Abstract
A diagnosis of schizophrenia is associated with a heterogeneous psychopathology including positive and negative symptoms. The disconnection hypothesis, an early pathophysiological framework conceptualizes the diversity of symptoms as a result of disconnections in neural networks. In line with this hypothesis, previous neuroimaging studies of patients with schizophrenia reported alterations within the default mode network (DMN), the most prominent network at rest. The aim of the present study was to investigate the functional connectivity during rest in patients with schizophrenia and with healthy individuals and explore whether observed functional alterations are related to the psychopathology of patients. Therefore, functional magnetic resonance images at rest were recorded of 35 patients with schizophrenia and 41 healthy individuals. Independent component analysis (ICA) was used to extract resting state networks. Comparing ICA results between groups indicated alterations only within the network of the DMN. More explicitly, reduced connectivity in the precuneus was observed in patients with schizophrenia compared to healthy controls. Connectivity in this area was negatively correlated with the severity of negative symptoms, more specifically with the domain of apathy. Taken together, the current results provide further evidence for a role DMN alterations might play in schizophrenia and especially in negative symptoms such as apathy.
Similar content being viewed by others
Introduction
The diagnostic category of schizophrenia summarizes heterogeneous symptoms that range from so-called positive symptoms, which refer to an excess of function with prominent examples such as hallucinations and delusions, to so-called negative symptoms, which refer to a diminishment of function such as anhedonia, affective blunting, and apathy1. Furthermore, a key feature of schizophrenia is an alteration in the subjective experience of a continuous sense of self, which has been argued to account for both positive and negative symptoms2. Regarding the pathophysiology of schizophrenia, an early and influential hypothesis proposes that core symptoms of schizophrenia can be regarded as dysfunctional interactions between different brain regions rather than pathological changes within localized areas3,4. Furthermore, it has been suggested that these alterations in the connectivity of neural circuits can account for the heterogeneity of symptoms that are associated with a diagnosis of schizophrenia as they are thought to affect the coordination of mental processes5.
Interactions in the brain can be described by means of complex networks. A neural network, when applied to functional magnetic resonance imaging (fMRI), is composed of cortical areas that are functionally connected with each other. Functional connections describe temporal correlations of distributed neural areas. Brain networks are commonly computed on data of individuals who are laying without a specific task instruction in an MRI scanner, a so-called resting-state (rs-fMRI) where the blood-oxygen-level-dependent (BOLD) contrast is measured. Brain networks at rest represent intrinsic or “default” brain activity and have been argued to be of fundamental importance for the understanding of the human brain6. Although fMRI recordings during the performance of a cognitive task (task-fMRI) are widely used and accepted as a valid tool to investigate neural correlates of specific behaviours, the energy consumption during a task is associated with an increase of less than 5% from the brain’s baseline energy consumption7 - indicating that the brain is constantly active at rest. Importantly, task and resting state fMRI can be understood as complementary rather than opposing modalities that are focusing on different theoretical and technical aspects: task-fMRI investigates cortical activation sites and rs-fMRI focuses on temporal aspects and correlations among sites to build brain networks. Rs-fMRI may be a promising method to study the pathophysiology of schizophrenia as it is comparatively easy to acquire in a clinical population, it is not affected by differences in task performance that might potentially confound group fMRI analyses8, and it has been related to a fundamental aspect of human experience, the sense of self9. Previous fMRI studies that investigated functional connectivity during rest in patients diagnosed with schizophrenia report altered neural connectivity10,11,12. However, the results indicate both increases and decreases in connectivity which might be explained by the various analysis techniques that were used.
Analysis techniques that are used to calculate functional connectivity (FC) during rest employ measures such as Pearson’s correlation coefficient, wavelet correlation, mutual information and independent component analysis (ICA) to calculate connectivity between brain areas. For most of these analysis brain areas can either be selected by using all voxels or choosing regions of interest (ROIs) from pre-defined templates. The most popular method is called seed functional connectivity (SeedFC)13, where ROIs and voxels are considered: typically a ROI (seed) is chosen and the connectivity (using Pearson’s correlation) is calculated between the seed and all voxels in the brain. Brain networks can also be extracted using ICA, which, as opposed to the methods mentioned above, does not rely on parcellation nor on the choice of a seed. ICA is a reliable method borrowed from engineering to extract the brain’s resting state networks. This method blindly recovers source signals from a mixture of sources14. One advantage of this method is that it is data-driven and requires no prior assumptions, as is the case for seed-based functional connectivity13. Therefore, we used ICA in the present study to investigate differences in the functional connectivity during rest in patients with schizophrenia and matched healthy individuals. ICA can be illustrated by means of the classic cocktail party problem: microphone recordings that contain simultaneous conversations of people can be used to recover individual voices of people by employing the method of ICA. The same principle can be applied to brain signals in rsfMRI – ICA decomposes the brain’s activity into multiple sources (components). Each component (source) that is retrieved by ICA is a spatial grouping of voxels with temporally coherent activity. Depending on the spatial grouping of voxels, the components are associated with sources that are either related to brain activity or to noise such as movement, blinking, breathing, and heartbeat. The activity-related sources that can be retrieved with ICA “closely resemble discrete cortical functional networks”15. These sources, called resting state networks (RSN), comprise of: default mode (DMN), basal ganglia, auditory, visuospatial, sensory-motor, salience, executive control, language and visual networks. The DMN became the most studied RSN comprising key cortical regions such as the posterior cingulate cortex, precuneus, medial prefrontal cortex, hippocampus, insula, and inferior parietal cortices. The term “default mode” has been coined based on the observation that these brain region exhibit increased and coherent neural activation during resting-state conditions compared to conditions requiring an external attention focus. Therefore it is conceptualized as the baseline of brain functioning at rest.
Rs-fMRI studies employing different methodological approaches have shown various functional alterations in this DMN in patients diagnosed with schizophrenia8,11,16. Studies that applied the data-driven method of ICA to resting-state fMRI data of patients diagnosed with schizophrenia and healthy controls reported mixed functional connectivity results within the spatial maps of the DMN including decreases17,18, increases19, both increases and decreases20,21,22, and no differences23,24,25 between the groups.
Based on these inconclusive results, the aim of the present study was to further investigate the functional connectivity in resting-state networks using the method of ICA in a larger group of patients diagnosed with schizophrenia and of matched healthy controls than included in previous studies. Considering that ICA is a data-driven method that allows us to uncover various resting state networks, and previous ICA studies observed various alterations in the DMN of patients with schizophrenia, we hypothesized connectivity alterations within the DMN. Furthermore, we set out to explore whether these alterations are related to clinical symptoms of patients.
Methods
Participants
In total, 76 participants were included in the reported analysis, of which 41 were healthy individuals and of which 35 individuals met the criteria for a diagnosis of schizophrenia following the International Classification for Diseases and Related Health Problems (ICD-10). Patients diagnosed with schizophrenia were recruited at St. Hedwig Hospital, Department for Psychiatry and Psychotherapy of the Charité-Universitätsmedizin Berlin (Germany). Healthy individuals were recruited using online advertisements and flyers and did not meet the criteria for any psychiatric disorder based on information acquired with the Mini International Neuropsychiatric Interview (MINI)26 and were not in current or past psychotherapy of an ongoing mental health-related problem. MRI exclusion criteria such as claustrophobia, neurological disorders and metallic implants applied to all participants. Healthy individuals matched the group of patients in terms of age, sex, handedness and level of education (Table 1). Handedness was acquired with Edinburgh Handedness Inventory27 (n = 75), cognitive functioning was tested using the Brief Assessment of Cognition in Schizophrenia28 (n = 65) and verbal intelligence with a German Vocabulary Test29 (n = 72). Supplemental information provides details regarding the medication of patients. All procedures of the study were approved by the ethics committee of the Charité-Universitätsmedizin Berlin. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Assessment of psychopathology
Trained clinicians rated the severity of symptoms with the Scale for Assessment of Negative Symptoms (SANS)30 and the Scale for Assessment of Positive Symptoms (SAPS)31. Table 1 includes details of patients’ psychopathology. The SANS assesses negative symptoms within the domains of affective blunting, alogia, avolition-apathy, anhedonia-asociality, attentional impairment and the SAPS assesses positive symptoms in the domains of hallucinations, delusions, bizarre behavior, and positive formal thought disorder. Both scales rate severity of symptoms on a scale from 0 (absent) to 5 (severe). For both scales a composite score of all items was computed. Additionally, both scales contain a global assessment item of each subdomain.
MRI data acquisition
Images were collected on a Siemens Tim Trio 3 T scanner (Erlangen, Germany) using a 12-channel head coil. Structural images were obtained using a T1-weighted magnetization prepared gradient-echo sequence (MPRAGE) based on the ADNI protocol (TR = 2500 ms; TE = 4.77 ms; TI = 1100 ms, acquisition matrix = 256 × 256 × 176; flip angle = 7°; 1 × 1 × 1 mm3 voxel size). Whole brain functional resting state images during 5 minutes were collected using a T2*-weighted EPI sequence sensitive to BOLD contrast (TR = 2000 ms, TE = 30 ms, image matrix = 64 × 64, FOV = 216 mm, flip angle = 80°, slice thickness = 3.0 mm, distance factor = 20%, voxel size 3 × 3 × 3 mm3, 36 axial slices). Before resting state data acquisition was started, participants were in the scanner for about 10 minutes, during which a localizer and anatomical images were acquired so that subjects could get used to the scanner noise. During resting state data acquisition, participants were asked to close their eyes and relax.
Preprocessing of resting state data
To ensure for steady-state longitudinal magnetization, the first 5 images were discarded. The acquired data was corrected for slice timing and realigned. Structural individual T1 images were coregistered to functional images and segmented into gray matter, white matter, and cerebrospinal fluid. Data was then spatially normalized to the MNI template and spatially smoothed with a 6-mm FWHM to improve signal-to-noise ratio. All steps of data preprocessing were performed using SPM12. In addition, to control for motion, we used the voxel-specific mean frame-wise displacement (FD)32. FD values were below the default threshold of 0.5 for control and patient group (0.15 ± 0.02 and 0.17 ± 0.02, t-test p = 0.42).
Independent component analysis (ICA)
ICA is a data-driven analysis tool in which source signals are blindly recovered14 from mixtures of sources. ICA was calculated using GIFT software (http://icatb.sourceforge.net/)33 in Matlab 2012b using Infomax algorithm to estimate independent sources. ICA was run 20 times and the results were clustered by GIFT toolbox ICASSO min cluster size of 16 and max of 20 (number of runs) and RandInit and Bootstrap were selected. Preprocessed data from all subjects in both groups were used. The optimal number of spatially independent resting-state networks (N) to be extracted was estimated by the software (N = 21; see Supplemental Material Fig. S1). Only components with ICASO stability index Iq of >0.9 were considered and the related spatial maps. The networks were identified using predefined templates in GIFT and a-posteriori by experts C.G.F and S.K. Among the ICA components, the default mode network was identified and taken to the second level analysis in SPM12 using a mask for individual networks provided by http://findlab.stanford.edu/functional_ROIs.html. To make use of the full potentiality of the method, we conducted an exploratory analysis selecting the following networks that were concomitantly extracted by ICA (Fig. S1 in Supplemental Material): basal ganglia, visual, salience, auditory, executive control, and visuospatial.
Differences between groups were calculated using a two-sample t-test (p = 0.001 uncorrected), significant threshold was set to p < 0.05 corrected for multiple comparison using family wise error (FWE) at cluster-level. Mean FD32, gender and age were used as covariates. In those clusters where we found significant group differences, we extracted the mean absolute ICA values (spatial maps) for each subject for a posteriori correlation analysis of functional connectivity with psychopathology.
Correlation with psychopathology
To investigate whether these differences in resting state networks between healthy individuals and patients with schizophrenia were related to the psychopathology of patients, Spearman’s correlation coefficients between resting state network connectivity and severity of symptoms were calculated. The resting state functional connectivity was extracted from the cluster in the DMN in which we found group differences: for each subject we extracted the mean absolute ICA value (spatial maps). In a first step, we correlated the functional connectivity in the DMN cluster with the composite scores of the SANS and SAPS. In case of a significant correlation, we conducted further exploratory correlations with the global ratings of the associated symptom domains. Statistical analysis was performed using SPSS 22. For the correlation with the SAPS and SANS composite scores, a Bonferroni adjusted significance level of 0.025 (0.05/2) was applied. For the correlations with subdomains we used a Bonferroni corrected alpha value of 0.0125 (0.05/4) for the SAPS and of 0.01 (0.05/5) for the SANS.
Results
Independent component analysis (ICA)
Default mode network analysis
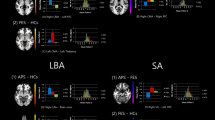
From 21 components chosen automatically in GIFT, the DMN was identified by experts C.G.F and S.K and taken to 2nd level analysis in SPM 12 (p < 0.001 uncorrected; significant threshold was set to p < 0.05 corrected for multiple comparison (FWE at cluster-level) using masks for individual networks provided by http://findlab.stanford.edu/functional_ROIs.html. The results indicated a significant decrease in the connectivity within the precuneus in patients with schizophrenia compared to healthy individuals (MNI coordinates = 4–60 36, T = 4.23, ppeak-level FWE corrected = 0.037; cluster size (in voxels) = 35; Fig. 1A).
Group differences between patients with schizophrenia and healthy individuals in the DMN. (A) Group comparison revealed decreased connectivity in the precuneus, as part of the DMN in patients with schizophrenia. (B) Functional connectivity during rest in the precuneus was significantly related to the severity of negative symptoms (assessed with the SANS) and more specifically with the severity of symptoms regarding avolition-apathy.
Exploratory analysis of the remaining resting state network
Besides the DMN, ICA method allows to uncover other resting state networks. In order to explore the full potentiality of ICA, we performed further exploratory analysis in additional networks extracted from our dataset. From the total of 21 components automatically estimated, additionally to the DMN, we identified, Basal Ganglia, Visual, Salience, Auditory, Executive control and Visuospatial networks (see supplements Fig. S1). Following the same procedure for the DMN, the networks were taken to the second level analysis in SPM12 using masks for individual networks provided by http://findlab.stanford.edu/functional_ROIs.html. No significant group differences were found.
Correlational analysis with psychopathology
In order to investigate whether the functional connectivity in the precuneus cluster in the DMN, were related to psychopathology of patients with schizophrenia, absolute ICA values (spatial maps) of the precuneus cluster were extracted and correlated with the composite score of the SAPS (r(34) = 0.106, p = 0.549) and of the SANS (r(35) = −0.398, p = 0.018, Fig. 1B). Based on the significant relationship between negative symptoms of the SANS and functional connectivity of the precuneus, we explored in a next step whether a specific domain of negative symptoms was associated with the functional connectivity in the precuneus. We observed a significant negative relationship between the functional connectivity of the precuneus in the DMN and avolition-apathy (apathy: r(35) = −0.504, p = 0.002, Fig. 1B) but not any of the other domains (anhedonia r(35) = −0.314, p = 0.066, affective blunting r(35) = −0.165, p = 0.343; alogie r(35) = −0.235, p = 0.175; attention r(35) = 0.239, p = 0.167). Please see Supplemental Material for additional analysis controlling for the influence of age, illness duration, sex, and medication.
In addition to the SANS domain of apathy that assesses behavioural aspects of apathy such as “grooming and hygiene”, “impersistence at school or work”, “physical anergia”, we calculated a second and multidimensional apathy score to further explore the association between apathy and precuneus. Following the suggestion of Bortolon and colleagues34, we built a multidimensional apathy score that additionally includes the SANS items “recreational interests and activities” and “affective nonresponsivity” to account for cognitive and emotional aspects of apathy. That multidimensional apathy score was also significantly correlated with the precuneus (r(35) = −0.469, p = 0.004).”
Discussion
The DMN network can be considered the baseline of brain processing at rest6,35 and is assumed to play a crucial role in schizophrenia8,10,11. In the presented study, the DMN was extracted from a group of patients diagnosed with schizophrenia and matched healthy individuals using ICA, a powerful data-driven method to infer connectivity that does not require an a priori definition of regions of interest. In addition, an exploratory analysis was conducted with all remaining resting state networks concomitantly extracted by ICA. Significant group differences were found solely in the DMN. The group comparison revealed significantly reduced functional connectivity in the DMN in patients diagnosed with schizophrenia. This result is consistent with two previous studies that employed the same ICA method to investigate rs-fMRI spatial maps and reported decreased functional connectivity within the DMN in patients with schizophrenia17,18 and thus supports the dysconnection hypothesis3,4. The present result of decreased connectivity within the DMN also supplements the inconsistent literature of previous ICA studies that observed both decreases and increases20,21,22, only increases19 as well as no differences in the spatial maps of the DMN at all23,24,25. In the context of previous inconclusive findings we would like to emphasize that we included more patients in the reported analysis (N = 35) than previous rs-fMRI studies employing ICA. More specifically, we observed reduced functional connectivity in the precuneus in patients with schizophrenia. This is in line with a previous ICA study where patients with schizophrenia also exhibited decreased connectivity during rest in the precuneus within the DMN as compared to healthy individuals20. However, another rs-fMRI ICA study reported both; increased connectivity in the right precuneus and decreased connectivity in the left precuneus but in a smaller group of patients diagnosed with schizophrenia21. Interestingly, the precuneus functions as a central hub within the DMN36. Furthermore, we observed that alterations in the functional connectivity of the precuneus in the DMN were negatively related to the severity of negative symptoms. More specifically, we found the avolition-apathy domain to be negatively related to precuneus connectivity in patients. Apathy is a multidimensional syndrome that presents itself as the most common negative symptom of schizophrenia34 and is also present in other neuropsychiatric diseases and in Alzheimer’s disease37,38. Apathy is described as a disorder of motivation with reduced or loss of goal-directed behavior, goal-directed cognitive processes, and emotion37,38. Whereas Andreasen30 refers to apathy as a decline in energy and drive and thus mainly covers behavioral aspects of apathy in the SANS assessment, Bortolon and colleagues34 emphasize the multidimensional aspects of apathy. Following the proposal of these authors34 as well as the diagnostic domains proposed by Robert and colleagues38, we calculated in addition to the SANS avolition-apathy score a second apathy score that allows a multidimensional assessment of apathy (see methods). Of note, also the multidimensional apathy score was negatively correlated with the functional connectivity in the precuneus during rest-state further supporting the observed relationship. In sum, the present findings show that patients diagnosed with schizophrenia exhibited reduced functional connectivity in the precuneus of the DMN and that this dysconnectivity was more pronounced in patients with more severe symptoms of apathy, a so-called negative symptom of schizophrenia.
To our knowledge, we are the first to observe a relationship between reduced functional connectivity in the precuneus and apathy. The precuneus has repeatedly been observed to play a central role in remembering events of one’s personal past, referred to as autobiographical memory, as part of the episodic memory system that is linked to the storage and recollection of events that contain a strong self-refs. 39,40. However, the precuneus is not only involved in remembering one’s past but also in imagining one’s future41. Also during episodes of rest when the precuneus becomes activated as a functional hub of the DMN, the human mind engages in self-generated thoughts that relate to past and future events42,43. On a behavioral level previous studies showed impairments in autobiographical memory in patients diagnosed with schizophrenia44,45,46 as well as in generating personal and specific future events47,48,49. Especially interesting in the light of the current result is the finding that deficits in imagining pleasant future events were found to be linked to symptoms of apathy in individuals with schizophrenia49. Raffard and colleagues49 thus argue that impairments in the capacity to generate future projection of one’s behavior, especially in preferable future situations, might underlie partially the reduced motivation to engage in goal-directed behavior – as is observed in apathy. In this study we observe that the precuneus that is commonly engaged in future-oriented thoughts shows disconnectivity in patients diagnosed with schizophrenia; and that this dysconnectivity is related to the severity of apathy that is defined as a reduction of goal-directed behavior. We therefore argue that our finding adds support from a neuroscientific perspective to the notion that impairments in future projections might partly underlie motivational decline to engage in goal-directed behavior49.
However, it is not possible to draw causal inferences based on the reported result as the directionality of this relationship still remains unclear. It is also conceivable that the impoverished life circumstances of patients might reduce their motivation to engage in future simulations48 or that diminished goal-directed behavior and cognition, which are characteristic for apathy, might diminish future-oriented thoughts49, which are potentially associated with the observed precuneus dysconnectivity during resting-state. Future studies are needed to further investigate the direction of this relationship.
Limitations of the study
A first limitation concerns the fact that 31 of the 35 patients were taking antipsychotic medication. As antipsychotic treatment was shown to affect functional connectivity in patients with schizophrenia50, we addressed this potential confound by calculating and controlling the performed correlation analysis for Chlorpromazine-equivalent (CPZ)51 (see supplements). Despite the fact that the results remained significant when controlling for CPZ, it would be beneficial for future studies to include antipsychotic-naive patients to exclude medication as a potential confound. A second limitation is that post-hoc correlational analysis with different psychopathology scales might have increased Type I error rates. Therefore, we applied Bonferroni correction for all correlational analysis. A further limitation is that we cannot infer a causal direction of the observed DMN dysconnectivity results, emphasizing the need of future longitudinal studies that investigate functional connectivity during rest over the course of illness. Finally, the number of components that is chosen in ICA is an arbitrary parameter. To overcome this issue, before running ICA, the optimal number of components were automatically identified by GIFT toolbox.
Conclusion
The results of the present study indicate altered resting-state functional connectivity in the DMN in patients diagnosed with schizophrenia compared to matched healthy individuals. This result adds further empirical evidence for early and influential theories, suggesting neural network disconnections account for symptoms associated with the diagnosis of schizophrenia4,5, and also complements previous studies reporting DMN alterations in schizophrenia. More specifically, we observed that reduced connectivity in the precuneus of the DMN was related to the severity of negative symptoms, more explicitly to the domain of apathy. In summary, the current findings emphasize the crucial role alterations in the DMN might play in schizophrenia and especially in a common negative symptom, namely apathy.
Data availability
The data cannot be stored in public repository as it was not part of the ethics statement and therefore the participants were not informed that the data would be made public. For more information about how to obtain the data please contact the authors.
References
Andreasen, N. C. Positive vs. negative schizophrenia: A critical evaluation. Schizophr. Bull. 11, 380–389 (1985).
Sass, L. A. & Parnas, J. Schizophrenia, Consciousness and the Self. Schizophr. Bull. 29, 427–444 (2003).
Friston, K., Brown, H. R., Siemerkus, J. & Stephan, K. E. The dysconnection hypothesis (2016). Schizophr. Res. 176(2–3), 83–94 (2016).
Friston, K. J. & Frith, C. D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 3, 89–97 (1995).
Andreasen, N. C., Paradiso, S. & O’Leary, D. S. "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 24, 203–18 (1998).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA 98, 676–682 (2001).
Raichle, M. E. & Mintun, M. A. Brain Work And Brain Imaging. Annu. Rev. Neurosci. 29, 449–476 (2006).
Whitfield-Gabrieli, S. & Ford, J. M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 8, 49–76 (2012).
Gusnard, D. A. & Raichle, M. E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2, 685–694 (2001).
Calhoun, V. D., Eichele, T. & Pearlson, G. Functional brain networks in schizophrenia: A review. Front. Hum. Neurosci. 3, 17 (2009).
Karbasforoushan, H. & Woodward, N. D. Resting-State Networks in Schizophrenia. Curr. Top. Med. Chem. 12, 2404–14 (2012).
Yu, Q., Sui, J., Kiehl, K. A., Pearlson, G. & Calhoun, V. D. State-related functional integration and functional segregation brain networks in schizophrenia. Schizophr. Res. 150(2–3), 450–458 (2013).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 (2007).
McKeown, M. J., Hansen, L. K. & Sejnowsk, T. J. Independent component analysis of functional MRI: what is signal and what is noise? Curr. Opin. Neurobiol. 13, 620–9 (2003).
Beckmann, C. F., DeLuca, M., Devlin, J. T. & Smith, S. M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 360, 1001–1013 (2005).
Hu, M. L. et al. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci. Bull. 33, 73–84 (2017).
Orliac, F. et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr. Res. 148, 74–80 (2013).
Rotarska-Jagiela, A. et al. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 117, 21–30 (2010).
Galindo, L. et al. Default mode network aberrant connectivity associated with neurological soft signs in schizophrenia patients and unaffected relatives. Front. Psychiatry 8, 298 (2018).
Li, P. et al. Altered Brain Network Connectivity as a Potential Endophenotype of Schizophrenia. Sci. Rep. 7, 5483 (2017).
Mannell, M. V. et al. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum. Brain Mapp. 31, 424–437 (2010).
Ongür, D. et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 183, 59–68 (2010).
Chahine, G., Richter, A., Wolter, S., Goya-Maldonado, R. & Gruber, O. Disruptions in the left frontoparietal network underlie resting state endophenotypic markers in Schizophrenia. Hum. Brain Mapp. 38, 1741–1750 (2017).
Camchong, J. et al. Altered functional and anatomical connectivity in schizophrenia. Schizophr. Bull. 37, 640–650 (2011).
Wolf, N. D. et al. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J. Psychiatry Neurosci. 36, 366–74 (2011).
Ackenheil, M., Stotz-Ingenlath, G., Dietz-Bauer, R. & Vossen, A. MINI Mini International Neuropsychiatric Interview, German Version 5.0.0, DSM IV. Psychiatr. Univ. München, Ger., https://doi.org/10.1016/0968-0004(94)90058-2 (1999).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, https://doi.org/10.1016/0028-3932(71)90067-4 (1971).
Keefe, R. S. E. et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr. Res. 102, 108–115 (2008).
Schmidt, K. & Metzler, P. Wortschatztest. Weinheim:Beltz (1992).
Andreasen, N. C. Scale for the Assessment of Negative Symptoms (SANS). Br. J. Psychiatry 7, 49–58 (1989).
Andreasen, N. The Scale for the Assessment of Positive Symptoms (SAPS). Univ. Iowa, https://doi.org/10.4236/oalib.1100693 (1984).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–54 (2012).
Calhoun, V. D. Group ICA/IVA of fMRI Toolbox (GIFT) Manual. (2004).
Bortolon, C., Macgregor, A., Capdevielle, D. & Raffard, S. Apathy in schizophrenia: A review of neuropsychological and neuroanatomical studies. Neuropsychologia 118(Part B), 22–33 (2018).
Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 100, 253–8 (2003).
Utevsky, A. V., Smith, D. V. & Huettel, S. A. Precuneus is a functional core of the default-mode network. J. Neurosci. 34, 932–40 (2014).
Mulin, E. et al. Diagnostic criteria for apathy in clinical practice. Int. J. Geriatr. Psychiatry 26, 158–165 (2011).
Robert, P. et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur. Psychiatry 24, 98–104 (2009).
Cabeza, R. & St Jacques, P. Functional neuroimaging of autobiographical memory. Trends Cogn. Sci. 11, 219–27 (2007).
Cavanna, A. E. & Trimble, M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129(Pt 3), 564–83 (2006).
Schacter, D. L., Addis, D. R. & Buckner, R. L. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 8, 657–661 (2007).
Andrews-Hanna, J. R., Smallwood, J. & Spreng, R. N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 1316, 29–52 (2014).
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R. & Buckner, R. L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 65, 550–62 (2010).
Danion, J. M. et al. Conscious recollection in autobiographical memory: An investigation in schizophrenia. Conscious. Cogn. 14, 535–47 (2005).
Riutort, M., Cuervo, C., Danion, J. M., Peretti, C. S. & Salamé, P. Reduced levels of specific autobiographical memories in schizophrenia. Psychiatry Res. 117, 35–45 (2003).
Wood, N., Brewin, C. R. & McLeod, H. J. Autobiographical memory deficits in schizophrenia. Cogn. Emot. 20, 536–547 (2006).
D’Argembeau, A., Raffard, S. & Van der Linden, M. Remembering the past and imagining the future in schizophrenia. J. Abnorm. Psychol. 117, 247–251 (2008).
de Oliveira, H., Cuervo-Lombard, C., Salamé, P. & Danion, J. M. Autonoetic awareness associated with the projection of the self into the future: An investigation in schizophrenia. Psychiatry Res. 169, 86–87 (2009).
Raffard, S., Esposito, F., Boulenger, J. P. & Van der Linden, M. Impaired ability to imagine future pleasant events is associated with apathy in schizophrenia. Psychiatry Res. 209, 393–400 (2013).
Lui, S. et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by ‘resting state’ functional magnetic resonance imaging. Arch. Gen. Psychiatry 67, 783–92 (2010).
Gardner, D. M., Murphy, A. L., O’Donnell, H., Centorrino, F. & Baldessarini, R. J. International consensus study of antipsychotic dosing. Am. J. Psychiatry 167, 686–93 (2010).
Acknowledgements
This work was supported by Evangelisches Studienwerk Villigst to L.K., the German Science Foundation (SFB 936/C7 to C.G.F. and S.K. and DFG KU 3322/1-1 to S.K.), the European Union (ERC-2016-StG-Self-Control-677804 to S.K.), and a Fellowship from the Jacobs Foundation (JRF 2016-2018 to S.K.).
Author information
Authors and Affiliations
Contributions
Data acquisition: L.K., J.B., L.S., P.G., M.F., N.S., C.M., S.K., J.G. Data analysis: C.G.F., L.K., S.K. Manuscript writing: C.G.F., L.K., S.K. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forlim, C.G., Klock, L., Bächle, J. et al. Reduced Resting-State Connectivity in the Precuneus is correlated with Apathy in Patients with Schizophrenia. Sci Rep 10, 2616 (2020). https://doi.org/10.1038/s41598-020-59393-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59393-6
This article is cited by
-
Resting-state global brain activity affects early β-amyloid accumulation in default mode network
Nature Communications (2023)
-
A significant, functional and replicable risk KTN1 variant block for schizophrenia
Scientific Reports (2023)
-
Effects of NMDA-receptor blockade by ketamine on mentalizing and its neural correlates in humans: a randomized control trial
Scientific Reports (2023)
-
Opposite effects of positive and negative symptoms on resting-state brain networks in schizophrenia
Communications Biology (2023)
-
Clinical anatomy of the precuneus and pathogenesis of the schizophrenia
Anatomical Science International (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.