSV infection. Studies in the Golden Syrian hamster model support the importance of IFN signaling in disease prevention as STAT2 knockout hamsters are highly susceptible to SFTSV infection while wild-type hamsters are not97. Further highlighting the importance of IFN signaling in protection against SFTSV, in vitro studies have found that SFTSV can antagonize type-I IFN signaling by mechanisms involving virus sequestration of components of the IFN pathway including (but not exclusively) TBK1, IKKε, IRF3, STAT1, and STAT2 into inclusion bodies35,98.
Multiple studies have investigated the human immune response to SFTSV infection in an attempt to identify differential profiles of protective and non-protective immune signaling. Specifically, several cohort and case–control studies of SFTS patients in China compared serum levels of cytokines, chemokines, and immune cell subsets with those of healthy individuals, SFTS patients experiencing mild disease, and SFTS patients experiencing severe and/or fatal outcome. Generally, SFTSV infection of humans causes an increase in cytokine signaling that correlates with higher viral load and worsening disease outcome99,100,101,102. Although there was marked cytokine/chemokine upregulation in all SFTS patients, significant differences in immune signaling between SFTS patients experiencing mild versus severe disease have been identified99,100,101,102. Specifically, TNF-α, IFN-γ, IP-10, IL-10, IL-6, MIP-1α, IL-8, IL-15, granzyme B, HSP70, G-CSF, IL-1-RA, and MCP-199,100,101,102 were present at higher levels in patients with severe compared with mild disease. Not all studies identified statistically significant differences in each of the cytokines/chemokines listed above, however, there is agreement in the literature that severe disease is associated with a “cytokine storm” and increase in pro-inflammatory molecules such as TNF-α, IP-10, and IL-699,100,101,102. Several cytokines/chemokines are notably downregulated as a result of SFTSV infection. Specifically, tPAI-1, GRO, PDGF-BB, and RANTES have been detected at decreased expression in SFTS patients when compared with healthy individuals; however, some other studies have not observed any change in RANTES expression based on disease severity100,101,102. One study measured a significant decrease of IFN-β that negatively correlated with disease severity but did not identify similar correlations for IFN-α, IFN-γ, or IFN-λ99. The same study found that patients with worsening disease experienced a decrease in IL-1β over time99. In addition, this study measured host mRNAs in peripheral monocytes of patients with differing disease severity and found that expression of TLR3, IRF3, and IRF7 was each negatively correlated with disease severity, of which TLR3 exhibited the most downregulation in severe cases99.
The role of natural killer (NK) cells in disease outcome is still to be definitively established, as one study found that increased NK cell populations were associated with severe disease, whereas other studies found that NK cells were depleted throughout the first week of symptoms but then began to rise 2 weeks after disease onset103,104,105. One study has investigated the plasmacytoid DC (pDC) and myeloid DC (mDC) populations in patients and determined that levels of circulating pDCs were variable among SFTSV-infected patients but distinct patterns of mDCs could be observed that appeared to be associated with outcome99. Specifically, mDCs were significantly increased 1 week after disease onset but then declined in weeks 2 and 3, especially in patients with severe disease99. By 3 weeks post disease onset, the surviving patients had significantly more mDCs than deceased patients99.
A regulatory role of A20-binding inhibitor of NF-κB activation 2 (ABIN2), and tumor progression locus 2 (TPL2), have been identified in the inhibition of IFN signaling by SFTSV NSs106. Direct interactions between NSs and ABIN2 were shown to promote TPL2 complex formation, which subsequently resulted in increased IL-10 expression. Pharmacological inhibition of TPL2 signaling during SFTSV infection resulted in decreased IL-10 expression. Tpl2−/− mice, as well as IL-10−/− mice, survived challenge unlike their wild-type controls. These data show a key role of IL-10 signaling in SFTSV disease severity and outcome.
Adaptive immune response
Several studies have also measured differences in T- and B-cell subsets in SFTSV-infected humans. Generally, there is agreement in the literature that severe disease is associated with depleted T-cell populations103,104,105. Specifically, patients with severe disease have lower numbers of CD3+, CD4+, and CD8+ T cells throughout the acute phase of disease103,104,105. A study of Chinese SFTS patients reported that arginine deficiency might contribute to T-cell dysregulation during SFTSV infection107. In terms of B cells, there are reports that increased numbers of B lymphocytes relative to other types of lymphocytes were correlated with severe disease and fatality104,105.
Overall, adaptive immune mechanisms capable of protecting against SFTSV have not been fully identified. Studies with a related bunyavirus, RVFV, have suggested protective roles for neutralizing antibodies108,109. Both the nucleoprotein and the Gn/Gc glycoproteins of RVFV have been identified as CD8+ T-cell targets108,109 however, the contribution of T cells specific for these proteins to protection against RVFV is incompletely understood.
For SFTSV, there have been a number of studies investigating the induction of virus-specific antibodies, including IgM, IgG, and neutralizing antibodies, resulting from SFTSV infection of humans. A cohort study of 298 Chinese SFTS patients identified some specific patterns of antibody responses following infection that may have relevance to disease pathogenesis103. IgM seroconversion occurred between 4 and 21 days after disease onset, peaked after 4 weeks, and decreased significantly thereafter103. IgG seroconversion occurred between 2 and 9 weeks post disease onset, peaked at 6 months, and then waned with an estimated half-life of 11 months103. During the first 4 weeks of disease onset, low IgM levels were measured in patients who were older, had co-morbidities, had a higher initial viral load, and had severe disease. Moreover, another study of Chinese SFTS patients found that N-specific IgM antibodies were associated with lower viral load and less-severe disease, but N-specific IgG, Gn-specific IgM, and Gc-specific IgM did not have the same correlations103. The magnitude and durability of neutralizing antibody responses to SFTSV have not yet been studied in great detail, but a report from China indicates that SFTS patients develop low levels of neutralizing antibodies and although they wane over time, some patients retain neutralizing antibodies through at least 4 years post infection110. Of 25 patients tested, 50% plaque reduction neutralization test (PRNT50) titers varied between 1:5 and 1:640 during the first year of infection and by year 4 the titers generally decreased only slightly, to a range of 1:20 to 1:160110. Based on this 4-year follow-up data, it was predicted that SFTSV neutralizing antibodies could be protective for up to 9 years111.
A study in rhesus macaques found that SFTSV infection of non-human primates (NHPs) resulted in increased production of cytokines including IFN-γ and TNF-α similar to that observed in humans112. In addition, the study in NHPs found that SFTSV-specific IgM appeared ~5 days post infection (dpi) and declined after ~15 dpi, whereas SFTSV-specific IgG appeared ~7 dpi and plateaued ~15 dpi112. Neutralizing antibodies in NHPs were also present at low levels ranging from 1:32 to 1:128. Overall, the immune response in rhesus macaques appeared similar to that of humans but of lower magnitude consistent with the less-severe disease observed in NHPs.
Characterization of humoral responses against other bunyaviruses has indicated that neutralizing antibodies against the viruses typically recognize both Gn and Gc113. For SFTSV, neutralizing antibodies to the Gn protein have been identified while non-neutralizing antibodies recognize the N23,46,114,115. Using serum samples from four recovered patients as well as SFTSV antisera from mice and rabbits in hemagglutination inhibition (HI) assays, antibodies against SFTSV were found to be cross-reactive with HRTV, with an HI titer of 1:40 to 1:1280, and, to a lesser extent, BHAV, with an HI titer of 1:20 to 1:80116. Although the proteins that were bound by antibodies in this study were not specified, cross-reactivity of antibodies against SFTSV with HRTV was corroborated in another study where antibodies to the N from SFTSV bound to HRTV N117.
A neutralizing human monoclonal antibody (mAb) for SFTSV was engineered by generating an antibody library from lymphocytes isolated from five individuals infected with SFTSV and was found to bind to the α6 helix in domain III of the Gn protein46,114. The mAb was capable of neutralizing several strains of SFTSV, indicating that the epitope is conserved within SFTSV strains and provides a potential vaccine target against SFTSV46,114. This mAb was not found to be cross-reactive with RVFV, Forecariah virus (FORV), or PALV46,115. In addition, mAbs were derived from a phage display library of lymphocytes from two patients with SFTSV. These monoclonal antibodies bound to the N but had no neutralizing activity23. It was determined that the N-terminal region of the N is important for antibody binding as well as three epitopes within the N: Glu10 and Phe11; Lys40, Lys41, and Glu44; and Arg24323. Although antibodies that target the N have not been found to have neutralizing activity, they have been demonstrated to be an effective tool for diagnosing SFTSV infection through the recognition of virus in serum95,96,115.
Animal models of SFTSV
Since its discovery, various laboratory animals have been evaluated in an attempt to recapitulate the observed pathogenesis of human SFTSV infection. Table 1 provides an overview of these studies. Small animal models, including mice, rats, and hamsters, have been tested with various pathogenic outcomes. Newborn mice show high susceptibility to SFTSV infection through multiple routes, including intracranial (IC) and intraperitoneal inoculation (IP)118,119. Full characterization of newborn infections has yet to be performed though they may provide a lethal model for therapeutics testing. Immunocompetent adult mice generally show few clinical signs of infection83,97,118,119, though an in-depth characterization of SFTSV infection in C57BL/6 mice showed similar pathology to mild human infection120. Elevated liver enzymes, including AST and ALT, were observed in C57BL/6 mice that were inoculated with 105 TCID50 via the IP route and viral RNA was detected in their livers, spleens, and kidneys. To generate a lethal infection in C57BL/6 mice, a dose of the immunosuppressant Mitomycin C was administered120. In addition to immune suppression by drugs, immunocompromised knockout mice have also been used to study SFTSV infection. Both C57BL/6 mice deficient in the type-I IFNR (IFNα/βR−/−)83, and strain 129 mice deficient in type-I IFNR (A129)119 have been used for SFTSV model development owing to their immunocompromised status. In both models, lethality was observed with high levels of viral RNA detected in the brain, liver, kidneys and spleen83,119. However, these two models show different mean times to death of 3–4 dpi for A129 mice119 and 5–7 dpi for IFNα/βR−/− mice83. STAT1 and STAT2 knockout mice have been utilized with only STAT2 knockouts showing lethal infections121. More detail about these mouse models can be found in Table 1.
Rats have also been used to study SFTSV pathogenesis but appear to have limited value as a disease model. In the early characterization of the virus, newborn and adult Wistar rats were used for infections in an effort to recapitulate human disease, however, only newborn rats infected through the IC route showed universal mortality.
Hamsters have previously been employed in the study of other bunyaviruses, such as RVFV, suggesting that they may be a viable model for similar studies with SFTSV122. However, much like mouse models, immunocompetent hamsters do not succumb to lethal infections with SFTSV. STAT2 knockout hamsters were found to be susceptible to low doses of virus, succumbing after infection with 10 PFU introduced subcutaneously (SC)97.
The use of ferrets as a model has shown significant promise owing to their age-dependent disease manifestations123. Young ferrets (<2 years) show little to no clinical manifestations of SFTSV infection. Conversely, aged ferrets (>4 years) show high mortality and notable clinical signs that mirror human infection. Aged ferrets had a 94% mortality rate with severe thrombocytopenia and leukopenia. In addition, AST and ALT levels were increased in comparison with young ferrets123. Pathologically, viral antigen was observed in the liver, spleen, kidneys, brain, spinal cord, and intestine. This model shows promise as it is one of the few immunocompetent models to show lethality during infection. This lethality coincides with the observation that SFTSV has significantly more severe manifestations in older individuals suggesting that age is a direct correlate of disease severity123.
Infections of NHPs have been performed in an attempt to develop a more biologically relevant model. Two studies have reported the infection of NHPs with SFTSV, one using rhesus macaques112 and the other cynomolgus macaques83, though only the infection of rhesus macaques was reported in detail. In that study, elevated cytokines mirroring human levels, such as IFNγ100, were observed. Minor lesions in the liver and kidney were also observed in the later stages of the infection with viremia peaking at day 5 ~105 TCID50/mL and clearing by day 7. Although the rhesus macaques used in that study did become infected, their disease progression mirrored that of only acute SFTSV infection, including mild fever112.
To date, no animal model completely recapitulates all the observed clinical manifestations of SFTSV nor has a full characterization of any animal model in particular been performed to date. Consequently, further investigation into suitable animal models needs to be performed in order to best model disease progression and identify models for use in vaccine development.
Therapeutics and vaccines for SFTSV
Case management
At present, no approved vaccines or therapeutics are available to prevent or treat infections by SFTSV. Care varies from case to case depending on the patient’s manifestations. Treatment with antivirals and antisera22 have shown mixed results. The addition of pulsed steroidal therapy has been shown to have positive effects124. To date no standard of care has been established.
Therapeutics
Early in vitro studies suggested that the common antivirals, ribavirin, and favipiravir, show efficacy in inhibiting viral multiplication. Initially, ribavirin was tested in Vero cell cultures against various strains of SFTSV to determine the 99% effective concentration. Cells were pre-treated with Ribavirin before infection with SFTSV (100 TCID50) and cultured for 3 days before viral titers were determined125. Effective concentrations were found to range from 19 to 64 mg/ml depending on the strain125. However, treatment of cells with similar concentrations of Ribavirin after infection showed drastically diminished effects. From a few reported case studies, the employment of Ribavirin following the diagnosis of SFTSV infection has shown inconclusive effects on disease outcome21,126. CFRs and biomarkers of disease, such as viral load and platelet counts, did not show improvement between patients who were given ribavirin and those who were not21. These data may suggest that Ribavirin is not a suitable post-exposure treatment, although more studies are required to address its suitability as a prophylactic. However, other case reports in which Ribavirin was administered, indicate positive therapeutic effects and increased patient recovery21,126. These contradictory reports show the need for continued investigation into the therapeutic effects of Ribavirin in the treatment of SFTSV infection. Currently, a clinical trial to determine the efficacy of Ribavirin is taking place in China. In addition, two clinical trials are listed in the Chinese Clinical Trial Registry. The first is investigating Ribavirin only (http://www.chictr.org.cn/showprojen.aspx?proj=10477) and the other is to determine the efficacy of Ribavirin in combination with IFN alpha 2a (http://www.chictr.org.cn/showprojen.aspx?proj=11210).
Favipiravir, a purine analog, has also shown effective inhibition of SFTSV replication in vitro as well as in a susceptible animal model127. Using IFNα/βR−/− mice, it was shown that Favipiravir provided 100% protection to animals pre-treated with the drug. Animals infected SC with 1.0 × 106 TCID50 were dosed via either IP or oral routes with Favipiravir for 5 days. Surviving animals showed milder clinical signs with increased platelet counts and lower viremia, compared with the untreated groups127. A clinical trial was performed with Favipiravir to determine its ability to inhibit disease progression in infected patients. Patients were to given a dose of 1800 mg twice on the first day and 800 mg twice a day for the subsequent 9–13 days of treatment128. Data from this study are yet to be published but a smaller case study showed promise in the use of Favipiravir as a treatment. Two patients, both male, were given a 5-day course of Favipiravir at different stages of disease129. A notable decline in viral load was observed after administration, suggesting a potential effect of Favipiravir on disease course. Further data are required to show statistical significance of these findings.
In addition to these two common antivirals, several other treatments have been proposed for SFTSV infection. The use of recombinant IFN (α, β, and γ) has shown effects in a Vero cell model in a dose-dependent manner125. However, IFN administration has yet to be well-characterized in the context of patient infections. A clinical trial has been performed using recombinant human IFN α2, however, those results are not published130. Case studies employing l-arginine supplementation have also been performed and suggest that arginine supplementation may be beneficial107. In a retrospective clinical investigation, patients treated with calcium channel blockers (CCBs) showed significantly improved clinical outcomes in juxtaposition to patients not receiving CCBs131. Animal model testing confirmed that CCBs exhibit anti-SFTSV properties, suggesting that this class of compounds may be a viable option for treatment of patients. Several other molecules have been shown to inhibit SFTSV infection and are shown in Table 2.
Passive transfer of antisera from human survivors has been investigated in small animal models as well as in patients. Sera from survivors were transferred to SFTSV-infected IFNα/βR−/− mice and shown to increase survival and decrease viremia when given 1 hour after infection132. Specifically, sera containing neutralizing antibodies at a titer of 1:2000 were given once every 24 hours to mice infected with 106 pfu of the virus.
Several case reports have described the use of serum transfer as a treatment for SFTSV infection. One study found that the administration of serum from survivors to patients with rapidly progressing SFTSV infection had a positive effect on disease outcome. All patients treated with serum transfusion showed lower viremia levels as detected by RT-PCR and 13 of the 14 patients treated with serum transfusion survived132. These data suggest that serum transfer may be a viable treatment for patients with rapidly progressing SFTSV infection, but the mechanism of protection requires further study, although it is assumed to be neutralizing antibodies.
Overall, Favipiravir, IFN, and antiserum treatment have the potential to be possible therapies for SFTSV infection. Nonetheless, continued research into viable treatments is required and the development of new therapeutics is needed.
Vaccines
Much like therapeutics, SFTSV vaccine development has been limited and there are no licensed vaccines currently available. Multiple studies, utilizing various approaches, have shown varying levels of efficacy in preventing SFTSV-associated disease in animal models. Recombinant protein strategies133, DNA vaccine strategies134,135,136, and viral vector strategies137 have all been utilized and several of these studies are discussed below.
The first study evaluated recombinant SFTSV NSs (100ng) given in Freund’s adjuvant (which is not an acceptable adjuvant for humans) and was administered twice (14 days apart) to C57BL/6 mice, via the SC route. Mice were challenged with 3 × 107 pfu SFTSV via the IP route. Despite high titers of anti-NSs antibodies, no inhibition of viral replication nor accelerated viral clearance were observed in vaccinated mice133. The second study investigated a DNA vaccine candidate. Plasmids encoding the NSs and N genes were transfected into mice and immune responses were measured. Significant differences in the release of TNF-α from CD8+ and CD4+ cells were observed in mice transfected with NSs in comparison to N, and both groups of vaccinated animals had increased immunologic response compared with controls. Protective efficacy of this strategy cannot be assessed as a subsequent challenge with the virus was not reported134.
Two other DNA vaccine studies have shown the promise of this strategy. Kwak et al.135 developed multiple constructs that varied in their expression of each of the five SFTSV proteins. The glycoproteins of SFTSV were found to confer the highest protection when used in the lethal ferret model, with partial protection induced by the NSs, N, and RdRp vaccines135. Researchers found that doses as low as 40 µg of plasmid induced a strong SFTSV-specific response with robust protection against intramuscular challenges as high as 107.6 TCID50. All of the candidate vaccines induced robust T-cell responses and strong, antigen specific antibodies were developed.
Studies by Kang et al. showed that the inclusion of an IL-12 open reading frame in addition to SFTSV genes helped to induce strong cellular responses136. Owing to the stability and ease of production, as well as the efficacy observed in these studies, the use of a DNA-based vaccine may be a promising approach to address the need for a SFTSV vaccine.
Of the candidate vaccines reported to date, the use of the viral vector strategy appears to be the most promising. Two studies have demonstrated the effectiveness of viral vectors; one employing a recombinant vesicular stomatitis virus (rVSV) system and another utilizing a recombinant, rationally attenuated SFTSV. The rVSV system encodes the glycoproteins of SFTSV and was given as a single 1 × 104 pfu dose to IFNα/βR−/− mice137. Immunization with this recombinant VSV vaccine was performed via multiple routes including IP, IV, SC, and intranasal. All immunized animals were protected from an IP challenge of 2 × 104 pfu of the Wuhan strain of SFTSV137. In comparison with the unvaccinated control group that all succumbed to SFTSV infection, the immunized animals showed minimal clinical signs of infection.
The study of the rationally attenuated recombinant SFTSV demonstrated that two variants, a NSs knockout and a single amino-acid mutant can convey protection in a lethal ferret model138. Both variants inhibited viral replication after post vaccination challenge and decreased most clinical signs. High genetic stability of the NSs knockout virus was observed even after six passages, demonstrating a low likelihood of reversion138.
Work has been undertaken to investigate candidate strains of SFTSV on which to base future vaccines so as to maximize protective coverage. Strain HB29 was found to be cross neutralized by rabbit antibodies generated against eight other SFTSV strains, and with sera from 33 SFTS survivors, suggesting that it is broadly neutralized and may be an effective base for candidate vaccines139. Other groups have taken these studies further and begun to map the antigenic regions of SFTSV’s glycoproteins, including identifying subdomain III of Gn as a target for neutralizing antibodies46. Determining antigenic regions that also induce neutralizing antibodies may be key in the development of certain types of vaccines.
Overall, the fields of SFTSV therapeutics and vaccines are in early stages and a significant gap in knowledge remains. Determining the correlate(s) of protection, molecular mechanisms of immune subversion, and the development and characterization of effective therapeutics are crucial to combatting SFTSV infection.
Conclusions
At present, no licensed vaccines are available for SFTS, and research is in the discovery stage with only three published reports of vaccine candidates. Existing drugs are available that may be efficacious in the treatment of SFTSV infection, though more preclinical data and clinical studies are required. In addition, no specific vector control methodologies have been effective at curbing the expansion of SFTSV competent vectors. Animal models for SFTS appear to recapitulate many facets of mild and/or severe human disease, however, none of the models mirrors all clinical manifestations. There are major gaps in knowledge with insufficient data available on basic immunologic responses, the correlates of protection, and the determinants of severe disease by SFTSV and related viruses.
References
Yu, X.-J. et al. Fever with thrombocytopenia associated with a novel Bunyavirus in China. N. Engl. J. Med. 364, 1523–1532 (2011).
Abudurexiti, A. et al. Taxonomy of the order Bunyavirales: update 2019. Arch. Virol. 164, 1949–1965 (2019).
Liu, S. et al. Systematic review of severe fever with thrombocytopenia syndrome: virology, epidemiology, and clinical characteristics. Rev. Med. Virol. 24, 90–102 (2014).
Zhang, Y.-Z. et al. The ecology, genetic diversity, and phylogeny of Huaiyangshan virus in China. J. Virol. 86, 2864–8 (2012).
Mourya, D. T. et al. Malsoor virus, a novel bat phlebovirus, is closely related to severe fever with thrombocytopenia syndrome virus and heartland virus. J. Virol. 88, 3605–9 (2014).
Savage, H. M. et al. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am. J. Trop. Med. Hyg. 89, 445–452 (2013).
Filipe, A. R. et al. Palma virus, a new bunyaviridae isolated from ticks in Portugal. Intervirology 37, 348–51 (1994).
Shah, K. V. & Work, T. H. Bhanja virus: a new arbovirus from ticks Haemaphysalis intermedia Warburton and Nuttall, 1909, in Orissa, India. Indian J. Med. Res. 57, 793–8 (1969).
Jiang, X.-L. et al. [Isolation, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals]. Bing. Du Xue Bao 28, 252–7 (2012).
Yun, S.-M. et al. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 20, 1358–1361 (2014).
Luo, L.-M. et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg. Infect. Dis. 21, 1770–1776 (2015).
Yoo, J. R. et al. Family cluster analysis of severe fever with thrombocytopenia syndrome virus infection in Korea. Am. J. Trop. Med. Hyg. 95, 1351–1357 (2016).
Kim, W. Y. et al. Nosocomial transmission of severe fever with thrombocytopenia syndrome in Korea. Clin. Infect. Dis. 60, 1681–1683 (2015).
Zhan, J. et al. Current status of severe fever with thrombocytopenia syndrome in China. Virol. Sin. 32, 51–62 (2017).
Gai, Z.-T. et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J. Infect. Dis. 206, 1095–1102 (2012).
Weng, Y., Chen, N., Han, Y., Xing, Y. & Li, J. Clinical and laboratory characteristics of severe fever with thrombocytopenia syndrome in Chinese patients. Braz. J. Infect. Dis. 18, 88–91 (2014).
Ramirez, M. Multiple organ dysfunction syndrome. Curr. Probl. Pediatr. Adolesc. Health Care. https://doi.org/10.1016/j.cppeds.2013.10.003 (2013).
Ohagi, Y. et al. Mild clinical course of severe fever with thrombocytopenia syndrome virus infection in an elderly Japanese patient. Case Rep. Infect. Dis. 2014, 918135 (2014).
Kaneko, M. et al. Unusual presentation of a severely ill patient having severe fever with thrombocytopenia syndrome: a case report. J. Med. Case Rep. 11, 27 https://doi.org/10.1186/s13256-016-1192-0 (2017).
Kawaguchi, T. et al. Severe fever with thrombocytopenia syndrome with myocardial dysfunction and encephalopathy: a case report. J. Infect. Chemother. 22, 633–637 (2016).
Liu, W. et al. Case-fatality ratio and effectiveness of ribavirin therapy among hospitalized patients in china who had severe fever with thrombocytopenia syndrome. Clin. Infect. Dis. 57, 1292–1299 (2013).
Sup, W., Taek, S., Hyung, S. & Jun, W. Plasma exchange and ribavirin for rapidly progressive severe fever with thrombocytopenia syndrome. Int. J. Infect. Dis. 18, 84–86 (2014).
Yu, L. et al. Critical epitopes in the Nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS ONE 7, e38291 (2012).
Lu, J. et al. Expression of structural and non-structural proteins of severe fever with thrombocytopenia syndrome bunyavirus. Bing Du Xue Bao 27, 515–20 (2011).
Lu, Z. et al. Biological and phylogenetic analysis of first isolate of Tahyna virus in China. Bing Du Xue Bao 27, 97–102 (2011).
Yuan, F. & Zheng, A. Entry of severe fever with thrombocytopenia syndrome virus. Virol. Sin. 32, 44–50 (2017).
Plegge, T., Hofmann-Winkler, H., Spiegel, M. & Pöhlmann, S. Evidence that processing of the severe fever with thrombocytopenia syndrome virus Gn/Gc polyprotein is critical for viral infectivity and requires an internal Gc signal peptide. PLoS ONE 11, e0166013 (2016).
Garry, C. E. & Garry, R. F. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of Bunyaviruses are class II viral fusion protein (beta-penetrenes). Theor. Biol. Med. Model. 1, 10 (2004).
Halldorsson, S. et al. Structure of a phleboviral envelope glycoprotein reveals a consolidated model of membrane fusion. Proc. Natl Acad. Sci. USA 113, 7154–7159 (2016).
Brennan, B. et al. Reverse genetics system for severe fever with thrombocytopenia syndrome virus. J. Virol. 89, 3026–37 (2015).
Yun, S.-M. et al. Molecular genomic characterization of tick- and human-derived severe fever with thrombocytopenia syndrome virus isolates from South Korea. PLoS Negl. Trop. Dis. 11, e0005893 (2017).
Overby, A. K., Popov, V. L., Pettersson, R. F. & Neve, E. P. A. The cytoplasmic tails of Uukuniemi Virus (Bunyaviridae) G(N) and G(C) glycoproteins are important for intracellular targeting and the budding of virus-like particles. J. Virol. 81, 11381–11391 (2007).
Sun, Q. et al. Synaptogyrin-2 promotes replication of a novel tick-borne Bunyavirus through Interacting with viral nonstructural protein NSs. J. Biol. Chem. 291, 16138–16149 (2016).
Santiago, F. W. et al. Hijacking of RIG-I signaling proteins into virus-induced cytoplasmic structures correlates with the inhibition of type I interferon responses. J. Virol. 88, 4572–85 (2014).
Wu, X. et al. Evasion of antiviral immunity through sequestering of TBK1/IKK /IRF3 into viral inclusion bodies. J. Virol. 88, 3067–3076 (2014).
Chen, X. et al. Severe fever with thrombocytopenia syndrome virus inhibits exogenous Type I IFN signaling pathway through its NSs invitro. PLoS ONE 12, e0172744 (2017).
Zhang, S. et al. Suppression of type I and type III IFN signalling by NSs protein of severe fever with thrombocytopenia syndrome virus through inhibition of STAT1 phosphorylation and activation. J. Gen. Virol. 96, 3204–3211 (2015).
ZHANG, S. et al. NSs protein of severe fever with thrombocytopenia syndrome virus suppresses interferon production through different mechanism than Rift Valley fever virus. Acta Virol. 61, 289–298 (2017).
Hofmann, H. et al. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J. Virol. 87, 4384–4394 (2013).
Drake, M. J. et al. A role for glycolipid biosynthesis in severe fever with thrombocytopenia syndrome virus entry. PLOS Pathog. 13, e1006316 (2017).
Tani, H. et al. Characterization of severe fever with thrombocytopenia syndrome virus glycoprotein-mediated entry. J. Virol. 90, 5292–5301 (2016).
Sun, Y. et al. Nonmuscle myosin heavy chain IIA is a critical factor contributing to the efficiency of early infection of severe fever with thrombocytopenia syndrome virus. J. Virol. 88, 237–48 (2014).
Li, D. A highly pathogenic new bunyavirus emerged in China. Emerg. Microbes Infect. 2, e1 (2013).
Hornak, K., Lanchy, J.-M. & Lodmell, J. RNA encapsidation and packaging in the Phleboviruses. Viruses 8, 194 (2016).
Lam, T. T. Y. et al. Evolutionary and molecular analysis of the emergent severe fever with thrombocytopenia syndrome virus. Epidemics 5, 1–10 (2013).
Wu, Y. et al. Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope. Proc. Natl Acad. Sci. 114, E7564–E7573 (2017).
Fu, Y. et al. Phylogeographic analysis of severe fever with thrombocytopenia syndrome virus from Zhoushan Islands, China: implication for transmission across the ocean. Sci. Rep. 6, 19563 (2016).
Li, Z. et al. Increased prevalence of severe fever with thrombocytopenia syndrome in eastern china clustered with multiple genotypes and reasserted virus during 2010–2015. Sci. Rep. 7, 6503 (2017).
Shi, J. et al. Migration, recombination, and reassortment are involved in the evolution of severe fever with thrombocytopenia syndrome bunyavirus. Infect. Genet. Evol. 47, 109–117 (2017).
Liu, J.-W. et al. Molecular evolution and spatial transmission of severe fever with thrombocytopenia syndrome virus based on complete genome sequences. PLoS ONE 11, e0151677 (2016).
He, C.-Q. & Ding, N.-Z. Discovery of severe fever with thrombocytopenia syndrome bunyavirus strains originating from intragenic recombination. J. Virol. 86, 12426–30 (2012).
Liu, K. et al. A national assessment of the epidemiology of severe fever with thrombocytopenia syndrome, China. Sci. Rep. 5, 9679 (2015).
Takahashi, T. et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. 209, 816–27 (2013).
Tran, X. C. et al. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg. Infect. Dis. 25, 1029–1031 (2019).
Zohaib, A. et al. Serologic evidence of severe fever with thrombocytopenia syndrome virus and related viruses in Pakistan. Emerg. Infect. Dis. 26, 1513–1516 (2020).
TL, L. et al. The first discovery of severe fever With thrombocytopenia syndrome virus in Taiwan. Emerg. Microbes Infect. 9, 148–151 (2020).
Choi, S. J. et al. Severe fever with thrombocytopenia syndrome in South Korea, 2013–2015. 10, e0005264 (2016).
Kato, H., Yamagishi, T., Shimada, T. & Matsui, T. Epidemiological and clinical features of severe fever with thrombocytopenia syndrome in Japan, 2013 – 2014. 11, e0165207 (2016).
Li, P. et al. Seroprevalence of severe fever with thrombocytopenia syndrome virus in China: a systematic review and meta-analysis. 12, e0175592 (2017).
Liu, K. et al. Epidemiologic features and environmental risk factors of severe fever with thrombocytopenia syndrome. 8, 1–7 (2014).
Yu, X.-J. Risk factors for death in severe fever with thrombocytopenia syndrome. Lancet Infect. Dis. 18, 1056–1057 (2018).
McMullan, L., Folk, S., Kelley, A., MacNeil, A. & Goldsmith, C. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367, 834–841 (2012).
Denic, S. et al. Acute thrombocytopenia, leucopenia, and multiorgan dysfunction: the first case of SFTS bunyavirus outside China? Case Rep. Infect. Dis. 2011, 204056 (2011).
Anagnostou, V., Pardalos, G., Athanasiou-Metaxa, M. & Papa, A. Novel phlebovirus in febril child, Greece. Emerg. Infect. Dis. 17, 940–941 (2011).
Tian, H. et al. Severe fever with thrombocytopenia syndrome virus in humans, domesticated animals, ticks, and mosquitoes. Am. J. Trop. Med. Hyg. 96, 1346–1349 (2017).
Kang, J., Kim, H. & Chong, S. Detection of severe fever with thrombocytopenia syndrome virus from wild animals and ixodidae ticks. 16, 408–414 (2016).
Hayasaka, D. et al. Epidemiological survey of severe fever with thrombocytopenia syndrome virus in ticks in Nagasaki, Japan. 43, 159–164 (2015).
Hoogstraal, H., Roberts, F. H. S., Kohls, T. G. M. & Tipton, V. J. Review of Haemaphysalis (Kaiseriana) longicornis Neumann (Resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). Auth 54, 1197–1213 (2017).
Beard, C. B. et al. Multistate infestation with the exotic disease-vector tick haemaphysalis longicornis - United States, August 2017-September 2018. Morb. Mortal. Wkly. Rep. 67, 1310–1313 (2018).
Rainey, T., Occi, J. L., Robbins, R. G. & Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 55, 757–759 (2018).
Niu, G. et al. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. 19, 756–763 (2013).
Zhang, Y. & Xu, J. The emergence and cross species transmission of newly discovered tick-borne Bunyavirus in China. Curr. Opin. Virol. 16, 126–131 (2016).
Ni, H. et al. Apodemus agrarius is a potential natural host of severe fever with thrombocytopenia syndrome (SFTS)—causing novel bunyavirus. J. Clin. Virol. 71, 82–88 (2015).
Hwang, J., Kang, J., Oh, S., Chae, J. & Cho, Y. Molecular detection of severe fever with thrombocytopenia syndrome virus (SFTSV) in feral cats from Seoul, Korea. Ticks Tick. Borne. Dis. 8, 9–12 (2017).
Tabara, K., Fujita, H., Hirata, A. & Hayasaka, D. Investigation of severe fever with thrombocytopenia syndrome virus antibody among domestic bovines transported to slaughterhouse in Shimane Prefecture, 445–447 https://doi.org/10.7883/yoken.JJID.2015.624 (2016).
Hayasaka, D. et al. Seroepidemiological evidence of severe fever with thrombocytopenia syndrome virus infections in wild boars in Nagasaki, Japan. Trop. Med. Health 1–5 https://doi.org/10.1186/s41182-016-0009-6 (2016).
Suh, J.-H. et al. Detection of SFTS virus in ixodes nipponensis and amblyomma testudinarium (ixodida: ixodidae) collected from reptiles in the Republic of Korea. J. Med. Entomol. 53, 584–590 (2016).
Matsuno, K. et al. Fatal tickborne phlebovirus infection in captive cheetahs, Japan. Emerg. Infect. Dis. 24, 1726–1729 (2018).
Nam, S.-J. et al. Unusual case of severe fever with thrombocytopenia syndrome showing clinical manifestations in a companion dog. Vet. Med. Sci. https://doi.org/10.1002/vms3.261 (2020).
Tang, X., Wu, W., Wang, H., Du, Y. & Liu, L. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J. Infect. Dis. 207, 736–739 (2013).
Hu, J. et al. Preliminary fast diagnosis of severe fever with thrombocytopenia syndrome with clinical and epidemiological parameters. PLoS ONE 12, e0180256 (2017).
Zhang, Y.-Z. et al. Hemorrhagic fever caused by a novel bunyavirus in china: pathogenesis and correlates of fatal outcome. Clin. Infect. Dis. 54, 527–533 (2012).
Matsuno, K. et al. Animal models of emerging tick-borne phleboviruses: determining target cells in a lethal model of SFTSV infection. Front. Microbiol. 8, 104 (2017).
Saito, T., Fukushima, K., Umeki, K. & Nakajima, K. Severe fever with thrombocytopenia syndrome in japan and public health communication. Emerg. Infect. Dis. 21, 487–489 (2015).
Sun, Y. et al. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J. Clin. Virol. 53, 48–53 (2012).
Li, Z. et al. A two-tube multiplex real-time RT-PCR assay for the detection of four hemorrhagic fever viruses: severe fever with thrombocytopenia syndrome virus, Hantaan virus, Seoul virus, and dengue virus. Arch. Virol. 158, 1857–1863 (2013).
Li, Z. et al. Development and application of a one-step real-time RT-PCR using a minor-groove-binding probe for the detection of a novel bunyavirus in clinical specimens. J. Med. Virol. 85, 370–377 (2013).
Yoshikawa, T. et al. Sensitive and specific PCR systems for detection of both chinese and japanese severe fever with thrombocytopenia syndrome virus strains and prediction of patient survival based on viral load. J. Clin. Microbiol. 52, 3325–3333 (2014).
Chi, Y. et al. SFTSV RNA detection in sera of patients suffering from fever with thrombocytopenia syndrome. J. Shandong Univ. (Health Sciences). 50, 119–121 (2012).
Yang, G. et al. Development and evaluation of a reverse transcription loop-mediated isothermal amplification assay for rapid detection of a new SFTS bunyavirus. Arch. Virol. 157, 1779–1783 (2012).
Xu, H. et al. Establishment of a novel one-step reverse transcription loop-mediated isothermal amplification assay for rapid identification of RNA from the severe fever with thrombocytopenia syndrome virus. J. Virol. Methods 194, 21–25 (2013).
Cui, L. et al. Detection of severe fever with thrombocytopenia syndrome virus by reverse transcription-cross-priming amplification coupled with vertical flow visualization. J. Clin. Microbiol. 50, 3881–3885 (2012).
Wang, X. et al. Development of a colloidal gold kit for the diagnosis of severe fever with thrombocytopenia syndrome virus infection. Biomed. Res. Int. 2014, 1–6 (2014).
Lee, J. W. et al. Development of a real-time loop-mediated isothermal amplification method for the detection of severe fever with thrombocytopenia syndrome virus. J. Microbiol. https://doi.org/10.1007/s12275-020-0109-1 (2020).
Jiao, Y. et al. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J. Clin. Microbiol. 50, 372–377 (2012).
Yu, F. et al. Application of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for the detection of SFTSV-specific human IgG and IgM antibodies by indirect ELISA. Virol. J. 12, 117 (2015).
Gowen, B. B. et al. Modeling severe fever with thrombocytopenia syndrome virus infection in golden syrian hamsters: importance of STAT2 in preventing disease and effective treatment with favipiravir. J. Virol. 91, e01942–16 (2017).
Ning, Y. et al. Disruption of type I interferon signaling by the nonstructural protein of severe fever with thrombocytopenia syndrome virus via the hijacking of STAT2 and STAT1 into inclusion bodies. 89, 4227–4236 (2015).
Song, P. et al. Downregulation of interferon- β and inhibition of TLR3 expression are associated with fatal outcome of severe fever with thrombocytopenia syndrome. Sci. Rep. 7, 6532 (2017).
Deng, B. et al. Cytokine and chemokine levels in patients with severe ffever with thrombocytopenia syndrome virus. PLoS ONE 7, e41365 (2012).
Ding, Y.-P. et al. Prognostic value of clinical and immunological markers in acute phase of SFTS virus infection. Clin. Microbiol. Infect. 20, 870–878 (2014).
Sun, Y. et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J. Infect. Dis. 206, 1085–1094 (2012).
Lu, Q. et al. Characterization of immunological responses in patients with severe fever with thrombocytopenia syndrome: a cohort study in China. Vaccine 33, 1250–1255 (2015).
Liu, J. et al. Dynamic changes of laboratory parameters and peripheral blood lymphocyte subsets in severe fever with thrombocytopenia syndrome patients. Int. J. Infect. Dis. 58, 45–51 (2017).
Sun, L. et al. Detection and evaluation of immunofunction of patients with severe fever with thrombocytopenia syndrome. Clin. Exp. Med. 14, 389–395 (2014).
Choi, Y. et al. Severe fever with thrombocytopenia syndrome phlebovirus non-structural protein activates TPL2 signalling pathway for viral immunopathogenesis. Nat. Microbiol. 4, 429–437 (2019).
Li, X.-K. et al. Arginine deficiency is involved in thrombocytopenia and immunosuppression in severe fever with thrombocytopenia syndrome. Sci. Transl. Med. 10, eaat4162 (2018).
Lopez-Gil, E. et al. A single immunization with MVA expressing GnGc glycoproteins promotes epitope-specific CD8+-T cell activation and protects immune-competent mice against a lethal RVFV infection. PLoS Negl. Trop. Dis. 7, e2309 (2013).
Xu, W., et al. The nucleocapsid protein of Rift Valley fever virus is a potent human CD8+ T cell antigen and elicits memory responses. PLoS ONE 8, e59210 (2013).
Huang, Y. T. Y. et al. Neutralizing antibodies to severe fever with thrombocytopenia syndrome virus 4 years after hospitalization, China. Emerg. Infect. Dis. 22, 1985–1987 (2016).
Qi, R., Huang, Y. T. & Yu, X. J. Persistence and gender differences in protection against severe fever with thrombocytopaenia syndrome virus with natural infection: a 4-year follow-up and mathematical prediction study. Epidemiol. Infect. 147, e78 (2019).
Jin, C. et al. SFTS virus infection in nonhuman primates. J. Infect. Dis. 211, 915–925 (2015).
Arikawa, J., Schmaljohn, A. L., Dalrymple, J. M. & Schmaljohn, C. S. Characterization of Hantaan virus envelope glycoprotein antigenic determinants defined by monoclonal antibodies. J. Gen. Virol. 70, 615–24 (1989).
Guo, X. et al. Human antibody neutralizes severe Fever with thrombocytopenia syndrome virus, an emerging hemorrhagic Fever virus. Clin. Vaccine Immunol. 20, 1426–32 (2013).
Fukuma, A. et al. Severe fever with thrombocytopenia syndrome virus antigen detection using monoclonal antibodies to the nucleocapsid protein. PLoS Negl. Trop. Dis. 10, e0004595 (2016).
Matsuno, K. et al. Characterization of the Bhanja serogroup viruses (Bunyaviridae): a novel species of the genus Phlebovirus and its relationship with other emerging tick-borne phleboviruses. J. Virol. 87, 3719–3728 (2013).
Xing, Z. et al. Novel bunyavirus in domestic and captive farmed animals, Minnesota, USA. Emerg. Infect. Dis. 19, 1487–9 (2013).
Chen, X.-P. et al. Infection and pathogenesis of Huaiyangshan virus (a novel tick-borne bunyavirus) in laboratory rodents. J. Gen. Virol. 93, 1288–1293 (2012).
Liu, Y. et al. The pathogenesis of severe fever with thrombocytopenia syndrome virus infection in alpha/beta interferon knockout mice: insights into the pathologic mechanisms of a new viral hemorrhagic fever. J. Virol. 88, 1781–6 (2014).
Jin, C. et al. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc. Natl Acad. Sci. 109, 10053–10058 (2012).
Yoshikawa, R., Sakabe, S., Urata, S. & Yasuda, J. Species-specific pathogenicity of severe fever with thrombocytopenia syndrome virus is determined by anti-STAT2 activity of NSs. J. Virol. 93, e02226-18 (2019).
Scharton, D. et al. Rift valley fever virus infection in golden syrian hamsters. PLoS ONE 10, e0116722 (2015).
Park, S.-J. et al. Ferret animal model of severe fever with thrombocytopenia syndrome phlebovirus for human lethal infection and pathogenesis. Nat. Microbiol. 4, 438–446 (2019).
Nakamura, S. et al. Steroid pulse therapy in patients with encephalopathy associated with severe fever with thrombocytopenia syndrome. J. Infect. Chemother. 24, 389–392 (2018).
Shimojima, M. et al. Combination effects of ribavirin and interferons on severe fever with thrombocytopenia syndrome virus infection. Virol. J. 12, 181 (2015).
Park, I., Kim, H. I. & Kwon, K. T. Two treatment cases of severe fever and thrombocytopenia syndrome with oral ribavirin and plasma exchange. Infect. Chemother. 49, 72 (2017).
Tani, H. et al. Efficacy of T-705 (Favipiravir) in the treatment of infections with lethal severe fever with thrombocytopenia syndrome virus. mSphere 1, e00061–15 (2016).
Azuma, T. & Yasukawa, M. Clinical study of favipiravir for patients with severe fever with thrombocytopenia syndrome. JPRN-UMIN000022398. (2016).
Song, R., Chen, Z. & Li, W. Severe fever with thrombocytopenia syndrome (SFTS) treated with a novel antiviral medication, favipiravir (T-705). Infection 48, 295 (2020).
Liu, W. & Zhang, X.-A. Clinical efficacy of therapy with recombinant human Interferona2a in patients with severe fever with thrombocytopenia syndrome. ChiCTR-IPR-15006285. http://www.chictr.org.cn/showprojen.aspx?proj=11210 (2015).
Li, H. et al. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 29, 739–753 (2019).
Therapeutic effect of post-exposure treatment with antiserum on severe fever with thrombocytopenia syndrome (SFTS) in a mouse model of SFTS virus infection. Virology 482, 19–27 (2015).
Liu, R. et al. Immunization with recombinant SFTSV/NSs protein does not promote virus clearance in SFTSV-infected C57BL/6J mice. Viral Immunol. 28, 113–22 (2015).
Jung, D., Rejinold, N. S., Kwak, J.-E., Park, S.-H. & Kim, Y.-C. Nano-patterning of a stainless steel microneedle surface to improve the dip-coating efficiency of a DNA vaccine and its immune response. Colloids Surf. B Biointerfaces 159, 54–61 (2017).
Kwak, J.-E. et al. Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nat. Commun. 10, 3836 (2019).
Kang, J.-G. et al. Vaccination with single plasmid DNA encoding IL-12 and antigens of severe fever with thrombocytopenia syndrome virus elicits complete protection in IFNAR knockout mice. PLoS Negl. Trop. Dis. 14, e0007813 (2020).
Dong, F. et al. Single dose of a rVSV-based vaccine elicits complete protection against severe fever with thrombocytopenia syndrome virus. NPJ Vaccines 4, 5 (2019).
Yu, K.-M. et al. Cross-genotype protection of live-attenuated vaccine candidate for severe fever with thrombocytopenia syndrome virus in a ferret model. Proc. Natl Acad. Sci. USA 116, 26900 (2019).
Jia, Z. et al. Identification of a candidate standard strain of severe fever with thrombocytopenia syndrome virus for vaccine quality control in China using a cross-neutralization assay. Biologicals 46, 92–98 (2017).
Acknowledgements
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated. All reasonable precautions have been taken by the World Health Organization (WHO) to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed, or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall WHO be liable for damages arising from its use.
Author information
Authors and Affiliations
Contributions
N.B., J.A.K., A.E.S., A.D.T.B., D.W.C.B., V.B., G.N.M., M.P.P., and L.M.R. all made equal contributions to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.D.T.B is the editor-in-Chief of npj Vaccines. The remaining authors declare no competing
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bopp, N.E., Kaiser, J.A., Strother, A.E. et al. Baseline mapping of severe fever with thrombocytopenia syndrome virology, epidemiology and vaccine research and development. npj Vaccines 5, 111 (2020). https://doi.org/10.1038/s41541-020-00257-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-020-00257-5
This article is cited by
-
Ruxolitinib plus standard of care in severe hospitalized adults with severe fever with thrombocytopenia syndrome (SFTS): an exploratory, single-arm trial
BMC Medicine (2024)
-
Heterologous vaccination utilizing viral vector and protein platforms confers complete protection against SFTSV
Scientific Reports (2023)
-
Spatiotemporal analysis of severe fever with thrombocytopenia syndrome in Shandong Province, China, 2014–2018
BMC Public Health (2022)
-
The associations between fasting blood glucose levels and mortality of SFTS in patients
BMC Infectious Diseases (2021)