Abstract
Hippocampal abnormalities are an established finding in the neuroimaging study of schizophrenia. However, no studies have examined the possibility of regional hippocampal abnormalities specific to deficit schizophrenia (DS) and associations with the unique symptoms of this schizophrenia subtype. This study compared 33 DS and 39 non-deficit schizophrenia (NDS) patients and 38 healthy subjects for hippocampal subfield volumetry. Clinical symptoms were assessed by PANSS, cognition by the neurocognitive battery on the day of the MRI scan. The automatic hippocampal segmentation were preprocesses use FreeSurfer 7.2.0. Unfortunately, the associations between neurocognitive scores and hippocampal subfield volumes in the DS group were not significant after the Bonferroni correction. Our results did not support a causal relationship between hippocampal subregional atrophy and cognitive deficits in DS.
Similar content being viewed by others
Introduction
Deficit schizophrenia (DS) is considered a distinct disease entity within the schizophrenia spectrum due to unique clinical manifestations compared to other entities (collectively termed non-deficit schizophrenia or NDS). Notably, DS is characterized by predominant negative symptoms from onset and during clinical stabilization1. Many studies have provided evidence for the long-term stability of defect syndrome and the reliability of deficit/non-deficit classification in schizophrenia2. There is also evidence that DS can be differentiated from NDS by greater cognitive impairment, poorer treatment response, and worse prognosis3. Several studies have reported that patients with DS perform worse on cognitive domains associated with negative symptoms4, including executive function5, cognitive flexibility6, and sustained attention7. However, a recent meta-analysis found that DS patients performed at lower levels than NDS patients in all major cognitive domains, and that deficit syndrome severity was strongly associated with cognitive impairment4. These neuropsychological and clinicopathological findings suggest that deficit schizophrenia may be a distinct disorder with unique underlying brain pathologies8,9.
It is well established that the hippocampus is essential for various forms of declarative (explicit) memory10, including verbal memory11. Numerous studies conducted over many decades have consistently found that hippocampal damage or atrophy impairs performance on declarative memory tasks, including among patients with schizophrenia12,13,14,15. The hippocampus is thought to play a crucial role in mediating attention16, navigating physical space17, semantic fluency18 and executive function19. A recent meta-analysis concluded that hippocampal volume is reduced even at disease onset as well as during the chronic stage13, and studies of first-episode patients have suggested that this early atrophy contributes to pathogenesis20,21. The left hippocampal volume was reduced in individuals in a clinically high-risk psychotic state22. The first-degree relatives of schizophrenic patients had smaller hippocampal volumes than healthy control subjects23. A recent multicenter neuroimaging study also found that the most obvious structural anomalies in patients with schizophrenia were within the hippocampus24. However, no studies have examined the possibility of regional hippocampal abnormalities specific to DS and associations with the unique symptoms of this schizophrenia subtype.
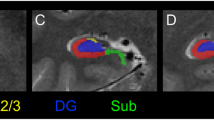
The hippocampus consists of distinct structurally and functionally distinct regions and subregions, including cornu ammonis (CA) fields 1 to 4 (CA1–CA4), the dentate gyrus (DG), and subiculum12,13,14. A recent systematic review of hippocampal neuroimaging studies concluded that the volumes of these hippocampal regions and subregions are differentially affected in schizophrenia, with CA1 most severely altered25. Schobel et al reported hypermetabolism in CA1 leading to atrophy in the early stages of schizophrenia26 and further studies on serotonin modulation of hippocampal functions suggested that the onset of schizophrenia symptoms is associated with hippocampal excitotoxicity triggered by stress-induced hyperactivity of serotonergic pacemaker cells in the dorsal raphe nucleus27,28. Consistent with distinct effects on hippocampal subregions during the disease course, Ho and colleagues found progressive disease-related volume loss extending initially from CA1 to all other subregions11. Hippocampal substructure volume atrophy has also been confirmed in first-episode drug-naive psychotic patients; moreover, the same study found a non-linear relationship between dentate gyrus/CA4 volume changes and antipsychotic dose after 12 weeks of risperidone or aripiprazole treatment29. Further, more recent studies have found significant associations of cognitive composite and declarative memory scores with the volumes of multiple hippocampal substructures in schizophrenia25,30.
Based on these findings, we hypothesized that subfield analysis would yield better predictors of symptom progression in DS compared to total hippocampal volume, and that DS patients would present with greater regional volume reductions compared to NDS patients, leading to more severe cognitive impairment.
Materials and methods
Participants
This cross-sectional comparative study included 72 patients with schizophrenia (33 DS and 39 NDS patients) and 38 healthy subjects from the Department of Psychiatric Rehabilitation, Wutaishan Hospital, Yangzhou City, Jiangsu Province, China. All patients were inpatients and right-handed males between 20 and 65 years of age diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria and the DSM-IV Structured Clinical Interview (SCID-I)31. Each patient was on antipsychotic medications and the regimen had not changed for at least 12 months prior to the study. In addition to study candidates with recent changes in medication, we excluded candidates with neurological diseases, previous head trauma, mental retardation, past or current history of alcohol or illicit drug abuse/dependence, and history of electroconvulsive therapy. Schizophrenic patients who meet the above criteria were jointly assessed as DS or NDS by two attending physicians using the Chinese version of the Deficiency Syndrome Scale (SDS)32. The diagnostic criteria for deficit schizophrenia are: 1. Symptomatologic criteria: six negative symptom clusters are included: (1) restricted affect, (2) diminished emotional range, (3) poverty of speech, (4) curbing of interests, (5) diminished sense of purpose, and (6) diminished social drive. The above symptom clusters can be grouped into two factors33,34: the affective expression factor (1-3) and the lack of motivation factor (4-6); 2. Severity and course criteria: two or more of the above negative symptoms have been clinically significant and have persisted for more than 12 months, and also persist during the clinical stabilization period; 3. The above symptoms are primary or idiopathic, not secondary to depression, anxiety, drug side effects, psychotic symptoms, or mental retardation; 4. The DSM-IV diagnosis of schizophrenia is met. Where each negative symptom cluster is scored on a 5-point scale of 0-4 (0: normal; 1: possible problem, but no significant abnormality, or there are some unusual manifestations, but they are still within the normal range of variation; 2 or 3 points: clinically, the symptom group of the patient is obviously abnormal; 4 points: the patient’s symptom group is extremely serious). Clinical significance is defined as the score of the symptom group is greater than or equal to 2 points. Those that do not meet this standard are NDS.
Healthy controls were matched for age, handedness, and education, and candidates with a history of cognitive, psychiatric, or physical co-morbidities were excluded.
The study was approved by the Institutional Ethics Committee for clinical research (approval No. 2018-056) and all participants provided written informed consent.
Clinical assessments
Clinical symptoms were assessed by a trained research psychiatrist using the Positive and Negative Syndrome Scale (PANSS) on the day of the MRI scan.
Neurocognitive assessments
We conducted the neurocognitive battery on all subjects, including the Digit Cancellation Test (DCT), Animal Naming Test (ANT), Controlled Oral Word Association Test (COWAT), Spatial Span Test (SS), two-part Trail Making Task (TMT-A and TMT-B), Block Design Test (BDT) and the Stroop Color-word Test (SCWT). All participants performed practice tests to ensure that they understood the instructions of the examiner.
MRI acquisition
All magnetic resonance images were acquired on a 3.0-T MRI scanner (GE HDx) using a T1-weighted high-resolution three-dimensional brain volume imaging (3D-BRAVO) sequence with the following parameters: TR = 11.94 ms; TE = 5.044 ms; flip angle = 15°; field of view = 240 × 240 mm; matrix size = 256 × 256; slice thickness = 1 mm without gaps; voxel size = 1 × 1 × 1 mm3; number of slices = 172. During the scans, participants were instructed to remain awake with their eyes closed, to lie still, and not to focus on anything specific. All reconstructed images are visually inspected by trained researchers, and the images filtered through visual inspection will enter the FreeSurfer processing.
Imaging data preprocessing
All images were preprocesses use FreeSurfer 7.2.0, which is available for free online download (http://surfer.nmr.mgh.harvard.edu/).The hippocampus was automatically segmented into 12 distinct regions: hippocampal tail, subiculum, CA1, hippocampal fissure, presubiculum, parasubiculum, molecular layer hippocampus (HP), granule cell and molecular layer of the dentate gyrus (GC-ML-DG), CA3, CA4, fimbria, and hippocampus-amygdala-transition-area (HATA). The partitioning algorithm is based on a computational atlas constructed using the ex vivo MRI data of the medial temporal lobe from cadaveric brains. The results of the program run have no error.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Participant demographics, clinical characteristics, and neurocognitive assessment scores are presented as mean ± standard deviation (SD). Means of demographic and clinical characteristics were compared among DS, NDS, and HC groups by one-way analysis of variance (ANOVA) and post-hoc pairwise comparison analyses, while differences in psychiatric symptoms were compared between DS and NDS groups by independent sample t tests. Hippocampal subregion volumes were compared among groups using analysis of covariance (ANCOVA) controlling for age, education, eTIV (estimated Total Intracranial Volume), and body mass index (BMI), and adjusted for multiple comparisons using the Bonferroni correction. Cognition except COWAT scores were compared among groups by ANCOVA controlling for age, education, eTIV, and BMI, with adjustment for multiple comparisons using Bonferroni correction, while COWAT results were compared by one-way ANOVA with Bonferroni correction. Datasets were first examined for homogeneity of variance using Welch’s test. A separate multiple linear regression model was constructed to investigate whether hippocampal subregion volumes were associated with cognitive tests scores. The P-value was set to 0.05. Bonferroni correction was applied to adjust for multiple comparisons. The Bonferroni-corrected P value for each PANSS score is the initial P value ×4. The Bonferroni-corrected P value for each hippocampal subfield volume is the initial P value ×44. The Bonferroni-corrected P value for each cognitive test score is the initial P value ×11. We will not do Bonferroni correction when the initial P value >0.05.
Results
Demographic data in patients and controls
The demographic and clinical characteristics of all 110 participants are summarized in Table 1. There were significant differences in years of education and BMI among DS, NDS, and HC groups, and post hoc analysis indicated that years of formal education was lowest in the DS group and that BMI was lowest in the HC group. Age at onset and CPZ equivalent dose did not differ significantly between the DS and NDS groups, but disease duration was significantly longer in the DS group. Total PANSS score was significantly higher in the DS group than the NDS group, indicating more severe clinical symptoms. In addition, negative syndrome and general psychopathological syndrome subscores of the PANSS were significantly higher in the DS group than the NDS group, while positive syndrome subscores did not differ between these groups.
Hippocampal subfield volumetry
The volumes of 8 subregions in the left hippocampus (left hippocampal GC-ML-DG body, left hippocampal CA1 head, left hippocampal molecular layer head, left hippocampal molecular layer body, left hippocampal fimbria, left hippocampal head, left hippocampal body, left hippocampus) and 12 subregions in the right hippocampus (right hippocampal GC-ML-DG head, right hippocampal GC-ML-DG body, right hippocampal CA4 head, right hippocampal CA4 body, right hippocampal CA1 head, right hippocampal CA1 body, right hippocampal molecular layer head, right hippocampal molecular layer body,
right hippocampal fimbria, right hippocampal head, right hippocampal body, right hippocampus) were significantly reduced in schizophrenia patients (combined DS and NDS group) compared to HCs (all P < 05 by ANCOVA controlling for age, education, eTIV and BMI, and with Bonferroni correction for multiple comparisons). The post-hoc-comparisons found that the volumes of hippocampal substructure in DS and NDS groups were significantly smaller than that in the normal control group, but there was no significant difference between DS and NDS groups (Table 2).
Neuropsychological assessment
Schizophrenia patients performed significantly worse than HCs on all cognitive tests, and the DS group performed significantly worse than the NDS group (Table 3).
Associations of neurocognitive scores and hippocampal subfields volumes
In the DS group, multiple regression analysis controlling for age, education, and BMI revealed that smaller right hippocampal CA1 head volume (p = 0.004) was significantly associated with worse DCT performance, while smaller left hippocampal tail volume (p = 0.003) and smaller right hippocampal HATA volume (p = 0.021) were associated with worse category fluency scores. In addition, smaller right hippocampal molecular layer head volume was associated with worse Stroop-colors task performance (p = 0.016). Unfortunately, none of the above correlations were significant after the Bonferroni correction. In the HC group, there were no significant associations after Bonferroni correction.
Discussion
Morphometric analysis of structural magnetic resonance images revealed significantly reduced CA1, CA4, molecular layer, fimbria, and GC-ML-DG volumes in chronic schizophrenia patients compared to matched healthy controls. Unfortunately, in DS patients, atrophy of hippocampal subfield volumes was not associated with neurocognitive scores after the Bonferroni correction. Our results did not support a causal relationship between hippocampal subregional atrophy and cognitive deficits in DS.
This study identifies several regions of hippocampal atrophy that could serve as biomarkers of cognitive dysfunction in schizophrenia and as targets for future investigations on disease pathogenesis. Decreased hippocampal volume is a consistent finding in neuroimaging studies of patients with schizophrenia, although the cellular bases for these volume changes are still unclear. A meta-analysis of autopsy studies found that the volumes of multiple left hippocampal subregions were significantly reduced in schizophrenia patients and that these volume decreases were associated with reduced neuronal size but not density35, while no changes were found in the right hippocampus, in contrast to the present study. A study of individuals in a clinically high-risk psychotic state eventually progressing to schizophrenia suggested that left hippocampal volume is reduced before onset and continues to shrink thereafter22. Consistent with our findings, Buchanan et al.36 found that both schizophrenic subgroups had smaller hippocampal volumes than the normal subjects and there were no differences between the two schizophrenic subgroups in hippocampal volumes. Although there is strong evidence that regional hippocampal atrophy is associated with cognitive impairment in schizophrenics37, it is regrettable that we have not found a relationship between regional hippocampal atrophy and specific cognitive impairment in DS patients. The volumes of hippocampal substructure in the DS and NDS groups were significantly smaller than that in the normal control group. The volume of hippocampal substructure in the DS group showed a trend of decrease compared with that in the NDS group (0.06-0.08), but it did not reach statistical significance. The illness duration may significantly impact the magnitude of hippocampal volume deficits in chronic schizophrenia38,39. we will conduct a follow-up and longitudinal study to explore the effect of the disease course on the pathological changes of the hippocampus in DS.
Performance on all cognitive domains was significantly lower in schizophrenia patients than healthy controls, and the performance of DS patients was significantly worse than that of NDS patients. These findings are consistent with previous studies showing that DS patients performed worse than NDS patients in every neuropsychological measure and cognitive domain examined7,40. The evidence presented in this study supports the hypothesis that DS may be a distinct disease entity within the schizophrenia spectrum. It is still unclear, however, whether these cognitive deficits are pervasive or more severe within certain specific domains. In this regard, one recent study utilizing eye movement analysis suggested that the major deficits were related to attention41, while another suggested that DS patients had significantly impaired overall cognitive abilities, possibly associated with chronic neuroinflammation42. While the true extent of cognitive deficits in DS remains uncertain, the current study supports more pervasive deficits, at least in patients with a long (several decade) disease history.
This article is the first to report associations between regional hippocampal atrophy and specific cognitive impairments in DS patients. We investigated multiple cognitive functions including attention and executive functions. But it is well known that the hippocampus is essential for human declarative memory10. The hippocampus is involved in memory encoding and regulation of emotional behavior among other high-order behavioral processes43. Information about new experiences, stimulus exposure, and various associations is thought to be processed in the hippocampus and stored elsewhere in the brain, enabling people to consciously recall personal events (episodic memory) and facts (semantic memory)43. The major input–out neurons of the hippocampus are glutamatergic pyramidal cells that span all four CA subregions (CA1, CA2, CA3, CA4) and have distinct input and projection patterns within and outside the hippocampal formation44. The key input pathway is called the perforant pathway from the entorhinal cortex45, which transmits signals from other brain regions, including the neocortex, to various neuronal populations in the subiculum, CA1, CA3, and dentate gyrus46, while output fibers leave the hippocampus via the fornix through the alveus and fimbria47.
In principle, any or all of these circuits could be disrupted in schizophrenia, possibly through altered connectivity or deficient synaptic plasticity. Alternatively, global regulation of these circuits by dopaminergic and serotonergic inputs could be dysfunctional. We found that more pronounced CA1 atrophy was associated with worse performance on the DCT in the DS group, while there were no significant correlations between CA1 volume and cognitive function in the NDS or HC groups. In accord with these results, Alden and colleagues reported a correlation between hippocampal deformation and verbal working memory impairments in patients with schizophrenia48 while other have reported a correlation between poorer executive function and CA1 volume atrophy in DS patients40,42. The DCT assesses focused, sustained, and selective attention49, information processing speed, and executive function. Studies have shown that as an attention assessment tool, DCT is associated with prefrontal cognitive function50,51. Our findings suggest that CA1 atrophy in DS patients may disrupt the circuit between the hippocampus and frontal cortex.
Findings could be reinforced by larger samples, especially increasing the size of DS patients. The disadvantage of automatic hippocampal segmentation is the concern about the accuracy of mapping subfield boundaries; the advantage is that it is unbiased, less labor-intensive, and easier to reproduce. In addition, there is considerable heterogeneity in the different antipsychotic drugs used by patients. Subgroup and multivariate analyses of sufficient size are needed to hedge against these limitations. Growing preclinical52 and clinical53 evidence suggests that hippocampal pathology in schizophrenia is localized to the anterior hippocampus. The anterior/posterior gradient of the hippocampus is not discerned. This study attempts to restrict variance due to confounders including gender, illness duration, fluctuation of mental symptoms, and social environment. Future research should also consider out-patients, women, and longitudinal data. Because automatic segmentation of the hippocampus of schizophrenic patients using FreeSurfer may fail54, future research should include manual correction of automatic segmentation in case of failure. Some studies have found that cariprazine55 and roluperidone56 can treat negative symptoms. We will try to intervene in negative symptoms in future studies.
Conclussion
To date, few studies have investigated the pattern of hippocampal atrophy at the subregional level in DS patients. We found that the cognitive function of the HC, NDS, DS groups showed a horizontal step-wise decline, accompanied by a subregional level of hippocampal atrophy. This subregion-level volume shrinkage was associated with poor performance on tests of cognitive function. Studying hippocampal subregional atrophy patterns has important implications for gaining a deeper understanding of the pathophysiology of cognitive deficits in DS. An immediate clinical implication is the identification of subregional atrophy patterns in DS, thereby enabling clinicians to identify patients with first-episode schizophrenia who are at higher risk for cognitive impairment.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Matsumoto, A. K. et al. Deficit schizophrenia and its features are associated with PON1 Q192R genotypes and lowered paraoxonase 1 (PON1) enzymatic activity: effects on bacterial translocation. CNS Spectrums 26, 406–415 (2021).
Kirkpatrick, B., Mucci, A. & Galderisi, S. Primary, Enduring Negative Symptoms: An Update on Research. Schizophr Bull 43, 730–736 (2017).
Giordano, G. M. et al. Investigating the Relationship between White Matter Connectivity and Motivational Circuits in Subjects with Deficit Schizophrenia: A Diffusion Tensor Imaging (DTI) Study. J. Clin. Med. 11, 61 (2021).
Bora, E., Binnur Akdede, B. & Alptekin, K. Neurocognitive impairment in deficit and non-deficit schizophrenia: a meta-analysis. Psychol. Med. 47, 2401–2413 (2017).
Polgár, P. et al. Executive function in deficit schizophrenia: what do the dimensions of the Wisconsin Card Sorting Test tell us? Schizophr Res. 122, 85–93 (2010).
Réthelyi, J. M. et al. General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 262, 107–115 (2012).
Yu, M. et al. Neurocognitive Impairments in Deficit and Non-Deficit Schizophrenia and Their Relationships with Symptom Dimensions and Other Clinical Variables. PLoS One 10, e0138357–e0138357 (2015).
Kirkpatrick, B., Gürbüz Oflezer, Ö., Delice Arslan, M., Hack, G. & Fernandez-Egea, E. An Early Developmental Marker of Deficit versus Nondeficit Schizophrenia. Schizophr Bull 45, 1331–1335 (2019).
Alabaf, S., Kirkpatrick, B., Chen, S., Cardinal, R. N. & Fernandez-Egea, E. Early versus late risk factors for deficit and nondeficit schizophrenia. Rev. Psiquiatr. Salud. Ment. (Engl Ed), S1888-9891(21)00033-1 (2021).
Small, S. A., Schobel, S. A., Buxton, R. B., Witter, M. P. & Barnes, C. A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 12, 585–601 (2011).
Ho, N. F. et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol. Psychiatry 22, 142–152 (2017).
Tamminga, C. A., Stan, A. D. & Wagner, A. D. The hippocampal formation in schizophrenia. Am. J. Psychiatry 167, 1178–1193 (2010).
Adriano, F., Caltagirone, C. & Spalletta, G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist 18, 180–200 (2012).
Haukvik, U. K. et al. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol. Psychiatry 77, 581–588 (2015).
Ho, N. F. et al. Progressive Decline in Hippocampal CA1 Volume in Individuals at Ultra-High-Risk for Psychosis Who Do Not Remit: Findings from the Longitudinal Youth at Risk Study. Neuropsychopharmacology 42, 1361–1370 (2017).
Aly, M. & Turk-Browne, N. B. Attention Stabilizes Representations in the Human Hippocampus. Cereb Cortex 26, 783–796 (2016).
Montagrin, A., Saiote, C. & Schiller, D. The social hippocampus. Hippocampus 28, 672–679 (2018).
Glikmann-Johnston, Y., Oren, N., Hendler, T. & Shapira-Lichter, I. Distinct functional connectivity of the hippocampus during semantic and phonemic fluency. Neuropsychologia 69, 39–49 (2015).
Nguyen, T. V. et al. Sex-specific associations of testosterone with prefrontal-hippocampal development and executive function. Psychoneuroendocrinology 76, 206–217 (2017).
Hasan, A. et al. Hippocampal integrity and neurocognition in first-episode schizophrenia: a multidimensional study. World J. Biol. Psychiatry 15, 188–199 (2014).
Vargas, T. et al. Hippocampal Subregions Across the Psychosis Spectrum. Schizophr Bull 44, 1091–1099 (2018).
Sasabayashi, D. et al. Reduced Hippocampal Subfield Volume in Schizophrenia and Clinical High-Risk State for Psychosis. Front. Psychiatry 12, 642048 (2021).
Boos, H. B. M., Aleman, A., Cahn, W., Pol, H. H. & Kahn, R. S. Brain Volumes in Relatives of Patients With Schizophrenia: A Meta-analysis. Arch. Gen. Psychiatry 64, 297–304 (2007).
van Erp, T. G. et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 21, 585 (2016).
Haukvik, U. K., Tamnes, C. K., Söderman, E. & Agartz, I. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 104, 217–226 (2018).
Schobel, S. A. et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78, 81–93 (2013).
Eggers, A. E. An explanation of why schizophrenia begins with excitotoxic damage to the hippocampus. Med. Hypotheses 81, 1056–1058 (2013).
Bombardi C., et al. Chapter 3 - Serotonin modulation of hippocampal functions: From anatomy to neurotherapeutics. In: Progress in Brain Research (eds Di Giovanni G., De Deurwaerdere P.). (Elsevier, 2021).
Rhindress, K. et al. Hippocampal subregion volume changes associated with antipsychotic treatment in first-episode psychosis. Psychol. Med. 47, 1706–1718 (2017).
Yasuda, K. et al. Hippocampal Subfield Volumes and Cognitive Function in Schizophrenia and Mood Disorders. Neuropsychobiology 81, 204–214 (2022).
Michael B., Williams, Janet B. W., Spitzer, Robert L. User’s Guide For The Structured Clinical Interview For Dsm-5 Disorders - Clinician Version (scid-5-cv). User’s guide for the Structured clinical interview for DSM-III-R: (2015).
Wang, X., Yao, S., Kirkpatrick, B., Shi, C. & Yi, J. Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Psychiatry Res. 158, 195–205 (2008).
Kimhy, D., Yale, S., Goetz, R. R., McFarr, L. M. & Malaspina, D. The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophr Bull 32, 274–278 (2006).
Nakaya, M. & Ohmori, K. A two-factor structure for the Schedule for the Deficit Syndrome in schizophrenia. Psychiatr. Res. 158, 256–259 (2008).
Roeske, M. J., Konradi, C., Heckers, S. & Lewis, A. S. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: a systematic review and meta-analysis of postmortem studies. Mol. Psychiatry 26, 3524–3535 (2021).
Buchanan, R. W. et al. Structural abnormalities in deficit and nondeficit schizophrenia. Am. J. Psychiatry 150, 59–65 (1993).
Antoniades, M. et al. Verbal learning and hippocampal dysfunction in schizophrenia: A meta-analysis. Neurosci. Biobehav. Rev. 86, 166–175 (2018).
Velakoulis, D. et al. Hippocampal and Amygdala Volumes According to Psychosis Stage and Diagnosis: A Magnetic Resonance Imaging Study of Chronic Schizophrenia, First-Episode Psychosis, and Ultra–High-Risk Individuals. Arch. Gen. Psychiatr. 63, 139–149 (2006).
McHugo, M. et al. Hippocampal volume in early psychosis: a 2-year longitudinal study. Transl. Psychiatry 10, 306 (2020).
Tang, X. et al. Serum BDNF and GDNF in Chinese male patients with deficit schizophrenia and their relationships with neurocognitive dysfunction. BMC Psychiatry 19, 254–254 (2019).
Zhang, L. et al. Eye movement characteristics in male patients with deficit and non-deficit schizophrenia and their relationships with psychiatric symptoms and cognitive function. BMC Neurosci. 22, 70–70 (2021).
Pan, L.-H., Qian, M., Qu, W., Tang, Q. & Yan, Y. Serum C-Reactive Protein in Patients with Deficit Schizophrenia and the Relationship with Cognitive Function. Neuropsychiatr. Dis. Treat 16, 2891–2897 (2020).
Jeffery, K. J. The Hippocampus: From Memory, to Map, to Memory Map. Trends Neurosci. 41, 64–66 (2018).
Harris, K. M. et al. Dendritic Spine Density Scales with Microtubule Number in Rat Hippocampal Dendrites. Neuroscience 489, 84–97 (2022).
Fogwe L. A., Reddy V., Mesfin F. B. Neuroanatomy, Hippocampus. In: StatPearls). (StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022).
Chauhan P., Jethwa K., Rathawa A., Chauhan G., Mehra S. The Anatomy of the Hippocampus. In: Cerebral Ischemia (ed Pluta R.). Exon Publications Copyright: The Authors.; The authors confirm that the materials included in this chapter do not violate copyright laws. Where relevant, appropriate permissions have been obtained from the original copyright holder(s), and all original sources have been appropriately acknowledged or referenced. (2021).
AbuHasan Q., Reddy V., Siddiqui W. Neuroanatomy, Amygdala. In: StatPearls). (StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022).
Alden, E. C. et al. Shape features of working memory-related deep-brain regions differentiate high and low community functioning in schizophrenia. Schizophr Res. Cogn. 29, 100250–100250 (2022).
Takeshi, H., Kazuhito, Y., Yasuhiro, I., Mitsuhito, M. & Hidehiro, K. Reliability And Validity Of The Digit Cancellation Test, A Brief Screen Of Attention. PSYCHOLOGIA 55, 246–256 (2013).
Cheng, J. X. et al. Effects of transcranial direct current stimulation on performance and recovery sleep during acute sleep deprivation: a pilot study. Sleep Med. 79, 124–133 (2021).
Hatta, T. et al. Relation between the prefrontal cortex and cerebro-cerebellar functions: evidence from the results of stabilometrical indexes. Appl. Neuropsychol. 11, 153–160 (2004).
Lodge, D. J. & Grace, A. A. Developmental pathology, dopamine, stress and schizophrenia. Int. J. Dev. Neurosci. 29, 207–213 (2011).
McHugo, M. et al. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. NeuroImage: Clinical 20, 1106–1114 (2018).
Roeske, M. J. et al. Incomplete hippocampal inversion in schizophrenia: prevalence, severity, and impact on hippocampal structure. Mol. Psychiatry 26, 5407–5416 (2021).
Fleischhacker, W. et al. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur. Psychiatry 58, 1–9 (2019).
Davidson, M. et al. Efficacy and Safety of Roluperidone for the Treatment of Negative Symptoms of Schizophrenia. Schizophr Bull 48, 609–619 (2022).
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This study was supported by Suzhou Municipal Sci-Tech Bureau Program (SS202070), Suzhou Municipal Committee of Health and Family Planning (SZYJTD201812), Suzhou clinical Medical Center for mood disorders (No.Szlcyxzx202109 to X.B.Z.), Jiangsu Provincial Commission of Health and Family Planning (No.H2018041), Key Diagnosis and treatment Program of Suzhou (LCZX201919).
Author information
Authors and Affiliations
Contributions
J.L. and X.Z. recruited subjects, collected clinical data, performed schizophrenia symptom assessment, analyzed data, and wrote the manuscript. H.Y. and M.Y. conducted a schizophrenia symptom assessment and analyzed the data. Hongyan Sun designed the study and prepared the manuscript. All authors participated in the preparation of the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Zhang, X., Yang, H. et al. Lack of correlation between hippocampal substructure atrophy and attention dysfunction in deficit schizophrenia. Schizophr 9, 24 (2023). https://doi.org/10.1038/s41537-023-00354-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-023-00354-z