Abstract
Hippocampal volume is smaller in schizophrenia, but it is unclear when in the illness the changes appear and whether specific regions (anterior, posterior) and subfields (CA1, CA2/3, dentate gyrus, subiculum) are affected. Here, we used a high-resolution T2-weighted sequence specialized for imaging hippocampal subfields to test the hypothesis that anterior CA1 volume is lower in early psychosis. We measured subfield volumes across hippocampal regions in a group of 90 individuals in the early stage of a non-affective psychotic disorder and 70 demographically similar healthy individuals. We observed smaller volume in the anterior CA1 and dentate gyrus subfields in the early psychosis group. Our findings support models that implicate anterior CA1 and dentate gyrus subfield deficits in the mechanism of psychosis.
Similar content being viewed by others
Introduction
Structural abnormalities of the hippocampus are robust findings in both post-mortem [1] and neuroimaging [2] studies of schizophrenia. Total hippocampal volume is smaller in chronic [3, 4] and early stages [5] of the illness and may decline with illness progression [6, 7]. Smaller volume is already apparent in individuals at high risk for psychosis [8], but it is unclear whether hippocampal volume predicts transition to psychosis [9]. Understanding the specificity and time course of hippocampal volume change is a critical step toward developing better staging models for psychosis.
Current neurobiological models of schizophrenia propose hippocampal dysfunction as a key mechanistic factor of the illness [10, 11], with a focus on pathology in cornu ammonis sectors CA1 [12, 13] and CA2/3 [14] and the dentate gyrus (DG) [15]. Hypermetabolism within these subfields is posited to drive the onset of psychosis and hippocampal volume loss due to excitotoxic spreading [10, 12, 16,17,18]. Persons with chronic schizophrenia show smaller volume across multiple hippocampal subfields [19,20,21,22,23,24,25,26], a pattern that may also be present in early-stage individuals [16, 27]. However, several studies of at-risk and early-stage individuals show evidence of an initial volume deficit in two subfields: the CA1 sector [19, 28,29,30] and the dentate gyrus [16, 22, 24, 31], reviewed in [32]. The mechanism behind spreading of hippocampal hyperactivity may depend on neurotransmission between CA1 and the dentate gyrus [33]. Consequently, identifying when structural changes occur within these subfields is necessary to inform development of targeted treatments to reduce hyperactivity [34] .
A comprehensive account of hippocampal volume deficits must also consider anterior-posterior gradients of hippocampal function along the long axis [35]. The anterior hippocampus has distinct connectivity with regions involved in processing emotions and motivation and represents more global aspects of the environment compared to the posterior hippocampus [36]. The anterior CA1 region in particular may be more vulnerable to oxidative stress [37], hypoxia [38], and excitability [39] due to differences in gene and NMDA receptor expression patterns [40]. Anterior CA1 hippocampal hypermetabolism is consistently found in psychosis [12, 41,42,43,44] and the resulting excitotoxicity may lead to disproportionately lower volume observed in the anterior region [45,46,47,48,49]. Limited evidence suggests that the anterior hippocampus is more affected in the early stages of psychosis [30, 45, 48, 50], but widespread changes across both anterior and posterior regions are present in chronic schizophrenia [21, 25, 51,52,53] consistent with neuroprogressive pathology [33, 54].
Few studies have examined subfield changes along the hippocampal long axis in early psychosis. While two reports from our group point to selective reduction in the anterior CA subfields [21, 30], a recent study in a large early psychosis cohort suggests that volume deficits may also be present in the posterior subiculum [50] and none of these reports separately measured the CA1 and CA2/3 subfields. High-resolution (sub-millimeter voxels in the coronal plane along the hippocampal long axis), T2-weighted scans acquired at 3 T or 7 T field strength are being used to establish harmonized definitions of subfield boundaries across disciplines [55, 56]. These scans have greater gray/white matter contrast that permits differentiation of the cornu ammonis and dentate gyrus subfields [57] and provide a complementary method to analyses of standard (1mm3) resolution images. To our knowledge, only a single study has examined subfield volumes using high in-plane resolution imaging of the hippocampus in a small group of schizophrenia patients, finding smaller CA1 and dentate gyrus volumes [20], but did not consider differences in anterior and posterior regions. High-resolution imaging of individuals in the early stage of psychosis will enable more precise anatomical characterization of the timing of subfield-specific changes, may help to resolve discrepancies between existing studies, and yield greater insight into the impact of aging [58] and antipsychotic medication [59, 60].
Taken together, current evidence suggests that the anterior CA1 subfield is affected in the early stages of schizophrenia. So far, this has only been tested with standard resolution neuroimaging. Here, we use high-resolution structural MRI in a large group of individuals in the early stage of psychosis to test the hypothesis of smaller anterior CA1 volume in the early stage of psychosis. Incomplete hippocampal inversion, a marker of atypical neurodevelopment, has been shown to impact hippocampal volume in large-scale studies of healthy individuals [61] and in schizophrenia [62, 63]. Consequently, we carried out a secondary analysis to test the effect of incomplete hippocampal inversion on hippocampal subfield volumes. Finally, we conducted exploratory analyses to examine whether subfield volume deficits observed in early psychosis were associated with psychosis symptoms, illness duration, or memory deficits.
Methods
Participants
Participants were 90 individuals in the early stage of a non-affective psychotic disorder (EP) and 70 demographically similar healthy control individuals (HC), recruited between May 2013 and January 2020 for a prospective longitudinal study on hippocampal structure and function in the early stages of psychosis. EP individuals were recruited from the inpatient and outpatient clinics at Vanderbilt University Medical Center Psychiatric Hospital. HC individuals were recruited from the surrounding community through advertisements. Inclusion criteria were (1) age 13–40; (2) estimated premorbid IQ greater than or equal to 75; (3) <2 years of psychotic illness; and 4) meeting criterion A for schizophrenia (at least two of the following symptoms for a minimum duration of one month: delusions, hallucinations, disorganized speech, disorganized or catatonic behavior, and negative symptoms). Exclusionary criteria for all participants included the presence of significant head injury, major medical illnesses, or pregnancy. EP participants were excluded for active substance abuse or dependence in the past month or diagnosis of a psychotic disorder due to a medical condition. HC participants were excluded if they met criteria for any Axis I disorder or had a first-degree relative with a known psychotic disorder. Data from a subset of participants in this cohort have been included in previous reports on hippocampal volume (N overlap = 122) [21, 30] and incomplete hippocampal inversion (N overlap = 131) [62, 64], but the T2-weighted scans and analyses presented here are novel. All participants provided written informed consent and received monetary compensation for their time. The Vanderbilt University Institutional Review Board approved the study.
Clinical and cognitive characterization
Demographic and clinical characteristics of participants included in statistical analyses are summarized in Table 1. Psychiatric diagnoses were assessed with the Structured Clinical Interview for DSM-IV, TR (SCID [65]). Data obtained from in-person interviews were augmented by extensive review of all available medical records. Taking into account all available information, diagnostic consensus meetings were held, and final diagnoses were made by psychiatrist SH. The Positive and Negative Symptom Scale (PANSS [66]) was used to characterize clinical symptoms at the time of the scan. Premorbid IQ was estimated using the Wechsler Test of Adult Reading (WTAR [67]). The onset of psychosis was determined through the SCID and Symptom Onset in Schizophrenia Inventory, a standardized measure for rating prodromal versus psychotic symptoms [68]. The duration of psychosis was calculated as the amount of time between the date of onset of psychosis and the scan. Chlorpromazine equivalents were calculated using the formulas from Gardner et al. [69] and Leucht et al. [70] (mean = 329.01, sd = 176.38).
Relational memory measures
We examined the relationship of volume in the EP group with two measures of relational memory, a hippocampal-dependent cognitive function that is impaired in schizophrenia [71]. A subset of participants completed two tasks to measure relational memory: the face-scene binding task, which uses implicit eye movements to measure relational binding ability, and the associative inference task, which measures the ability to make explicit inferences about previously bound pairs of items (detailed descriptions of each task and how behavioral measures were calculated are presented in [72]). From the face-scene binding task, we calculated viewing slope (n = 74; smaller slope = worse performance; mean slope=11.63, sd = 12.14). For associative inference, we examined inference pair accuracy in participants who achieved at least 80% accuracy during training (n = 71 included; mean accuracy = 0.74, sd = 0.19; data from an additional 14 EP participants were excluded for failing to pass training criteria).
Data acquisition and processing
Structural imaging data were collected on one of two identical 3 T Philips Intera Achieva scanners with a 32-channel head coil (Philips Healthcare, Inc., Best, The Netherlands) at the Vanderbilt University Institute of Imaging Sciences. We acquired a 3D T1-weighted image (voxel size = 1mm3; field of view = 256 mm2; number of slices = 170; gap = 0 mm; TE = 3.7 ms; TR = 8.0 ms) and a T2-weighted turbo spin echo scan with oblique coronal slices oriented perpendicular to the hippocampal long axis (in-plane resolution = 0.5mm2; slice thickness = 2 mm; field of view = 230 mm x 184 mm; number of slices =26; gap = 0 mm; TE = 90 ms; TR = 2375 ms).
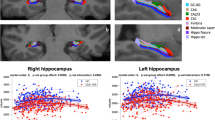
Hippocampal subfield volumes were obtained from the T2 and T1-weighted images using the Automated Segmentation of Hippocampal Subfields (ASHS) software with the Penn PMC atlas [73]. ASHS uses a library of manually segmented atlas images to automatically label hippocampal subfields in a participant’s native space (Fig. 1). Each participant’s T2 and T1 images are rigidly aligned and then registered to a T1 template image. All T2 atlas images are then registered to the participant’s T2 image. Multi-atlas joint label fusion is used to produce an initial consensus segmentation of the participant T2 image and corrective learning is used as a post-processing step to adjust segmentation errors. The resulting hippocampal segmentations were manually divided into anterior and posterior regions based on the presence of an anatomical landmark, the uncus [36, 74, 75]. The final slice of the anterior region was defined as the last coronal slice in which there were two cuts visible through the hippocampus, such that the uncus itself would have been included in the anterior region. Quality control consisted of visual inspection by author MM. T1 and T2-weighted images were visually inspected for motion artifacts and hippocampal segmentations were inspected for mislabeling. Segmentations that had voxels labeled outside the hippocampus (in the amygdala, adjacent white matter, or ventricles) or in which the hippocampus was not completely labeled were reviewed with expert rater SH before being excluded (N excluded = 4 EP and 3 HC individuals). No manual editing of segmentations was carried out. We obtained estimated total intracranial volume using Freesurfer 6 [76, 77].
The presence and severity of incomplete hippocampal inversion were determined using previously published criteria [61] by a single rater (MR). Each hippocampus was scored for incomplete inversion (range 0–10, higher score = more severe incomplete hippocampal inversion) and categorized as having incomplete hippocampal inversion (IHI) based on a cutoff score of ≥3.75 (described in [62]). We identified 29 cases of unilateral left hemisphere IHI, 2 cases of unilateral right hemisphere IHI, and 8 cases of bilateral IHI in our sample; 114 individuals did not have IHI (Table 2).
Statistical analysis
Statistical analysis of hippocampal subfield volume was carried out using linear mixed models in R (R Core Team, 2019) with the packages lmerTest [78], emmeans [79], and car [80]. To test whether there are regionally specific subfield volume deficits in early psychosis, we fitted a model with Volume as the outcome variable and Group (healthy control, early psychosis), Hemisphere (left, right), Region (anterior, posterior), Subfield (CA1, DG, subiculum, CA2/3), and their interaction as fixed effects, and participant as a random effect (Model 1: Volume ~ Group x Hemisphere x Region x Subfield + Age + Sex + ICV + Scanner + (1|Participant)). Age, sex, intracranial volume, and scanner were included as covariates of no interest. We conducted significance tests on the fixed effects in each model using analysis of variance (ANOVA) on the model output. Significant effects were followed up with contrasts adjusted for multiple comparisons using Bonferroni correction. Model assumptions were examined using fitted vs. residual plots, scale location plots, quantile-quantile plots, and the variance inflation factor (all values < 2). The assumptions of normality of residuals and homogeneity of variance were violated in the full model. Although linear mixed models are robust to violations of these assumptions [81], we fitted separate linear mixed models for each subfield (four models: CA1, CA2/3, DG, Subiculum) that examined the effects of Group, Hemisphere, and Region and are detailed in the supplement. After correction for four multiple comparisons, the results did not differ from those of the full model. A secondary model including incomplete hippocampal inversion score was fitted to examine whether it contributes to hippocampal subfield volume differences in early psychosis (Model 2: Volume ~ Group × Hemisphere × Region x Subfield + IHI + Age + Sex + ICV + Scanner + (1|Participant)).
Exploratory analyses examining the relationship between volume with clinical and cognitive characteristics in the EP group were carried out using linear regression. We used separate linear regression models for anterior CA1 and DG to test for an association between volume and positive, negative, and general PANSS scores, duration of psychosis, chlorpromazine equivalents, FSB viewing slope, and AI accuracy. All analyses included intracranial volume and scanner as covariates of no interest.
Results
Hippocampal subfield volume analysis
We found group differences in subfield volumes in the anterior region of the hippocampus (Fig. 2; Group × Region × Subfield interaction: F3,2261 = 5.66, p < 0.001). Individuals in the early stage of psychosis showed lower volume than healthy controls in the anterior CA1 (t960 = −5.06, p < 0.001) and DG (t960 = −3.96, p = 0.001) subfields, but not in CA2/3 (t968 = −0.80, p = 1.00) or the subiculum (t960 = −1.20, p = 1.00). We did not find evidence for group differences in volume for any subfields in the posterior hippocampus (all p’s > 0.92). In a secondary analysis with incomplete hippocampal inversion included in the model, we found a main effect of incomplete hippocampal inversion (F1,1048 = 8.69, p = 0.003), but our primary results remained unchanged (Group × Region × Subfield interaction: F3,2260 = 5.67, p < 0.001).
Associations with clinical characteristics and memory performance
We did not find evidence that anterior CA1 and DG volumes were associated with psychosis symptoms, duration of psychosis, chlorpromazine equivalents, or relational memory performance in the EP group (Table 3).
Discussion
In a large cross-sectional study, we show that hippocampal volume deficits are limited to the CA1 and DG subfields in the anterior region in the early stage of psychosis. To our knowledge, this is only the second study in schizophrenia to use high-resolution structural imaging designed to maximize visualization of anatomical detail within the hippocampus [20] and the first of its kind in an early psychosis cohort. First, we will discuss the value of high-resolution imaging in the study of hippocampal volume in schizophrenia. Then we will review functional implications of regionally specific hippocampal volume changes in the early stage of psychosis.
A post-mortem study of schizophrenia was the first to report smaller hippocampal volume [82]. The initial wave of CT and MRI studies confirmed smaller total hippocampal volume in schizophrenia [5], which is now recognized as the largest effect size among the numerous structural brain abnormalities observed in patients [3]. In a second wave, MRI studies explored hippocampal subfield volumes in schizophrenia [26]. But in contrast to post-mortem studies, which employed cytoarchitectural criteria to study hippocampal subfields, such detail is not available for neuroimaging studies. Therefore, researchers employed protocols for automated segmentation of subfields in the human hippocampus. The initial versions of these protocols arrived at volume estimates that are not compatible with the known anatomy of the human hippocampus [83]. Subfield volumes derived from 1mm3 resolution images is further limited by an inability to visualize internal details of hippocampal structure necessary for differentiation of the cornu ammonis and dentate gyrus subfields and automated methods applied to these data may primarily reflect differences in overall volume [57]. Consequently, some reports of subfield-specific deficits of hippocampal volume in schizophrenia from this second wave need to be interpreted with caution.
Our study belongs to the third wave of neuroimaging studies exploring hippocampal volume differences in schizophrenia using advanced imaging and segmentation methods. Accurate inferences regarding the nature of subfield-specific volume deficits in schizophrenia require valid, reliable, and reproducible methods that can be applied to large-scale datasets. T2-weighted coronal images of the hippocampus with high in-plane resolution (0.4–0.5 mm) enable delineation of the cornu ammonis and dentate gyrus and are recommended by consensus groups dedicated to harmonizing subfield segmentation protocols across laboratories [55]. While manual segmentation of subfields on such images remains the current best practice, the use of automated methods that are validated against manual segmentations will facilitate research on subfield structure in large datasets [57]. We included a high-resolution protocol using T1 and T2-weighted images, employed an accepted anatomical criterion to define anterior–posterior regions, and included the classification of incomplete hippocampal inversion, a variant in the development of the human hippocampus. Future meta-analytic studies of hippocampal volume in schizophrenia will need to consider the confounding effects of data acquisition and hippocampal segmentation.
In contrast to most previous studies of hippocampal volume in schizophrenia, we specifically tested for volume differences in the anterior region. Recent work has highlighted distinct functions of both hippocampal subfields (transverse axis) and hippocampal regions (longitudinal axis) [36, 84]. In fact, a full account of hippocampal function may be described best by gradients across both axes [35, 40]. Our data indicate smaller subfield volumes in the anterior but not posterior region in schizophrenia. Recent data-driven parcellations of the hippocampus based on both functional activation and gene expression patterns have provided convergent evidence that the anterior hippocampus is primarily involved in processing affective, motivational, and self-relevant information [85, 86]. Dysregulation of mood and affect are among the earliest symptoms to emerge during the prodromal period [87] and disturbance of self-related cognition may be a core feature of schizophrenia [88]. While we have taken an approach of using anatomically defined markers of hippocampal subregions in the current work, future studies are needed that jointly consider individual-specific parcellations of structural and functional data and their relationship to psychosis symptoms and cognition.
We confirm a prior study that highlighted the importance of CA1 and DG subfield abnormalities in the early stage of psychosis [32]. Our finding of smaller CA1 and DG volumes is of significant functional importance and lends support to several existing models of hippocampal dysfunction in schizophrenia. CA1 hyperactivity arising from glutamatergic dysfunction [12] or deficits in GABAergic interneurons [89] is thought to lead to positive symptoms and cognitive impairments. Early hyperactivity within CA1 may then spread in an excitotoxic cascade to adjacent subfields with illness progression, ultimately leading to concomitant volume deficits [17, 54]. A separate line of research suggests that reduced glutamatergic signaling in the DG gives rise to memory deficits and positive symptoms [15]. However, we did not find evidence for CA2/3 subfield changes [14, 90] in our cohort. Future longitudinal studies of hippocampal function, in concert with the type of structural neuroimaging presented here, are needed to fully determine a causal link between hippocampal hyperactivity and atrophy [29].
While incomplete hippocampal inversion explained substantial variance in hippocampal subfield volumes, we did not find evidence that it differentially impacted our primary finding of volume deficits within the anterior CA1 and DG subfields of EP individuals. Although we had a large cohort of individuals in the early stage of psychosis in the present study, the relatively low incidence of incomplete hippocampal inversion may have precluded our ability to observe a differential effect by group. Hemispheric asymmetry in the hippocampus is well-described [74] and incomplete hippocampal inversion contributes to asymmetry across healthy individuals and those with psychosis [62]. Unfortunately, because of the low incidence of right incomplete hippocampal inversion (2 HC, 8 EP), the current sample is underpowered to fully examine the question of hemispheric differences in subfield volumes across groups and anterior–posterior regions. Analysis of incomplete hippocampal inversion in larger or consortia datasets is needed to examine its relationship to hippocampal subfield volumes across the anterior-posterior axis.
We did not find evidence of an association between anterior CA1 or DG subfield volumes and clinical characteristics or memory performance in this sample. As we have discussed previously [30], our sample was identified very early in the illness (mean duration of psychosis ~8 months). It is possible that there is too little variation within the variables examined to observe a relationship with the subtle volume deficits that are present in the early stage of illness. The relationship between hippocampal dysfunction, psychosis symptoms, and memory impairment is likely complex and multifactorial. Future studies are needed that examine information about hippocampal structure and function (e.g., connectivity, perfusion, task-based fMRI) together with clinical and cognitive measures.
While high-resolution imaging in a large cohort is a strength of our study, there are also limitations. First, the cross-sectional data presented here are from a cohort of individuals in the early stage of illness. Not all non-affective psychotic disorder patients included in our cohort will progress to schizophrenia [91]. Additionally, hippocampal volume continues to change throughout the lifespan, particularly within the cornu ammonis and dentate gyrus subfields [92] and may exhibit nonlinear changes that differ in anterior and posterior regions in the age range represented in this sample [93, 94]. Longitudinal imaging is needed to clarify how hippocampal subregion volumes vary with clinical and diagnostic trajectory and the extent to which the differences in hippocampal subfield volumes from the present study reflect early neurodevelopmental processes or ongoing illness progression in psychosis. Second, while we did not find evidence for volume changes in the CA2/3 or subiculum, segmentation of these small subfields is challenging, even with manual segmentation [73]. Ultra-high-resolution 7 T imaging is needed to confirm the present findings. Finally, the majority of patients in our study were on antipsychotic medication and we cannot rule out their impact on hippocampal volume [95]. Although antipsychotic treatment may have greater effects within the dentate gyrus [60], data from antipsychotic-naïve individuals suggests that lower volume is not due solely to medication [16].
In summary, we find compelling evidence for subfield-specific changes in the anterior, but not posterior, hippocampus in the early stage of non-affective psychosis. These findings indicate that the more pervasive changes of hippocampal structure present in chronic schizophrenia are not yet established in the first two years of illness. Novel interventions and treatments aimed at normalizing hippocampal function may offer a pathway to preserving hippocampal volume and improving functional outcomes in non-affective psychosis [10].
Data availability
The data used in the current study are available by reasonable request to the corresponding author.
References
Roeske MJ, Konradi C, Heckers S, Lewis AS. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: a systematic review and meta-analysis of postmortem studies. Mol Psychiatry. 2021;26:3524–35.
Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38.
van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53.
Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016;21:1460–6.
Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist. 2012;18:180–200.
Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96.
Velakoulis D, Wood SJ, Wong MTH, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–49.
Vissink CE, Winter-van Rossum I, Cannon TD, Fusar-Poli P, Kahn RS, Bossong MG. Structural brain volumes of individuals at clinical high risk for psychosis: a meta-analysis. Biol Psychiatry Glob Open Sci. 2022;2:147–52.
Hinney B, Walter A, Aghlmandi S, Andreou C, Borgwardt S. Does hippocampal volume predict transition to psychosis in a high-risk group? A meta-analysis. Front Psychiatry. 2021;11:614659.
Knight S, McCutcheon R, Dwir D, Grace AA, O’Daly O, McGuire P, et al. Hippocampal circuit dysfunction in psychosis. Transl Psychiatry. 2022;12:13.
Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–42.
Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93.
Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529−53.
Benes FM. Building models for postmortem abnormalities in hippocampus of schizophrenics. Schizophr Res. 2015;167:73–83.
Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–93.
Briend F, Nelson EA, Maximo O, Armstrong WP, Kraguljac NV, Lahti AC. Hippocampal glutamate and hippocampus subfield volumes in antipsychotic-naive first episode psychosis subjects and relationships to duration of untreated psychosis. Trans Psychiatry. 2020;10:137.
Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–302.
Mancini V, Saleh MG, Delavari F, Bagautdinova J, Eliez S. Excitatory inhibitory imbalance underlies hippocampal atrophy in individuals with 22q11.2 Deletion Syndrome with psychotic symptoms. Biol Psychiatry. 2023. https://doi.org/10.1016/j.biopsych.2023.03.021.
Ho NF, Iglesias JE, Sum MY, Kuswanto CN, Sitoh YY, De Souza J, et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry. 2017;22:142–52.
Ota M, Sato N, Hidese S, Teraishi T, Maikusa N, Matsuda H, et al. Structural differences in hippocampal subfields among schizophrenia patients, major depressive disorder patients, and healthy subjects. Psychiatry Res Neuroimaging. 2017;259:54–59.
McHugo M, Talati P, Woodward ND, Armstrong K, Blackford JU, Heckers S. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. Neuroimage Clin. 2018;20:1106–14.
Wannan CMJJ, Cropley VL, Chakravarty MM, Van Rheenen TE, Mancuso S, Bousman C, et al. Hippocampal subfields and visuospatial associative memory across stages of schizophrenia-spectrum disorder. Psychol Med. 2019;49:2452−62.
Nakahara S, Turner JA, Calhoun VD, Lim KO, Mueller B, Bustillo JR, et al. Dentate gyrus volume deficit in schizophrenia. Psychol Med. 2019;50:1267–77.
Sasabayashi D, Yoshimura R, Takahashi T, Takayanagi Y, Nishiyama S, Higuchi Y, et al. Reduced hippocampal subfield volume in schizophrenia and clinical high-risk state for psychosis. Front Psychiatry. 2021;12:642048.
del Re EC, Zeng V, Alliey-Rodriguez N, Lizano P, Bolo N, Lutz O, et al. Anterior-posterior axis of hippocampal subfields across psychoses: a B-SNIP study. Biomark Neuropsychiatry. 2021;5:100037.
Haukvik UK, Tamnes CK, Söderman E, Agartz I. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: A systematic review and meta-analysis. J Psychiatr Res. 2018;104:217–26.
Hoang D, Lizano P, Lutz O, Zeng V, Raymond N, Miewald J, et al. Thalamic, Amygdalar, and hippocampal nuclei morphology and their trajectories in first episode psychosis: a preliminary longitudinal study. Psych Res Neuroimaging. 2021;309:111249.
Ho NF, Holt DJ, Cheung M, Iglesias JE, Goh A, Wang M, et al. Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology. 2017;42:1361–70.
Provenzano FA, Guo J, Wall MM, Feng X, Sigmon HC, Brucato G, et al. Hippocampal pathology in clinical high-risk patients and the onset of schizophrenia. Biol Psychiatry. 2020;87:234–42.
McHugo M, Armstrong K, Roeske MJ, Woodward ND, Blackford JU, Heckers S. Hippocampal volume in early psychosis: a 2-year longitudinal study. Trans Psychiatry. 2020;10:306.
Park MTM, Jeon P, Khan AR, Dempster K, Chakravarty MM, Lerch JP, et al. Hippocampal neuroanatomy in first episode psychosis: a putative role for glutamate and serotonin receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2021:110:110297.
Nakahara S, Matsumoto M, van Erp TGM. Hippocampal subregion abnormalities in schizophrenia: a systematic review of structural and physiological imaging studies. Neuropsychopharmacol Rep. 2018;38:156–66.
Bauer JP, Rader SL, Joffe ME, Kwon W, Quay J, Seanez L, et al. Modeling intrahippocampal effects of anterior hippocampal hyperactivity relevant to schizophrenia using chemogenetic excitation of long axis–projecting mossy cells in the mouse dentate gyrus. biological psychiatry: global open. Science 2021;1:101–11.
Tregellas JR. Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry. 2014;76:111–9.
Genon S, Bernhardt BC, La Joie R, Amunts K, Eickhoff SB. The many dimensions of human hippocampal organization and (dys)function. Trends Neurosci. 2021. https://doi.org/10.1016/J.TINS.2021.10.003.
Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–40.
Wang X, Pal R, Chen X, Limpeanchob N, Kumar KN, Michaelis EK. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol. 2005;140:120–6.
Bartsch T, Döhring J, Reuter S, Finke C, Rohr A, Brauer H, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35:1836–45.
Dougherty KA, Islam T, Johnston D. Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. J Physiol. 2012;590:5707–22.
Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–69.
Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ, et al. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014;5:359–64.
McHugo M, Talati P, Armstrong K, Vandekar SN, Blackford JU, Woodward ND, et al. Hyperactivity and reduced activation of anterior hippocampus in early psychosis. Am J Psychiatry. 2019;176:1030–8.
Allen P, Azis M, Modinos G, Bossong MG, Bonoldi I, Samson C, et al. Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: replication in a second cohort. Schizophr Bull. 2018;44:1323–31.
Modinos G, Egerton A, McMullen K, McLaughlin A, Kumari V, Barker GJ, et al. Increased resting perfusion of the hippocampus in high positive schizotypy: a pseudocontinuous arterial spin labeling study. Hum Brain Map. 2018;39:4055−64.
Kalmady SV, Shivakumar V, Arasappa R, Subramaniam A, Gautham S, Venkatasubramanian G, et al. Clinical correlates of hippocampus volume and shape in antipsychotic-naïve schizophrenia. Psychiatry Res Neuroimaging. 2017;263:93–102.
Thoma RJ, Monnig M, Hanlon FM, Miller GA, Petropoulos H, Mayer AR, et al. Hippocampus volume and episodic memory in schizophrenia. J Int Neuropsychol Soc. 2009;15:182–95.
Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009;114:110–8.
Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, et al. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190–7.
Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–85.
Choi S, Kim M, Park H, Kim T, Moon S-Y, Lho SK, et al. Volume deficits in hippocampal subfields in unaffected relatives of schizophrenia patients with high genetic loading but without any psychiatric symptoms. Schizophr Res. 2022;240:125–31.
Maller JJ, Daskalakis ZJ, Thomson RHS, Daigle M, Barr MS, Fitzgerald PB. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil’s in De-Tail. Hippocampus. 2012;22:9–16.
Rametti G, Segarra N, Junqué C, Bargalló N, Caldú X, Ibarretxe N, et al. Left posterior hippocampal density reduction using VBM and stereological MRI procedures in schizophrenia. Schizophr Res. 2007;96:62–71.
Weiss AP, Dewitt I, Goff D, Ditman T, Heckers S. Anterior and posterior hippocampal volumes in schizophrenia. Schizophr Res. 2005;73:103–12.
Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–72.
Olsen RK, Carr VA, Daugherty AM, La Joie R, Amaral RSC, Amunts K, et al. Progress update from the hippocampal subfields group. Alzheimer’s Dement Diagn Assess Dis Monit. 2019;11:439–49.
Yushkevich PA, Amaral RSCC, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, et al. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: Towards a harmonized segmentation protocol. NeuroImage. 2015;111:526–41.
Wisse LEM, Chételat G, Daugherty AM, Flores R, Joie R, Mueller SG, et al. Hippocampal subfield volumetry from structural isotropic 1 mm 3 MRI scans: a note of caution. Hum Brain Map. 2020;42:539−50.
de Flores R, La Joie R, Chételat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience. 2015;309:29–50.
Li W, Li K, Guan P, Chen Y, Xiao Y, Lui S, et al. Volume alteration of hippocampal subfields in first-episode antipsychotic-naïve schizophrenia patients before and after acute antipsychotic treatment. NeuroImage Clin. 2018;20:169–76.
Rhindress K, Robinson DG, Gallego JA, Wellington R, Malhotra AK, Szeszko PR. Hippocampal subregion volume changes associated with antipsychotic treatment in first-episode psychosis. Psychol Med. 2017;47:1706–18.
Cury C, Toro R, Cohen F, Fischer C, Mhaya A, Samper-González J, et al. Incomplete hippocampal inversion: a comprehensive MRI study of over 2000 Subjects. Front Neuroanat. 2015;9:1–12.
Roeske MJ, McHugo M, Vandekar S, Blackford JU, Woodward ND, Heckers S. Incomplete hippocampal inversion in schizophrenia: prevalence, severity, and impact on hippocampal structure. Mol Psychiatry. 2021;26:5407−16.
Cachia A, Cury C, Brunelin J, Plaze M, Delmaire C, Oppenheim C, et al. Deviations in early hippocampus development contribute to visual hallucinations in schizophrenia. Transl Psychiatry. 2020;10:102.
Roeske MJ, Lyu I, McHugo M, Blackford JU, Woodward ND, Heckers S. Incomplete hippocampal inversion: a neurodevelopmental mechanism for hippocampal shape deformation in schizophrenia. Biol Psychiatry. 2022;92:314–22.
First M, Spitzer R, Miriam G, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/PSY SCREEN). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Wechsler D. Wechsler test of adult reading. San Antonio, TX: Pearson; 2001.
Perkins DO, Leserman J, Jarskog LF, Graham K, Kazmer J, Lieberman JA. Characterizing and dating the onset of symptoms in psychotic illness: the Symptom Onset in Schizophrenia (SOS) inventory. Schizophr Res. 2000;44:1–10.
Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93.
Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40:314–26.
Raucher-Chéné D, Lavigne KM, Lepage M. Episodic Memory and Schizophrenia: From Characterization of Relational Memory Impairments to Neuroimaging Biomarkers. In: Barch DM, Young JW, editors. Cognitive functioning in schizophrenia: leveraging the RDoC framework, Cham: Springer International Publishing; 2023. p. 115–36.
Avery SN, Armstrong K, McHugo M, Vandekar S, Blackford JU, Woodward ND, et al. Relational memory in the early stage of psychosis: a 2-year follow-up study. Schizophr Bull. 2021;47:75–86.
Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, et al. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Map. 2015;36:258–87.
Woolard AA, Heckers S. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res Neuroimaging. 2012;201:48–53.
Duvernoy HM, Cattin F, Risold P-Y. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Berlin: Springer-Verlag; 2013.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–94.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207.
Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: tests in linear mixed effects models. J Stat Softw. 2017;82:1–26.
Lenth R. emmeans: estimated marginal means, aka least-squares means. 2018. https://cran.r-project.org/package=emmeans.
Fox J, Weisberg S. An R companion to applied regression. 2nd ed. Thousand Oaks: SAGE; 2011.
Schielzeth H, Dingemanse NJ, Nakagawa S, Westneat DF, Allegue H, Teplitsky C, et al. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol Evol. 2020;11:1141–52.
Bogerts B, Meertz E, Schönfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia: a morphometric study of brain volume and shrinkage. Arch Gen Psychiatry. 1985;42:784–91.
Wisse LEM, Biessels GJ, Geerlings MI. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front Aging Neurosci. 2014;6:261.
Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19.
Plachti A, Eickhoff SB, Hoffstaedter F, Patil KR, Laird AR, Fox PT, et al. Multimodal parcellations and extensive behavioral profiling tackling the hippocampus gradient. Cereb Cortex. 2019. https://doi.org/10.1093/cercor/bhy336.
Vogel JW, La Joie R, Grothe MJ, Diaz-Papkovich A, Doyle A, Vachon-Presseau E, et al. A molecular gradient along the longitudinal axis of the human hippocampus informs large-scale behavioral systems. Nat Commun. 2020;11:960.
Yung AR, McGorry PD. Prediction of psychosis: setting the stage. Br J Psychiatry. 2007;191:s1–s8.
Sass L, Borda JP, Madeira L, Pienkos E, Nelson B. Varieties of self disorder: a bio-pheno-social model of schizophrenia. Schizophr Bull. 2018;44:720–7.
Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11.
Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S. Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull. 2012;38:927–35.
Fusar-Poli P, Cappucciati M, Bonoldi I, Hui LMC, Rutigliano G, Stahl DR, et al. Prognosis of brief psychotic episodes a meta-analysis. JAMA Psychiatry. 2016;73:211–20.
Daugherty AM, Bender AR, Raz N, Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26:220–8.
Schlichting ML, Guarino KF, Schapiro AC, Turk-Browne NB, Preston AR. Hippocampal structure predicts statistical learning and associative inference abilities during development. J Cogn Neurosci. 2017;29:37–51.
DeMaster D, Pathman T, Lee JK, Ghetti S. Structural development of the hippocampus and episodic memory: developmental differences along the anterior/posterior axis. Cereb Cortex. 2014;24:3036–45.
Bodnar M, Malla AK, Makowski C, Chakravarty MM, Joober R, Lepage M. The effect of second-generation antipsychotics on hippocampal volume in first episode of psychosis: longitudinal study. BJPsych Open. 2016;2:139–46.
Acknowledgements
This work was supported by the Charlotte and Donald Test Fund, NIMH grants R01-MH70560 (SH) and 1R01MH123563-01 (SNV), the Vanderbilt Psychiatric Genotype/Phenotype Project, and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH).
Author information
Authors and Affiliations
Contributions
MM and SH designed the study and drafted the manuscript. MM, MJR, SNV, KA, and SNA contributed to data analysis. All authors contributed to important manuscript revisions and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McHugo, M., Roeske, M.J., Vandekar, S.N. et al. Smaller anterior hippocampal subfields in the early stage of psychosis. Transl Psychiatry 14, 69 (2024). https://doi.org/10.1038/s41398-023-02719-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-023-02719-5