Abstract
Negative symptoms (NS) are a core component of schizophrenia affecting community functioning and quality of life. We tested neural correlates of NS considering NS factors and consensus subdomains. We assessed NS using the Clinical Assessment Interview for Negative Symptoms and the Scale for Assessment of Negative Symptoms. Arterial spin labeling was applied to measure resting-state cerebral blood flow (rCBF) in 47 schizophrenia patients and 44 healthy controls. Multiple regression analyses calculated the relationship between rCBF and NS severity. We found an association between diminished expression (DE) and brain perfusion within the cerebellar anterior lobe and vermis, and the pre-, and supplementary motor area. Blunted affect was linked to fusiform gyrus and alogia to fronto-striatal rCBF. In contrast, motivation and pleasure was not associated with rCBF. These results highlight the key role of motor areas for DE. Considering NS factors and consensus subdomains may help identifying specific pathophysiological pathways of NS.
Similar content being viewed by others
Introduction
Negative symptoms (NS) form a core component of schizophrenia, characterized by deficits in emotion and behavior, hampering treatment compliance and leading to social withdrawal, reduced quality of life and poor functional outcome1,2.
Previous studies investigated NS as a heterogeneous single dimension, often disregarding the hypothesized specific mechanistic differences of NS subdomains including blunted affect, alogia, anhedonia, asociality and avolition3. In fact, investigations of the structure of NS led to a comprehensive agreement to classify NS as two distinct factors: diminished expression (DE) and motivation and pleasure (MAP)4,5,6,7. DE includes the NIMH-MATRICS consensus subdomains blunted affect and alogia and is characterized by reduced outward expression of emotion and speech. The second factor MAP, includes anhedonia, asociality and avolition and represents symptoms related to impairments in motivation, goal-directed behavior and decision-making6. Different terms have been used in the literature for both NS factors DE (e.g. expressive factor, Expressive Deficit domain) and MAP (e.g. apathy, avolition-apathy, experiential factor)8. Throughout the manuscript, we will be referring to the two factors as diminished expression (DE), and motivation and pleasure (MAP). Importantly recent studies report partly distinct cognitive, behavioral and neural correlates for each factor. For example, deficits in DE are mostly linked to cognitive impairments and everyday life skills8,9,10. Conversely, MAP is strongly linked to poor interpersonal relationships, functional outcome and depression4,7,10,11.
To date, neural correlates of NS in schizophrenia are not fully understood. Previous studies investigated NS as a heterogeneous single dimension, rarely were neural correlates of DE explored as a NS factor. Hitherto evidence resulting from both functional and structural imaging (rs-fMRI, sMRI) suggests, that blunted affect is associated with aberrant limbic and paralimbic brain activity which includes the amygdala, parahippocampal, anterior cingulate, orbitofrontal, medial and ventrolateral prefrontal, premotor, motor and parietal areas, as well as, the fusiform gyrus (FFG)12,13,14,15,16. Alogia, the second subdomain of DE has been linked to abnormal activity within the anterior cingulate cortex (ACC)17 and areas of the basal ganglia, including pallidum and caudate18,19. In contrast, more studies focused on the neural correlates of MAP by applying rs-fMRI, sMRI and investigating the anticipation of reward in different fMRI tasks. Several studies and meta-analytic evidence suggest hypoactivation of the ventral striatum as a neuronal correlate of MAP (i.e. during the anticipation of reward)20,21,22,23,24,25. Further evidence suggests the dorsal caudate as an additional striatal area to be involved in reward anticipation21,26. Besides the central role of striatal areas, some studies also linked MAP severity to reduced activation in the cingulate cortex and insula23, as well as, in the ventromedial prefrontal cortex22 and the inferior frontal gyrus27. Importantly, studies on NS subdomains do not directly compare these subdomains in respect of the neuronal correlates. Thus, studies focusing on both NS factors to examine neuronal correlates are needed.
In sum, areas relevant for cognitive and emotional processing in motor and fronto-limbic networks seem to play an essential role in the pathophysiology of DE. In contrast, MAP is thought to be associated with changes in prefrontal-striatal areas28. The majority of these studies however present differences in the blood-oxygen-level-dependent (BOLD) signal between rewarding and non-rewarding stimuli. Studies reflecting absolute activations in different brain areas and NS subdomains are rare. In particular, arterial spin labeling uses arterial water as a tracer to measure resting-state cerebral blood flow (rCBF). Therefore, ASL results in an absolute quantification of rCBF with high spatial and temporal resolution rendering rCBF an essential correlate of brain functioning29. Thus while BOLD contrast primarily detects changes that indirectly reflect changes in CBF, ASL perfusion techniques directly quantify CBF, and are believed to be directly linked to neuronal activity in contrast to BOLD signal changes30. Therefore, ASL measures may exhibit decreased inter-subject variability compared to BOLD. Few studies have used rCBF quantifications to investigate NS as a uniform construct with conflicting results. In detail, one study found a positive association between NS and increased perfusion in the left inferior temporal gyrus and insula31, while two other studies detected decreased perfusion in bilateral superior temporal gyrus (STG) and left middle and inferior frontal gyrus (IFG)32,33. The first two studies used the Positive and Negative Syndrome Scale (PANSS) to assess NS, while Pinkham and colleagues used the global score of the Scale for the Assessment of Negative Symptoms (SANS). The current conceptualization of NS however suggests the use of newer assessments such as the Clinical Assessment Interview for Negative Symptoms (CAINS) and the Brief Negative Symptom Scale (BNSS) to measure NS, or omitting the global scales when using the SANS34,35,36. Further, more recent evidence advocates the examination of the five consensus subdomains separately, which might fit the psychometric data more adequately34,35,36. Regarding NS and brain perfusion, only two small studies focused on NS factors. In particular, Liemburg and colleagues reported less task-related rCBF in fronto-parietal, motor and thalamic regions37 associated with MAP. Schneider and colleagues showed higher striatal perfusion associated with MAP performing region of interest analyses38. To our knowledge, neither study has investigated neural correlates of the NS factors nor the five consensus subdomains using ASL whole brain resting-state perfusion analyses.
The current study therefore aims to investigate the association of severity of DE, MAP and the related five consensus subdomains and whole brain changes in rCBF in schizophrenia patients. We conceptualize NS considering the most recent guidelines34,35,36 using the CAINS and SANS. Based on the current literature, we hypothesize, that DE and its related subdomains will be associated with altered perfusion in motor and fronto-limbic areas, while MAP and its related subdomains will be associated with aberrant perfusion in prefrontal-striatal networks.
Results
Patients showed moderately severe psychotic symptoms according to PANSS total scores. Patients and controls did not differ in age, sex, or duration of education. Within 24 h of the testing eight patients were treated with antidepressants, eight were taking benzodiazepines and 15 received anticonvulsant medication.
Negative symptom factor DE is associated with perfusion in the supplementary motor area and the anterior cerebellum
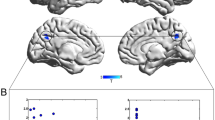
We observed an association between severity of DE and rCBF bilaterally in the pre-supplementary and supplementary motor area (SMA) as well as the anterior lobe of the cerebellum and the cerebellar vermis (Fig. 1). Specifically, severe reduced emotional and speech expression (DE) was associated with higher perfusion in these regions. Conversely, we observed no significant association between brain perfusion and the second main negative symptom factor (MAP). In addition, comparing patients and controls independent of NS identified lower rCBF in patients within the right planum temporale (see Table S2).
Negative symptom subdomain alogia is associated with perfusion within the supplementary motor area, ventral premotor area and the anterior cerebellum
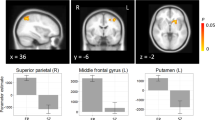
Similarly, and in accordance with the main negative symptom factor DE we detected an association between severity of the subdomain alogia and rCBF bilaterally most prominent within the anterior and posterior lobe as well as the cerebellar vermis and again the pre-SMA/SMA cluster. In addition, we found an association within the bilateral anterior cingulate cortex (ACC), the right insula, head of the caudate, putamen and nucleus accumbens, as well as within the triangularis part of the left inferior frontal gyrus (IFG), dorsal and ventral premotor area (PMd/PMv), postcentral gyrus (PoG) and lingual gyrus, left supramarginal gyrus (SMG), rolandic operculum and superior temporal gyrus (STG) (Fig. 2, Table 1:2). Importantly, these results differ only marginally when applying either scale (SANS or CAINS, see Table S1). In addition, the subdomain blunted affect was associated with brain perfusion bilaterally within the anterior lobe and vermis of the cerebellum and the right FFG (Fig. 3, Table 1:3). However, we detected no associations when testing the three MAP subdomains (anhedonia, asociality, and avolition).
Correlations between resting-state perfusion and negative symptom severity according to the Clinical Assessment Interview for Negative Symptoms (CAINS). Depicted clusters are significant at p(FWE-corr) < 0.05 at peak or cluster level. IFG Inferior frontal gyrus, MFG Middle frontal gyrus, SMA Supplementary motor area, PMd Dorsal premotor area, PMv Ventral premotor area, ACC Anterior cingulate cortex, LiG Lingual gyrus, NAcc Nucleus accumbens, STG Superior temporal gyrus, PoG Postcentral gyrus, SMG Supramarginal gyrus.
Discussion
Here we investigated the relationship between resting-state perfusion and negative symptom (NS) severity of the recently proposed NS factors and subdomains. As hypothesized, we observed increased perfusion in motor areas and the anterior lobe of the cerebellum to be associated with the factor diminished expression (DE). In addition, and in line with the hypothesis, we detected fronto-striatal resting-state perfusion associated with the subdomain alogia and perfusion of the right FFG to be associated with blunted affect. The results differ only marginally when applying either of the two NS scales SANS and CAINS. However, we found no association with brain perfusion and the second NS factor motivation and pleasure (MAP), or the subdomains anhedonia, asociality, and avolition. Our results suggest hyperactivation of motor areas and fronto-striatal brain areas to contribute to DE in patients with schizophrenia and point to the importance of focusing on NS factors and subdomains to identify brain alterations that match pathophysiological concepts of DE2,6,34,39.
Perfusion of motor areas is associated with the NS factor DE of emotion and speech
Our main results show that patients with DE of emotion and speech present with higher perfusion within the supplementary motor area (SMA), the pre-SMA and the cerebellum. The SMA, and the most anterior portion commonly termed pre-SMA, are key players of the motor system and have repeatedly been shown to be associated with motor symptoms in schizophrenia40,41,42. In fact, there is considerable conceptual overlap of negative symptoms and motor symptoms in schizophrenia43,44,45,46. In particular, DE includes psychomotor symptoms of slowing such as decreased spontaneous movements and reduced facial expression. Not surprisingly an overlap in brain alterations relevant for negative symptoms and psychomotor symptoms has been suggested47,48. Hence our finding of increased SMA/pre-SMA perfusion at rest fits to the suggested key role of the SMA/pre-SMA in psychomotor symptoms in schizophrenia41 and the suggested role of motor symptoms for NS43,44,45,46,49,50.
Distinct structural and functional alterations in the motor system are associated with specific categories of motor dysfunction in schizophrenia51. In particular, the SMA is involved in selecting, preparing, and executing different modes of action. The SMA is via direct and indirect motor pathways tightly connected to the primary motor cortex, the pre-SMA, the striatum, the subthalamic nucleus (STN), the thalamus, and the corticospinal tract52. By contrast, the pre-SMA has extensive pre-frontal connectivity. Most importantly, SMA activity controls ongoing action via basal ganglia loops. The pre-SMA plays a critical role in exerting control over voluntary actions in situations of response conflict52. As a consequence, SMA and pre-SMA hyperperfusion may contribute to slowness in planning and execution of fine motor tasks and general hypokinesia41,42,53,54,55 and consequently NS such as decreased spontaneous movements and reduced facial expression56,57. Additionally, hyperperfusion of these key players of the motor system may contribute to higher motor impairments such as reduced gesturing as a further symptom of the NS factor DE. In detail, schizophrenia patients displayed hypoactivation during execution of familiar gestures within the premotor cortices (bilateral SMA, pre-SMA and cingulate motor areas)58. However, altered gesture production in schizophrenia has in addition been associated with altered activity of the praxis network58,59,60. Apart from the above mentioned key role in the motor system evidence suggests the pre-SMA/SMA to be highly relevant for cognitive functions such as attention, temporal processing61,62,63,64, problem solving and working memory capabilities in schizophrenia65,66, as well as in early psychosis67,68. Finally SMA dysfunction has been associated with altered sense of agency52,69,70,71.
The detected relatively large cluster within the cerebellum associated with DE includes most prominently the anterior lobe as well as the posterior lobe and the cerebellar vermis. In general, the role of the cerebellum in motor functions such as planning, coordination and fine tuning is well known and widely accepted72. In addition more recently, the relevance of cerebellar networks in emotional and cognitive processing such as timing and associative learning73, as well as, motivational and social behavior with regional specification has been discussed74,75. Both, the anterior (largely lobule V, but also lobules IV, and VI) and inferior posterior parts of the cerebellum (lobules VIIIa and VIIIb) are connected to the primary motor cortex and have been associated with sensorimotor processes, while the lateral and superior posterior cerebellum is suggested to be relevant for cognition and emotional processing47,76,77,78. In addition, the cerebellar vermis has also been linked to affective processing76,78. Bernard and Mittal advocate alterations within distinct motor and non-motor closed-loop circuits with the cerebral cortex as affected in schizophrenia79. Particularly, the shown alterations within the anterior and inferior posterior parts of the cerebellum may impact cerebellar-mediated motor behaviors. Thus, the cerebellar hyperperfusion may contribute to motor symptoms, and in addition reflect alterations in cerebellar networks relevant for emotional, cognitive and motivational behavior associated with DE. Likewise, some evidence also suggests involvement of the cerebellum in hallucinations and formal thought disorder80.
Finally, independent of NS factors altered brain activity of the SMA and the cerebellum has previously been suggested to be associated with overall negative symptoms. For instance, Vanes and colleagues showed reduced cerebellar and SMA task-based fMRI activation during a Stroop task associated with negative symptoms severity in particular in patients suffering from a first schizophrenia episode67. Independent of NS factors, Bernard and colleagues advocate that cerebellar motor networks might contribute to overall negative symptoms in schizophrenia patients81.
Taken together the detected hyperperfusion of the SMA and the cerebellum nicely fit to the framework on motor symptoms in schizophrenia41 and findings of studies investigating motor abnormalities and gesturing in schizophrenia42,45,58,59,60 as a highly relevant aspect of the NS factor DE5,8,28,44.
Importantly these findings fit to evidence showing an association of NS, motivation and motor symptoms at the behavior level in schizophrenia. In fact, patients with more negative symptoms showed reduced physical activity45,82, physical activity is reduced during the course of the disorder83, autonomous motivation was associated with physical84, and physical activity predicted the course of overall negative symptoms between episodes85.
Perfusion of motor areas, fronto-striatal areas and the FFG associated with the NS subdomains blunted affect and alogia
When looking at the association of brain perfusion and NS subdomains we detected effects associated with the subdomains suggested to build the DE factor (blunted affect and alogia). Not surprisingly, our results therefore mirror the described effects detected with DE. In particular, showing hyperperfusion in the SMA, the ventral premotor (PMv) and the dorsal premotor area (PMd) associated with alogia and hyperperfusion within the cerebellum associated with alogia and blunted affect. Moreover, additional brain areas emerged. Specifically, severe blunted affect was besides associated with perfusion of the right FFG. Severe symptoms of alogia were in addition associated with fronto- and paralimbic resting-state perfusion.
The role of the SMA and the cerebellum for NS has been discussed above. Likewise, the few studies on NS subdomains suggest the motor cortex to be associated with blunted affect. Lee and colleagues showed for instance severity of blunted affect to be associated with aberrant brain activity in premotor and motor cortex and the inferior parietal lobule using a facial emotion imitation task15. In line with previous imaging reports assessing task-based activity, severe symptoms of blunted affect were associated with increased perfusion at rest of the right FFG12,13,14. Although the exact functionality of the FFG is still disputed, there is relative consensus on its involvement in face emotion processing86,87, on which schizophrenia patients show deficits86,87 and NS are presumed to have influence88. In fact, facial recognition and memory difficulties were associated with severity of overall NS89,90, reduced FFG volume correlated significantly with higher NS but not with positive symptoms90,91, and patients with persistent NS show smaller GM volume of the right FFG compared to patients with non-persistent NS92. Consistent with these results evidence from PET (lower glucose metabolic rate in the FFG) and EEG (event related potential components of the left FFG cluster at P100) suggest overall NS to be associated with FFG activity88,93. However, other reports focusing on hallucinations advocate the relevance of the FFG for pathophysiological concepts on hallucinations94. Thus, our results fit to the suggested substantial role of the FFG in the development of NS91 and further specify the relevance for the NS subdomain blunted affect. In fact, it seems plausible that in particular reduced eye contact, one aspect of the NS subdomain blunted affect, may lead to deficits in facial recognition. The detected hyperactivation of the FFG may reflect a compensatory effect. However, this remains speculative, as it was not directly tested in the present study.
As stated and consistent with the NS factor DE alogia, the second subdomain of DE, was associated with hyperperfusion of the SMA and the cerebellum. In addition, alogia was linked to increased perfusion in the frontal cortex (IFG, MFG, ACC), motor and premotor areas (SMA, pre-SMA, the dorsal and ventral premotor area), basal ganglia and limbic and paralimbic brain areas including the insula, putamen, pallidum, caudate and nucleus accumbens (Table 1, Fig. 2). To our knowledge, so far no study tested whole brain perfusion associated with NS subdomains. In line with our results areas relevant for cognitive and emotional processing in motor and fronto-limbic networks as well as the caudate nuclei have been suggested to play an essential role in the pathophysiology of the NS factor DE12,13,14,15,16,17,18,19,28. When particularly looking at the subdomain alogia first task-based MRI evidence suggested alogia to be linked to abnormal activity within the anterior cingulate cortex (ACC)17 and areas of the basal ganglia, including pallidum and caudate18. In detail, Hager and colleagues investigated the neural effects of monetary reward performance using an n-back fMRI task. Their results revealed a negative correlation between severity of DE using the SANS NS scale and brain activity in the rostral ACC. Considering the role of the ACC in cognitive functioning including emotional processing, attention allocation and mood regulation, the authors advocate that especially symptoms in DE lead to difficulties in cognitive processing. This and our results support the cognitive resource limitation model proposing that emotional expression requires a considerate amount of cognitive resources95. These resources are thought to be insufficient for emotional expression in patients particularly during high cognitive demanding situations such as social interactions. Thus we provide additional evidence for the cognitive resource limitation model relevant for DE in schizophrenia supporting studies connecting cognitive impairment and DE9. Although prior evidence linking activation in the insula or the STG to the NS subdomain alogia is limited the detected link fits to previous reports showing functional and structural alterations in the insula cortex, the STG and overall negative symptom severity90,96,97,98,99,100,101,102,103. In addition, early evidence shows that insular lesions can lead to deficits in speech initiation and planning as well as emotional expression and comprehension104,105,106. Finally, the key role of the STG in language processing is widely accepted107. Therefore, an association with the NS subdomains involved in speech initiation (alogia) is plausible102.
Interestingly examining perfusion associations of the two consensus subdomains of DE (blunted affect and alogia) separately revealed distinct neural correlates between the two subdomains. This points to the fact that indeed and as recently suggested looking at subdomains may help identifying specific underlying mechanism34,35,36.
No association with the NS factor MAP and the subdomains anhedonia, asociality or avolition
Contrary to previous reports, we did not find any significant associations between perfusion and MAP severity including its consensus subdomains anhedonia, asociality or avolition. Brain abnormalities in corticostriatal areas have been linked to reward anticipation and motivation and its subdomains anhedonia, asociality and avolition6. We thus hypothesized an association between brain perfusion in prefrontal-striatal areas and severity of MAP, as suggested by numerous studies20,21,22,23,26 and meta-analysis24. One reason why the results do not support our assumption might be relevant methodological differences compared to previous reports. In particular, the majority of the previous studies examined differences in the blood-oxygen-level-dependent (BOLD) signal between rewarding and non-rewarding stimuli while we looked at whole brain absolute activations at rest using ASL. In fact only one previous study focused on investigating the association of NS factors and rCBF and observed a significant positive correlation between MAP and perfusion in the striatum38. In contrast to our study, Schneider and colleagues, however, used a region-of-interest approach focusing on the striatum.
Limitations
Our results extend previous findings detecting resting-state hyperperfusion in motor and fronto-limbic areas linked to DE in schizophrenia considering recent guidelines on the assessment of negative symptoms. Particularly, we used whole brain ASL to reflect absolute brain metabolism. However, some limitations require discussion. First, we cannot rule out possible effects of medication on our findings of altered rCBF108. In our sample, all but four patients were treated with antipsychotic medication at the time of scanning. In fact, reports on the impact of antipsychotic medication on rCBF yielded conflicting results. While some studies detect no effects of medication on rCBF109,110, others show CBF reduction111, regional different effects on rCBF112 or substance depending effects on rCBF113. However, we solely included patients on stable medication treated with first- or second-generation antipsychotics. In addition, we included antipsychotic medication dosage (OLZs) and dosage of benzodiazepines (diazepam equivalents), as a covariate of no-interest in all our analyses. Furthermore, as expected factors of NS were partially overlapping. This hampers the sharp distinction of effects of NS factors. Additionally, we used two independent measures resulting in marginal differences of the results. These differences may result from limitations of the first-generation measurement SANS. However we directly addressed these weaknesses by applying the most recent conceptualization of NS34,35,36 and implemented an improved rating of the SANS to measure NS factors. Finally, we cannot comment on possible associations of the detected brain activity (i.e. within the pre-SMA / SMA) with cognitive functions or the performance of neurocognitive tasks as this was not specifically tested in the study.
To conclude, investigating the association of NS factors or subdomains and resting-state whole brain perfusion revealed effects for DE and the subdomains blunted affect and alogia. In particular, we highlight the central role of motor areas for DE in schizophrenia. Furthermore, our results match the suggested relevance of fronto-limbic networks and the FFG in the development of NS and further specify the FFG to be key for the NS subdomain blunted affect. Our results support the relevance of the recently proposed NS factors and subdomains to unravel brain perfusion alterations that match pathophysiological concepts of NS. Future multimodal studies may further disentangle the link of task and resting-state based brain alterations associated with NS factors and subdomains in schizophrenia114,115,116. Finally, in-depth studies on NS pathophysiology will pave the way for novel treatment strategies, such as non-invasive brain stimulation techniques.
Methods
Participants
We included 47 patients with schizophrenia spectrum disorders and 44 age-and sex matched healthy controls. We diagnosed patients according to the DSM-5 diagnostic and statistical manual of mental disorders. Demographic and clinical characteristics are given in Table 2.
Schizophrenia patients were recruited from the inpatient and outpatient department of the University Hospital of Psychiatry and Psychotherapy in Bern, Switzerland. Exclusion criteria for all participants included head trauma or general exclusion criteria for MRI scans (e.g. metallic implants, claustrophobia and pregnancy), and a history of substance abuse or dependence other than nicotine as assessed during the patients’ interviews, careful chart review, and according the mini international neuropsychiatric interview (MINI)117. Additionally, exclusion criteria for healthy controls were history of any psychiatric disorder or any first-degree relatives with schizophrenia spectrum disorders according to the MINI117. We obtained written informed consent from all participants. The study protocol adhered to the declaration of Helsinki and was approved by the local Ethics Committee, Bern (KEK). All participants included in this study participated in previous studies58,118. We assessed antipsychotic medication and calculated olanzapine equivalents (OLZs) in accordance to Leucht et al119. All but four patients were treated with antipsychotic medication at the time of testing. Diazepam equivalents were calculated according to Ashton120.
Clinical assessments
We assessed symptom severity in patients using the Positive and Negative Syndrome Scale (PANSS)121, the Clinical Assessment Interview for Negative Symptoms (CAINS)57 and the Scale for the Assessment of Negative Symptoms (SANS)56.
The CAINS interview was particularly designed to assess the five negative symptom consensus subdomains (blunted affect, alogia, anhedonia, asociality and avolition). All 13 items are scored on a five-point scale ranging from 0 (no impairment) to 4 (severe deficit). We used the expert ratings of each single item of the CAINS to calculate means for the five subdomains according to Strauss and colleagues34. Next, we used the mean of alogia and blunted affect to calculate the mean of diminished expression (DE) and the mean of anhedonia, asociality and avolition to calculate the mean of motivation and pleasure (MAP) as these form the two main suggested negative symptom factors (Table 2).
Likewise, the SANS is an interview-based rating scale for negative symptoms. It consists of five subscales: affective flattening or blunting, alogia, avolition-apathy, anhedonia-asociality, and attention. Within each subscale, experts rate severity of 3–8 single symptoms from 0 (none) to 5 (severe). In accordance with the most recent guidelines, we removed SANS items with redundant or conflicting content to the current concept of NS (inappropriate affect, poverty of content of speech and inattention). Next, we calculated mean values for each of the five consensus subdomains34,36 and again calculated means of the two main negative symptom factors DE and MAP. In particular, the DE factor was calculated using the mean of blunted affect and alogia. MAP was calculated using the mean of anhedonia, asociality and avolition.
We provide means and standard deviations of the NS factors (DE and MAP) and the consensus subdomains (blunted affect, alogia, anhedonia, asociality and avolition) for the CAINS in Table 2 and ranges depicting negative symptom severity for both rating scales in the supplement (Table S3).
MRI acquisition
We performed structural and functional MRI on a 3 T MRI scanner (Siemens Magnetom Trio; Siemens Medical Solutions, Erlangen, Germany) using a 12-channel radio frequency head coil for signal reception. For all participants we obtained structural 3D-T1-weighted (Modified Driven Equilibrium Fourier Transform Pulse Sequence; MDEFT) images: 176 sagittal slices with 256 × 256 matrix points with a non-cubic field of view (FOV) of 256 mm, yielding a nominal isotopic resolution of 1 mm³ (i.e. 1 mm × 1 mm × 1 mm). Scanning parameters further contained, repetition time (TR) of 7.92 ms, echo time (TE) of 2.48 ms and a flip angle (FA) of 16°.
In order to obtain 110 functional images per participant, we applied a pseudo continuous arterial spin labeling (pCASL) sequence. This sequence provided 20 slices in ascending order, 64 × 64 matrix points with a non-cubic 230 mm FOV, yielding a nominal isotopic resolution of 4.27 mm³ (i.e. 3.6 mm × 3.6 mm × 6 mm), TR of 4000 ms, TE of 18 ms and a FA of 25°122.
Data processing
We analyzed structural and perfusion imaging data using SPM version 12 (The Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, London, UK; https://www.fil.ion.ucl.ac.uk/spm/). We applied an in-house written MATLAB program toolbox to preprocess perfusion images42,118,122,123. Briefly, we realigned the ASL images and implemented a voxel-wise calculation of the mean regional resting-state cerebral blood flow (rCBF). We stored these rCBF maps for each participant. Further, we applied coregistration of all rCBF maps to the structural images. Finally, we normalized and smoothed the data with 8 mm full-width at half-maximum (FWHM) kernel.
Statistical analyses
We analyzed demographic and clinical data using SPSS version 28 (SPSS Inc., Chicago, IL, USA). To test group differences in demographic and clinical variables we used two-sample t-tests and chi-square tests (χ2).
Our main analysis focused on the effect of NS factors on rCBF. Therefore, we applied two separate multiple regression analyses in SPM to test the association between brain perfusion and mean severity of DE and MAP as calculated from the CAINS. To test for consistency of the results we repeated the analyses for the second negative symptom scale (SANS), again within two separate multiple regression analyses.
In addition to the two main factors, we explored the association of NS severity within the five consensus subdomains. Again, we calculated the association between mean severity for each subdomain and rCBF by applying multiple regression analyses for both negative symptom scales. We thus performed exploratory analyses with five separate multiple regression analyses for the CAINS as well as for the SANS. Finally, to test group differences in rCBF independent of NS we performed a t-test comparing patients and controls.
For all imaging analyses, we included age, six motion parameters, olanzapine equivalents, PANSS positive subscore, duration of illness, years of education and diazepam equivalents as covariates of no-interest. In addition, voxels with <10 [ml/100 g/min] blood flow were excluded from all analyses to exclude voxels in white matter118. We applied a statistical threshold of p < 0.05 family wise error corrected for multiple testing (FWE-corr). We present results for the Clinical Assessment Interview for Negative Symptoms (CAINS) corrected at peak- or cluster level in the main manuscript. Additionally, results for the Scale for the Assessment of Negative Symptoms (SANS) corrected for multiple testing (FWE-corr) at peak- or cluster level are presented in the supplementary material (Table S1). We produced figures using SPM12 and the SPM xjView toolbox (https://www.alivelearn.net/xjview). For illustration purposes, we produced images at p < 0.001 uncorrected with a minimum cluster size of 350 voxels. The minimum cluster size of 350 voxels was chosen to guarantee that only brain regions surviving the strict FWE corrections <0.05 are displayed. We extracted the mean perfusion values post-hoc from significant clusters for all patients and healthy controls using MarsBaR124 and plotted extracted brain perfusion values and NS severity.
Data availability
The statistical maps that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to them containing information that could compromise research participant privacy or consent. Explicit consent to deposit raw-imaging data was not obtained from the patients.
References
Strauss, G. P., Sandt, A. R., Catalano, L. T. & Allen, D. N. Negative symptoms and depression predict lower psychological well-being in individuals with schizophrenia. Compr. Psychiatry 53, 1137–1144 (2012).
Galderisi, S., Merlotti, E. & Mucci, A. Neurobiological background of negative symptoms. Euro. Arch. Psychiatry Clin. Neurosci. 265, 543–558 (2015).
Kirkpatrick, B., Fenton, W. S., Carpenter, W. T. & Marder, S. R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 32, 214–219 (2006).
Strauss, G. P. et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J. Psychiatric Res. 47, 783–790 (2013).
Kaiser, S. et al. Individual negative symptoms and domains—relevance for assessment, pathomechanisms and treatment. Schizophr. Res. 186, 39–45 (2017).
Galderisi, S., Mucci, A., Buchanan, R. W. & Arango, C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 5, 664–677 (2018).
Galderisi, S. et al. Categorical and dimensional approaches to negative symptoms of schizophrenia: focus on long-term stability and functional outcome. Schizophr. Res. 147, 157–162 (2013).
Marder, S. R. & Galderisi, S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16, 14–24 (2017).
Hartmann-Riemer, M. N. et al. The association of neurocognitive impairment with diminished expression and apathy in schizophrenia. Schizophr. Res. 169, 427–432 (2015).
Giuliani, L. et al. Improving knowledge on pathways to functional outcome in Schizophrenia: main results from the Italian network for research on Psychoses. Front. Psychiatry 12, 791117 (2021).
Galderisi, S. et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 13, 275–287 (2014).
Fahim, C. et al. Brain activity during emotionally negative pictures in schizophrenia with and without flat affect: an fMRI study. Psychiatry Res. 140, 1–15 (2005).
Gur, R. E. et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch. Gen. Psychiatry 64, 1356–1366 (2007).
Lepage, M. et al. Emotional face processing and flat affect in schizophrenia: functional and structural neural correlates. Psychol. Med. 41, 1833–1844 (2011).
Lee, J. S., Chun, J. W., Yoon, S. Y., Park, H.-J. & Kim, J.-J. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr. Res. 152, 268–274 (2014).
Rahm, C. et al. Negative symptoms in schizophrenia show association with amygdala volumes and neural activation during affective processing. Acta Neuropsychiatr. 27, 213–220 (2015).
Hager, O. M. et al. Reward-dependent modulation of working memory is associated with negative symptoms in schizophrenia. Schizophr. Res. 168, 238–244 (2015).
Shaffer, J. J. et al. Neural correlates of Schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol. Neuropsychiatry 1, 191–200 (2015).
Liddle, P. F. et al. Patterns of cerebral blood flow in schizophrenia. Br .J. Psychiatry 160, 179–186 (1992).
Kirschner, M. et al. Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. J. Psychiatry Neurosci. 41, 152–161 (2016).
Stepien, M. et al. Investigating the association of ventral and dorsal striatal dysfunction during reward anticipation with negative symptoms in patients with schizophrenia and healthy individuals. PLoS One 13, e0198215 (2018).
Waltz, J. A. et al. Motivational deficits in Schizophrenia are associated with reduced differentiation between gain and loss-avoidance feedback in the striatum. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 3, 239–247 (2018).
Moran, E. K., Culbreth, A. J., Kandala, S. & Barch, D. M. From neuroimaging to daily functioning: a multimethod analysis of reward anticipation in people with schizophrenia. J. Abnorm. Psychol. 128, 723–734 (2019).
Radua, J. et al. Ventral striatal activation during reward processing in Psychosis: a neurofunctional meta-analysis. JAMA Psychiatry 72, 1243–1251 (2015).
Chase, H. W., Loriemi, P., Wensing, T., Eickhoff, S. B. & Nickl-Jockschat, T. Meta-analytic evidence for altered mesolimbic responses to reward in schizophrenia. Hum. Brain mapping 39, 2917–2928 (2018).
Mucci, A. et al. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med. 45, 1765–1778 (2015).
Kluge, A. et al. Combining actigraphy, ecological momentary assessment and neuroimaging to study apathy in patients with schizophrenia. Schizophr. Res. 195, 176–182 (2018).
Bègue, I., Kaiser, S. & Kirschner, M. Pathophysiology of negative symptom dimensions of schizophrenia—current developments and implications for treatment. Neurosci. Biobehav. Rev.116, 74–88 (2020).
Borogovac, A. & Asllani, I. Arterial Spin Labeling (ASL) fMRI: advantages, theoretical constrains, and experimental challenges in neurosciences. Int. J. Biomed. Imaging 2012, 818456 (2012).
Liu, T. T. & Brown, G. G. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J. Int. Neuropsychol. Soc. 13, 517–525 (2007).
Zhu, J. et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J. Psychiatric Res. 63, 28–35 (2015).
Ota, M. et al. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr. Res. 154, 113–118 (2014).
Pinkham, A. et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 194, 64–72 (2011).
Strauss, G. P. et al. The latent structure of negative symptoms in Schizophrenia. JAMA Psychiatry 75, 1271–1279 (2018).
Strauss, G. P., Ahmed, A. O., Young, J. W. & Kirkpatrick, B. Reconsidering the latent structure of negative symptoms in Schizophrenia: a review of evidence supporting the 5 consensus domains. Schizophr. Bull. 45, 725–729 (2019).
Galderisi, S. et al. EPA guidance on assessment of negative symptoms in schizophrenia. Euro. Psychiatry: J. Assoc. Euro. Psychiatrists 64, e23 (2021).
Liemburg, E. J. et al. Neural correlates of planning performance in patients with schizophrenia–relationship with apathy. Schizophr. Res. 161, 367–375 (2015).
Schneider, K. et al. Cerebral blood flow in striatal regions is associated with apathy in patients with schizophrenia. J. Psychiatry Neurosci. 44, 102–110 (2019).
Kaliuzhna, M. et al. How far to go in deconstructing negative symptoms? Behavioural and neural level evidence for the amotivation domain. Schizophr. Res. 236, 41–47 (2021).
Stegmayer, K. et al. Supplementary motor area (SMA) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Res. 223, 49–51 (2014).
Walther, S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 233, 293–298 (2015).
Walther, S. et al. Resting-state hyperperfusion of the supplementary motor area in Catatonia. Schizophr. Bull. 43, 972–981 (2017).
Peralta, V. & Cuesta, M. J. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr. Res. 47, 107–116 (2001).
Peralta, V., Jalón, E. Gde, Campos, M. S. & Cuesta, M. J. Covariation between motor signs and negative symptoms in drug-naive subjects with schizophrenia-spectrum disorders before and after antipsychotic treatment. Schizophr. Res. 200, 85–91 (2018).
Walther, S. et al. Low physical activity is associated with two hypokinetic motor abnormalities in psychosis. J. Psychiatric Res. 146, 258–263 (2022).
Nadesalingam, N. et al. The behavioral mapping of psychomotor slowing in psychosis demonstrates heterogeneity among patients suggesting distinct pathobiology. Schizophr. Bull. 49, 507–517 (2023).
Bernard, J. A. et al. Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum. Brain Mapping 35, 4064–4078 (2014).
Mucci, A., Galderisi, S., Amodio, A. & Dierks, T. In Neuroimaging of Schizophrenia and Other Primary Psychotic Disorders (eds Galderisi, S., DeLisi L. E. & Borgwardt, S.) 57–155 (Springer International Publishing, Cham, 2019).
Bègue, I. et al. Cerebellar and cortico-striatal-midbrain contributions to reward-cognition processes and apathy within the psychosis continuum. Schizophr. Res. 246, 85–94 (2022).
Brady, R. O. et al. Cerebellar-prefrontal network connectivity and negative symptoms in Schizophrenia. Am. J. Psychiatry 176, 512–520 (2019).
Walther, S. & Mittal, V. A. Motor system pathology in Psychosis. Curr. Psychiatry Rep. 19, 97 (2017).
Nachev, P., Kennard, C. & Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci.9, 856–869 (2008).
Walther, S. et al. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in Schizophrenia spectrum disorders. Schizophr. Bull. 43, 982–992 (2017).
Walther, S., Stegmayer, K., Wilson, J. E. & Heckers, S. Structure and neural mechanisms of catatonia. Lancet Psychiatry 6, 610–619 (2019).
Schröder, J., Wenz, F., Schad, L. R., Baudendistel, K. & Knopp, M. V. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br. J. Psychiatry 167, 197–201 (1995).
Andreasen, N. C. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry 155, 49–52 (1989).
Kring, A. M., Gur, R. E., Blanchard, J. J., Horan, W. P. & Reise, S. P. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am. J. Psychiatry 170, 165–172 (2013).
Stegmayer, K. et al. Structural brain correlates of defective gesture performance in schizophrenia. Cortex 78, 125–137 (2016).
Stegmayer, K. et al. Limbic interference during social action planning in Schizophrenia. Schizophr. Bull. 44, 359–368 (2018).
Viher, P. V. et al. The cortical signature of impaired gesturing: findings from Schizophrenia. NeuroImage. Clin. 17, 213–221 (2018).
Ortuño, F., Guillén-Grima, F., López-García, P., Gómez, J. & Pla, J. Functional neural networks of time perception: challenge and opportunity for schizophrenia research. Schizophr. Res. 125, 129–135 (2011).
Ortuño, F. M., Lopez, P., Ojeda, N. & Cervera, S. Dysfunctional supplementary motor area implication during attention and time estimation tasks in schizophrenia: a PET-O15 water study. NeuroImage 24, 575–579 (2005).
Alústiza, I., Radua, J., Pla, M., Martin, R. & Ortuño, F. Meta-analysis of functional magnetic resonance imaging studies of timing and cognitive control in schizophrenia and bipolar disorder: evidence of a primary time deficit. Schizophr. Res. 188, 21–32 (2017).
Davalos, D. B., Rojas, D. C. & Tregellas, J. R. Temporal processing in schizophrenia: effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophr. Res. 127, 123–130 (2011).
Picó-Pérez, M. et al. Multimodal meta-analysis of structural gray matter, neurocognitive and social cognitive fMRI findings in schizophrenia patients. Psychol. Med. 52, 614–624 (2022).
Zarghami, T. S., Zeidman, P., Razi, A., Bahrami, F. & Hossein-Zadeh, G.-A. Dysconnection and cognition in schizophrenia: a spectral dynamic causal modeling study. Hum. Brain Mapping 44, 2873–2896 (2023).
Vanes, L. D. et al. Neural correlates of positive and negative symptoms through the illness course: an fMRI study in early psychosis and chronic schizophrenia. Sci. Rep. 9, 14444 (2019).
Horne, C. M. et al. The role of cognitive control in the positive symptoms of psychosis. NeuroImage. Clin. 34, 103004 (2022).
Farrer, C. et al. Modulating the experience of agency: a positron emission tomography study. NeuroImage 18, 324–333 (2003).
Wolpe, N., Hezemans, F. H. & Rowe, J. B. Alien limb syndrome: a Bayesian account of unwanted actions. Cortex 127, 29–41 (2020).
Yomogida, Y. et al. The neural basis of agency: an fMRI study. NeuroImage 50, 198–207 (2010).
McCormick, D. A. Motor control. The cerebellar symphony. Nature 374, 412–413 (1995).
Andreasen, N. C. & Pierson, R. The role of the cerebellum in schizophrenia. Biol. Psychiatry 64, 81–88 (2008).
Schmahmann, J. D. The cerebellum and cognition. Neurosci. Lett. 688, 62–75 (2019).
Buckner, R. L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815 (2013).
Stoodley, C. J. & Schmahmann, J. D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46, 831–844 (2010).
Guell, X., Gabrieli, J. D. E. & Schmahmann, J. D. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. NeuroImage 172, 437–449 (2018).
Stoodley, C. J. & Schmahmann, J. D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage 44, 489–501 (2009).
Bernard, J. A. & Mittal, V. A. Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front. Psychiatry 5, 160 (2014).
Picard, H., Amado, I., Mouchet-Mages, S., Olié, J.-P. & Krebs, M.-O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr. Bull. 34, 155–172 (2008).
Bernard, J. A., Goen, J. R. M. & Maldonado, T. A case for motor network contributions to schizophrenia symptoms: evidence from resting-state connectivity. Hum. Brain Mapping 38, 4535–4545 (2017).
Walther, S., Ramseyer, F., Horn, H., Strik, W. & Tschacher, W. Less structured movement patterns predict severity of positive syndrome, excitement, and disorganization. Schizophr. Bull. 40, 585–591 (2014).
Walther, S. et al. Physical activity in Schizophrenia is higher in the first episode than in subsequent ones. Front. Psychiatry 5, 191 (2014).
Costa, R. et al. Autonomous motivation and quality of life as predictors of physical activity in patients with schizophrenia. Int. J. Psychiatry Clin. Practice 22, 184–190 (2018).
Walther, S. et al. The longitudinal course of gross motor activity in Schizophrenia—within and between episodes. Front. Psychiatry 6, 10 (2015).
Goghari, V. M., MacDonald, A. W. & Sponheim, S. R. Temporal lobe structures and facial emotion recognition in schizophrenia patients and nonpsychotic relatives. Schizophr. Bull. 37, 1281–1294 (2011).
Habel, U. et al. Neural correlates of emotion recognition in schizophrenia. Schizophr. Res. 122, 113–123 (2010).
Kim, D.-W., Kim, H.-S., Lee, S.-H. & Im, C.-H. Positive and negative symptom scores are correlated with activation in different brain regions during facial emotion perception in schizophrenia patients: a voxel-based sLORETA source activity study. Schizophr. Res. 151, 165–174 (2013).
Ohara, N. et al. Neurophysiological face processing deficits in patients with chronic Schizophrenia: an MEG study. Front. Psychiatry 11, 554844 (2020).
Nestor, P. G. et al. Dissociable contributions of MRI volume reductions of superior temporal and fusiform gyri to symptoms and neuropsychology in schizophrenia. Schizophr. Res. 91, 103–106 (2007).
Jung, S. et al. Fusiform gyrus volume reduction associated with impaired facial expressed emotion recognition and emotional intensity recognition in patients with schizophrenia spectrum psychosis. Psychiatry Res. Neuroimaging 307, 111226 (2021).
Benoit, A., Bodnar, M., Malla, A. K., Joober, R. & Lepage, M. The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Front. Psychiatry 3, 42 (2012).
Potkin, S. G. et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Positron emission tomography. Am. J. Psychiatry 159, 227–237 (2002).
Zhou, M. et al. Alterations in functional network centrality in first-episode drug-naïve adolescent-onset schizophrenia. Brain Imaging Behav. 16, 316–323 (2022).
Cohen, A. S., Morrison, S. C., Brown, L. A. & Minor, K. S. Towards a cognitive resource limitations model of diminished expression in schizotypy. J. Abnorm. Psychol. 121, 109–118 (2012).
Sigmundsson, T. et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am. J Psychiatry 158, 234–243 (2001).
Takahashi, T. et al. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophr. Res. 108, 49–56 (2009).
Lei, W. et al. Gray matter volume alterations in first-episode drug-naïve patients with deficit and nondeficit schizophrenia. Psychiatry Res. 234, 219–226 (2015).
Manoliu, A. et al. Insular dysfunction reflects altered between-network connectivity and severity of negative symptoms in Schizophrenia during psychotic remission. Front. Hum. Neurosci. 7, 216 (2013).
Cascella, N. G. et al. Gray-matter abnormalities in deficit schizophrenia. Schizophr. Res. 120, 63–70 (2010).
Lui, S. et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am. J Psychiatry 166, 196–205 (2009).
Anderson, J. E. et al. An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophr. Res. 58, 123–134 (2002).
Kim, J. J. et al. Morphology of the lateral superior temporal gyrus in neuroleptic nai;ve patients with schizophrenia: relationship to symptoms. Schizophr. Res. 60, 173–181 (2003).
Dronkers, N. F. A new brain region for coordinating speech articulation. Nature 384, 159–161 (1996).
Shuren, J. Insula and aphasia. J. Neurol. 240, 216–218 (1993).
Cancelliere, A. E. & Kertesz, A. Lesion localization in acquired deficits of emotional expression and comprehension. Brain Cogn. 13, 133–147 (1990).
Bhaya-Grossman, I. & Chang, E. F. Speech computations of the human superior temporal gyrus. Ann. Rev. Psychol. 73, 79–102 (2022).
Goozée, R., Handley, R., Kempton, M. J. & Dazzan, P. A systematic review and meta-analysis of the effects of antipsychotic medications on regional cerebral blood flow (rCBF) in schizophrenia: association with response to treatment. Neurosci. Biobehav. Rev. 43, 118–136 (2014).
Gur, R. E. et al. Brain function in psychiatric disorders. III. Regional cerebral blood flow in unmedicated schizophrenics. Arch. Gen. Psychiatry 42, 329–334 (1985).
Mathew, R. J., Duncan, G. C., Weinman, M. L. & Barr, D. L. Regional cerebral blood flow in Schizophrenia. Arch. Gen. Psychiatry 39, 1121–1124 (1982).
Goldstein, P. C., Brown, G. G., Marcus, A. & Ewing, J. R. Effects of age, neuropsychological impairment, and medication on regional cerebral blood flow in schizophrenia and major affective disorder. Henry Ford Hosp. Med. J.38, 202–206 (1990).
Handley, R. et al. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum. Brain Mapp. 34, 272–282 (2013).
Lahti, A. C., Weiler, M. A., Medoff, D. R., Tamminga, C. A. & Holcomb, H. H. Functional effects of single dose first- and second-generation antipsychotic administration in subjects with schizophrenia. Psychiatry Res. 139, 19–30 (2005).
Sabe, M. et al. Thirty years of research on negative symptoms of schizophrenia: a scientometric analysis of hotspots, bursts, and research trends. Neurosci. Biobehav. Rev. 144, 104979 (2023).
Galderisi, S. Negative symptoms of schizophrenia: Trying to answer unanswered research questions. Psychiatry Res. 320, 115043 (2023).
van der Meer, L., Kaiser, S. & Castelein, S. Negative symptoms in schizophrenia: reconsidering evidence and focus in clinical trials. Br. J. Psychiatry 219, 359–360 (2021).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33 (1998). quiz 34-57; Study.
Stegmayer, K. et al. Resting state perfusion in the language network is linked to formal thought disorder and poor functional outcome in schizophrenia. Acta Psychiatr. Scand. 136, 506–516 (2017).
Leucht, S. et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr. Bull. 41, 1397–1402 (2015).
Ashton, H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr. Ann. 25, 158–165 (1995).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Wu, W.-C., Fernández-Seara, M., Detre, J. A., Wehrli, F. W. & Wang, J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magnetic Resonance in Med. 58, 1020–1027 (2007).
Stegmayer, K. et al. Specific cerebral perfusion patterns in three schizophrenia symptom dimensions. Schizophr. Res. 190, 96–101 (2017).
Brett, M., Anton, J.-L., Valabregue, R. & Poline, J.-B. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conferance on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. NeuroImage 13, 210–217 (2002).
Acknowledgements
This study received funding from the Swiss National Science Foundation (Grant Nos. SNF: 152619 to S.W., and PZPGP3_180022 to K.S.), as well as the Bangerter-Rhyner Foundation (to S.W.) and the Foundation Adrian and Simone Frutiger (to K.S.).
Author information
Authors and Affiliations
Contributions
S.W. designed the study, wrote the protocol, acquired funding, supervised the data acquisition and edited the manuscript. K.S. acquired funding, performed data acquisition, analyzed data and edited the manuscript. N.G. analyzed data and wrote the first draft of the manuscript. All authors discussed findings and edited the final manuscript.
Corresponding author
Ethics declarations
Competing interests
S.W. received honoraria from Janssen, Lundbeck, Mepha and Neurolite. K.S. received honoraria from Janssen, Lundbeck, Mepha, and Sunovion. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gangl, N., Conring, F., Federspiel, A. et al. Resting-state perfusion in motor and fronto-limbic areas is linked to diminished expression of emotion and speech in schizophrenia. Schizophr 9, 51 (2023). https://doi.org/10.1038/s41537-023-00384-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-023-00384-7