Abstract
This retrospective study evaluated the benefit of following different long-acting injectable (LAI) initiation strategies based on the timing of behavioral and clinical events among Medicaid beneficiaries with schizophrenia. Adults with schizophrenia initiating oral antipsychotics (OAPs) after 12 months without antipsychotic use or schizophrenia-related inpatient/emergency room (ER) visits (index date) were identified. Patients were categorized into four event-driven LAI initiation strategy cohorts based on observed sequences of behavioral (i.e., OAP adherence) and clinical (i.e., schizophrenia-related inpatient/ER visits) events between index and LAI initiation or censoring—strategy #1: adherent to OAPs without schizophrenia-related inpatient/ER visits; strategy #2: nonadherent to OAPs without schizophrenia-related inpatient/ER visits; strategy #3: one schizophrenia-related inpatient/ER visit; strategy #4: ≥2 schizophrenia-related inpatient/ER visits. Clinical outcomes (i.e., all-cause inpatient/ER visits) were evaluated between OAP initiation and end of follow-up. Comparisons between LAI initiation strategy cohorts were conducted using a dynamic marginal structural model adjusting for baseline characteristics and time-varying confounders. Among 13,444 eligible patients, 13.1%, 53.6%, 15.7%, and 17.6% were following strategies #1–4, respectively; of these, 21.9%, 4.3%, 9.2%, and 6.5% started an LAI (the remaining were censored). Strategy #1 was associated with a greater clinical benefit, with 43%, 69%, and 80% fewer inpatient days (all p < 0.05); and 57%, 59%, and 79% fewer ER visits (all p < 0.01) vs strategies #2–4, respectively; the clinical benefit was also observed for strategy #2 vs #3–4. Therefore, starting an LAI prior to OAP nonadherence or occurrence of a schizophrenia-related inpatient/ER visit was associated with fewer all-cause inpatient days of inpatient stay and ER visits.
Similar content being viewed by others
Introduction
Antipsychotics are the main pharmacologic therapy for patients with schizophrenia1,2. Schizophrenia treatment guidelines recommend tailoring pharmacologic intervention based on patient’s disease stage, history of schizophrenia-related relapses, psychiatric and physical comorbidities, response to current or previous antipsychotic(s), potential side effects associated with antipsychotic medication and its tolerability profile, history of antipsychotic medication adherence, transition of care, and patient preference2,3,4,5,6,7.
An important challenge in schizophrenia care is adherence to therapy, which is crucial for reducing the risk of schizophrenia relapse and its negative consequences such as hospitalization and self-harm8,9,10,11,12. Studies have demonstrated that long-acting injectable antipsychotics (LAIs), which require less frequent administration than oral antipsychotics (OAPs), are associated with better adherence than OAPs13,14,15,16. Some studies have also found that treatment with LAIs was associated with significant reductions in healthcare resource utilization, with the magnitude of results varying depending on the study design and patient population17,18. Other recognized advantages of LAIs include ensured knowledge about adherence status; lower risk of relapse, hospitalization and mortality; and increased opportunity to receive psychosocial treatments during a state of greater stability8,14,17.
Barriers to using LAIs may exist. Surveys conducted among clinicians have identified some reasons underlying the infrequent LAI use, particularly as treatment following first-episode psychosis, including concerns over costs, adverse effects, stigmatization, presumed OAP adherence and nonacceptance of LAI treatment, and clinicians’ perception that LAIs were reserved for later use in the disease course19,20,21.
Despite these barriers, most guidelines recognize the various advantages of LAIs compared to OAPs; however, recommendations for the appropriate timing of LAI initiation with respect to events such as nonadherence and relapse vary considerably across guidelines and will differ based on patient experiences, preferences, and situations22. A recent systematic review found that among US guidelines for schizophrenia, recommendations include initiating LAIs as first-line therapy after tolerability and sufficient effectiveness have been assessed with the oral formulation of the same antipsychotic (Florida Medicaid Program)3, in patients with history of poor or uncertain adherence (American Psychiatric Association, Harvard South Shore Program)2,6, in patients presenting frequent relapses (American Association of Community Psychiatrists [AACP])23, after failure of ≥2 OAPs or in nonadherent patients (Florida Medicaid Program, AACP, Oregon Health Authority)5,23,24, or when patients prefer the LAI formulation to OAPs (Schizophrenia Patient Outcomes Research Team)4.
Given the lack of consensus regarding LAI recommendations, variations in clinical practice, and each patient’s unique journey, there is a need to understand the impact of different LAI treatment initiation strategies on clinical outcomes. More specifically, patients’ start of LAIs may be preceded by clinical events, such as relapses or nonadherence episodes. As such, LAI treatment initiation strategies may be identified based on the observed sequences of previous behavioral and clinical events, resulting in different timing of LAI initiation for each patient. This study therefore aimed to assess clinical outcomes (i.e., inpatient and emergency room [ER] visits) and healthcare costs among Medicaid beneficiaries (from six states) with schizophrenia following different event-driven LAI initiation strategies, identified based on patterns of OAP adherence and schizophrenia-related inpatient admissions or ER visits after initial OAP treatment. To assess the experience of various demographic subgroups on different LAI initiation strategies, analyses were also conducted among patient subgroups based on age, sex at birth, and race. Based on prior studies25,26,27, we hypothesized that starting LAIs while patients are adherent to their current OAP and prior to one or more schizophrenia-related inpatient admissions or ER visits would be associated with significantly better outcomes, and this benefit would be consistent across demographic subgroups.
Results
Sample size and strategy cohorts
A total of 13,444 eligible patients were included in this study (Fig. 1). To compare outcomes across different LAI initiation strategies, patients were categorized into four different LAI initiation strategy cohorts based on the observed sequence of behavioral (i.e., OAP adherence) and clinical events (i.e., schizophrenia-related inpatient admissions or ER visits) during the follow-up period (Fig. 2) between index and LAI initiation or censoring. LAI initiation strategy #1 (the most proactive strategy) comprised patients adherent to OAPs without schizophrenia-related inpatient/ER visits; strategy #2 comprised patients nonadherent to OAPs without schizophrenia-related inpatient/ER visits; strategy #3 comprised patients with exactly one schizophrenia-related inpatient/ER visit during the follow-up period; and strategy #4 (the most reactive strategy) comprised patients with ≥2 schizophrenia-related inpatient/ER visits ≥30 days apart (i.e., revolving door patients). At the end of the follow-up period, 1759 (13.1%), 7211 (53.6%), 2111 (15.7%), and 2363 (17.6%) patients were following event-driven LAI initiation strategies #1–4 cohorts, respectively (as defined in Fig. 2).
By the end of the follow-up period, 21.9% of patients in strategy #1 started an LAI and the remaining 78.1% were censored (i.e., patients still at risk of initiating LAI while being adherent to their current OAP and having no schizophrenia-related inpatient admissions or ER visits by the end of the follow-up period). The proportions of patients who started an LAI according to strategies #2–4 were 4.3%, 9.2%, and 6.5%, respectively, with the remaining patients censored (Table 1). This corresponded to an overall 7.8% of patients across cohorts who started an LAI during the follow-up period. By demographic subgroups, this proportion ranged from 6.0% to 11.9% and was higher among patients 18–35 years old (11.9% vs 6.6% in patients >35 years old), males (9.3% vs 6.0% in females), and Blacks (8.6% vs 6.8% in whites/Caucasians). Overall, more patients received a second-generation LAI than a first-generation LAI across all four studied cohorts, but the proportion of patients receiving a first-generation LAI was relatively higher in strategy #1 than in strategies #2–4 (Table 1).
Baseline demographic and clinical characteristics
During the 12 months prior to the index date (i.e., before the date of the first OAP claim), across cohorts, mean age was 47.8–48.9 (standard deviation [SD] = 14.0–15.5) years; 39.7–48.2% were female (51.8–60.3% were male); and 27.5–37.7% were Black (Table 2). Patients treated according to strategy #1 had a lower mean baseline Quan-Charlson Comorbidity Index (Quan-CCI) than those treated according to strategies #2–4 (0.7 vs 1.0–1.1, p < 0.0001) and had fewer unique mental health diagnoses (2.0 vs 2.3–2.5, p < 0.0001). During the baseline period, a lower proportion of patients treated according to strategy #1 had an all-cause inpatient admission than those treated according to strategies #2–4 (13.8% vs 21.8–24.5%, p < 0.0001). The same was true for ER visit (25.3% vs 38.4–42.7%, p < 0.0001). Meanwhile, patients treated according to strategy #1 had similar baseline all-cause total healthcare costs as those treated according to strategies #2–4 ($1255 vs $1181–$1199, p = 0.7981). Key baseline characteristics by demographic subgroup are shown in Supplementary Table 1.
Clinical outcomes
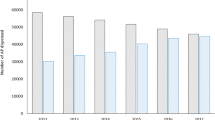
Beginning from the time the patients initiated OAP through the end of the follow-up period, including the period when patients transitioned to LAI, and after adjusting for baseline patient characteristics and time-varying confounders, starting an LAI prior to evidence of nonadherence or the occurrence of a schizophrenia-related inpatient/ER visit (strategy #1) was associated with greater clinical benefit (i.e., fewer inpatient admissions, days of inpatient stay, and ER visits) than initiating an LAI subsequent to nonadherence or schizophrenia-related inpatient/ER visit(s) (strategies #2–4). Specifically, strategy #1 was associated with 32% (p = 0.072), 53% (p = 0.004), and 73% (p < 0.001) fewer inpatient admissions; 43%, 69%, and 80% fewer days of inpatient stay (all p < 0.05); and 57%, 59%, and 79% fewer ER visits (all p < 0.01) during the follow-up period relative to strategy #2, #3, or #4, respectively (Fig. 3). Strategy #2 was associated with 27% and 59% fewer inpatient admissions (all p < 0.05), 42% and 64% fewer days of inpatient stay (all p < 0.05), and 2% (p = 0.946) and 52% (p < 0.05) fewer ER visits relative to strategy #3 or #4, respectively. Among demographic subgroups, similar reductions in inpatient admissions, days of inpatient stay, and ER visits were observed for strategies #1–2 relative to strategies #3–4 during the follow-up period (Supplementary Table 2).
CI confidence interval, ER emergency room, IP inpatient, LAI long-acting injectable, OAP oral antipsychotic, SCH schizophrenia. *Significant at the 5% level. aClinical benefit was measured by weighted all-cause IP admissions, IP stays, and ER visits per-patient-per-month. bStrategy cohorts were determined at transition to LAI or censoring for each patient by considering all information from the index date up until that point. The following definitions were used to categorize patients into event-driven LAI initiation strategies: Strategy #1: Patients with adherence and no SCH-related IP admissions or ER visits between OAP and either LAI initiation or censoring; Strategy #2: Patients with nonadherence and no SCH-related IP admissions or ER visits between OAP and either LAI initiation or censoring; Strategy #3: Patients with exactly one SCH-related IP admission or ER visit between OAP and either LAI initiation or censoring; Strategy #4: Patients with ≥2 SCH-related IP admissions or ER visits ≥30 days apart between OAP and either LAI initiation or censoring.
Cost outcomes
Compared to strategies #3–4, strategies #1–2 had lower or similar mean all-cause medical costs during the follow-up period, which resulted in lower or similar mean all-cause total healthcare costs (Table 3). Specifically, while strategy #1 was associated with higher mean monthly total healthcare costs than strategy #2, it was associated with numerically lower mean monthly total healthcare costs than strategies #3–4 (cost difference range: −$109 to −$227, p ≥ 0.05). Meanwhile, strategy #2 was associated with significantly lower mean monthly total healthcare costs than strategies #3–4 (−$494 to −$651, p < 0.05); the cost difference was mainly driven by a reduction in medical costs.
Discussion
This retrospective longitudinal cohort study found that among 13,444 eligible Medicaid beneficiaries in six states, the strategy of starting LAI prior to OAP nonadherence and a schizophrenia-related inpatient/ER visit (most proactive strategy) was associated with greater clinical benefits compared to starting LAI after nonadherence to OAP or experiencing a schizophrenia-related inpatient/ER visits (reactive strategies). This benefit included a reduction in the number of all-cause inpatient admissions and/or days of inpatient stay, and ER visits. This could potentially be explained by patients’ lower disease severity when they are still adherent or have not gone through periods of distress resulting in (one or multiple) schizophrenia-related inpatient/ER visits; for example, it has been shown that antipsychotic nonadherence is associated with greater severity of symptoms28. Moreover, results across all demographic subgroups similarly showed that LAI initiation prior to evidence of nonadherence and having a schizophrenia-related inpatient/ER visit was associated with greater clinical benefits than waiting to start LAI after one of these important clinical events.
Current schizophrenia treatment guideline recommendations vary considerably regarding LAI use2,3,4,5,6,7,24. Based on a systematic review of 19 schizophrenia guidelines worldwide, only one guideline recommends LAI use independent of OAP adherence to prevent future nonadherence-related relapses3. Ten schizophrenia practice guidelines suggest initiating LAI in patients with signs of nonadherence independent of, or prior to, adverse schizophrenia-related clinical events22. In addition, five clinical practice guidelines recommend LAI use as early as the first schizophrenia episode22. The current study found that LAI initiation among patients who are adherent to their current OAP and have not experienced a schizophrenia-related inpatient/ER visit resulted in better clinical outcomes. This is consistent with the Florida Medicaid Program recommendations3, which is the only guideline recommending the use of LAI even when patients are still adherent to their OAP22. Results of this study should help raise awareness among clinicians and payers about potential benefits associated with LAI initiation prior to OAP nonadherence or the occurrence of adverse schizophrenia-related clinical events and highlight the need to re-examine current schizophrenia treatment guideline recommendations pertaining to LAI initiation in a broader patient population.
The current approach compared event-driven treatment strategies all together in a same model (i.e., the marginal structural model [MSM]). This approach, which has been applied in other therapeutic areas29,30,31, allows the comparison of LAI initiation strategies based on different sequences of events by adjusting for the time-varying confounders during the follow-up period. Previous literature has evaluated the clinical outcomes of initiating LAIs relative to OAPs in different schizophrenia populations, including those who had experienced a relapse event or those aged 18–34 years (used to approximate timing of LAI start). Most studies demonstrated a clinical advantage with initiation of LAIs, which resulted in a neutral effect on costs8,13,18,32,33,34,35,36,37. However, results from these studies cannot inform on the best timing for LAI initiation over a patient’s journey to optimize outcomes. The current analysis addresses this need by further refining the categorization of timing of LAI start and comparing different event-driven LAI initiation strategies all together using the same adjusted model.
Overall, the rate of LAI use during the follow-up period was only about 7.8%, which was slightly lower than the national average of 13% recently reported by Patel et al. for the overall Medicaid population across 45 states in 201838. However, based on the Patel et al. study, rates of LAI use varied across the 45 states (4–26%), with the reported rate in the current study within this range; this may account for the difference in the overall rate found in the current study based on only six states. Furthermore, because the first qualifying index date (based on the inclusion/exclusion criteria) starting on 1 January 2007 was used for the study, a large proportion of patients initiated treatment in earlier years (2007–2010), when fewer LAI options were available, and this may also explain the difference between the proportion of LAI users found in the current study and data from Patel et al. The current study also found that the proportion of patients who started an LAI was higher among those 18–35 years old, male, and Black. Future studies are warranted to further evaluate health disparities related to LAI utilization and associated outcomes in these subgroups. Lastly, it should be noted that first-generation LAIs were more used in strategy #1 and second-generation LAIs were more used in other strategies. As some second-generation LAIs are recommended or approved by the Food and Drug Administration for patients with schizoaffective disorder7,39, the variations in first- vs second-generation LAI use may be partially due to the higher proportion of patients with schizoaffective disorder in patients treated according to strategies #3–4 than strategy #1 in the current study. In addition, given the higher proportion of patients with dual Medicaid/Medicare coverage in strategies #2–4, second-generation LAIs may have been more used by patients in these strategies than those in strategy #1, because the additional Medicare coverage results in reduced out-of-pocket spending and fewer cost-related barriers40. On the other hand, as the proportion of patients with dual Medicaid/Medicare coverage was lower in strategy #1, these patients may have opted for first-generation LAIs because of their lower price. However, future studies should be conducted to further investigate this finding.
The overall low rate of LAI use in the current study is in line with previous reports that LAIs are underused in US clinical practice41,42, and this contradicts the documented effectiveness of LAIs in schizophrenia14,43,44. Indeed, LAI use for first-episode or newly diagnosed schizophrenia has been associated with reduced relapse rates27, better adherence37, and increased patient satisfaction relative to OAPs45. In addition, a retrospective cohort study showed that LAI initiation ≤1 year after the first recorded schizophrenia diagnosis resulted in lower hospitalization rates and healthcare costs compared with later initiators46.
Collectively, the above literature evidence indicates that LAI use early in the disease course has the potential to improve treatment outcomes. The current study expanded this existing literature by demonstrating the greater clinical benefit associated with LAI initiation prior to OAP nonadherence and the occurrence of a schizophrenia-related clinical event, and that such clinical benefit translated into a neutral effect on or reduction in healthcare costs.
Some limitations should be considered when interpreting the results of the current study. First, this study included beneficiaries with Medicaid coverage from six states only, which could limit the generalizability of findings. Second, we assumed claims for OAPs were taken as prescribed, which may lead to a potential overestimation of OAP adherence. Misclassification of the timing of LAI initiation strategy was also possible since antipsychotics given via patient sample programs were not recorded in claims. Third, the onset date of schizophrenia could not be confirmed in the claims; hence, the duration of the disease could not be assessed and adjusted for in the analyses and patients in this study could have included a mix of new antipsychotic users and patients re-starting antipsychotic treatment that may or may not be patients with recent-onset schizophrenia. Fourth, the current categorization of cohorts for strategies #3–4 was based on schizophrenia-related clinical events and patients could have been either OAP-adherent and OAP-nonadherent patients; therefore, these two strategies may be confounded by patient’s adherence status. Likewise, the time to LAI initiation for each cohort in this study was driven by study design. As a longer follow-up time would be needed for each additional behavioral or clinical event to occur prior to LAI initiation, strategy cohorts that needed patients to meet requirements for more events would likely have a prolonged time between the index date and LAI initiation; this is reflected by the increasing mean time to LAI initiation from strategies #1 to #4. Fifth, patients were selected based on the earliest qualifying index date, resulting in more index dates in the earlier years of data availability (2007–2010). Sixth, this study may have been subject to residual confounding due to unmeasured confounders. In addition, while many confounders were adjusted for in the MSM analysis to balance cohorts, the number of confounders used was limited by sample size (particularly the number of patients starting LAI) and confounders that did not change over time or had unusual patterns over time resulted in unstable weights; therefore, to avoid extreme weights, extremely small and large weights were trimmed. To further mitigate this limitation, doubly robust adjustment was made to incorporate characteristics that could not be balanced using MSM.
In conclusion, starting an LAI prior earlier—that is before there is any evidence of nonadherence to current OAP treatment and prior to any adverse schizophrenia-related clinical events (inpatient admission/ER events)—was associated with the greatest clinical benefit, as evidenced by significantly fewer all-cause inpatient admissions, days of inpatient stay, and ER visits. The second most optimal event-driven LAI initiation strategy was after evidence of OAP nonadherence but prior to any adverse schizophrenia-related clinical events. The least favorable strategy was starting LAI after ≥2 schizophrenia-related inpatient/ER visits. Results were consistent across demographic groups, suggesting that starting LAI prior to nonadherence or schizophrenia-related inpatient/ER visits regardless of the patient age, race, or sex can optimize patient outcomes. The findings of this study may inform clinical decision-making and future schizophrenia treatment guidelines pertaining to timing of initiating LAI in patients diagnosed with schizophrenia.
Methods
Data source
Medicaid data from Iowa (2006Q1-2017Q1), Kansas (2006Q1-2019Q1), Mississippi (2006Q1-2019Q1), Missouri (2006Q1-2019Q1), New Jersey (2006Q1-2014Q1) and Wisconsin (2006Q1-2013Q4) were used. The Medicaid database includes information on enrollment eligibility, physician visits, hospitalizations, long-term care services, prescription drugs, and other services reimbursed by Medicaid. Cost information is pre-rebate and represents the actual amount paid by Medicaid for services rendered. Data are de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act; therefore, no reviews by an institutional review board were required.
Study design
This was a retrospective longitudinal cohort study. The index date was the date of the first OAP claim on or after 1 January 2007 after applying a 12-month washout period without any antipsychotic claims (i.e., no OAPs, short-acting injectables, or LAIs) or schizophrenia-related inpatient admissions or ER visits, to capture new starts and re-starts of OAPs. The baseline period was defined as the 12-month period pre-index (i.e., prior to the date of first OAP claim, same as the washout period) and the follow-up period (which was used to evaluate study outcomes) was defined as the period from the index date until the earliest among the following events: LAI discontinuation (last day before a period >180 days without LAI treatment), the date of the first claim for clozapine, end of continuous eligibility, end of data availability, or death (Supplementary Fig. 1).
Patient selection criteria
Patients were included if they were ≥18 years of age at the index date; had ≥2 claims with a diagnosis for schizophrenia on different dates any time prior to or within 30 days after the index date (International Classification of Diseases, 9th Revision [ICD-9]: 295.X [excluding 295.7]; ICD-10: F20.X, F21); had ≥1 claim for an OAP on/after 1 January 2007; and had ≥12 and ≥3 months of continuous Medicaid eligibility before and after the index date, respectively. Patients were excluded if they had ≥1 claim for an antipsychotic during the 12-month washout period or for clozapine at any time prior to the index date; had ≥1 schizophrenia-related inpatient admission or ER visit during the 12-month washout period; and had ≥2 claims on different dates with a diagnosis for bipolar disorder (ICD-9: 296.4, 296.5, 296.6, 296.7, 296.89; ICD-10: F31.X) during the baseline period or within 30 days after the index date (Fig. 1).
Patient cohorts
The different LAI initiation strategy cohorts were determined based on adherence and schizophrenia-related inpatient admissions or ER visits observed after OAP initiation, until LAI initiation or censoring. The LAI initiation strategies can be considered proactive (i.e., switching to LAI while still adherent to current OAP) or reactive (i.e., switching to LAI after experience of nonadherence, or after experience of multiple relapses). LAI initiation strategy #1 comprised patients adherent to OAPs without schizophrenia-related inpatient/ER visits; strategy #2 comprised patients nonadherent to OAPs without schizophrenia-related inpatient/ER visits; strategy #3 comprised patients with exactly one schizophrenia-related inpatient/ER visit; and strategy #4 comprised patients with ≥2 schizophrenia-related inpatient/ER visits ≥30 days apart (Fig. 2). In the analyses, patients not yet initiating an LAI by the end of their follow-up, but still at risk of following a given strategy based on the sequence of events observed, were considered censored. Adherence was defined as having a proportion of days covered (PDC) by antipsychotics ≥80%, measured over the minimum of the period between index and LAI initiation/censoring or the last 12 months. For pharmacy claims, the number of days covered was taken from days of supply information. For medical claims, the number of days covered was imputed based on prescribing information for each LAI.
The proportion of patients initiating LAI and the time to LAI initiation in each cohort are shown in Table 1.
Study measures and outcomes
Demographic and clinical characteristics were assessed during the 12-month baseline period (i.e., prior to the date of the first OAP claim) and study outcomes were assessed during the follow-up period. Clinical outcomes included the number of inpatient admissions, number of days spent in an inpatient setting, and number of ER visits. Total healthcare costs, including pharmacy, inpatient, ER, outpatient, long-term care, and other medical service costs, were reported per-patient-per-month and expressed in 2019 US dollars using the medical care component of the Consumer Price Index.
Statistical analysis
Baseline characteristics were summarized using means, SDs, and medians for continuous variables, and frequencies and proportions for categorical variables. Unadjusted comparisons between the four event-driven LAI initiation strategies at the end of the follow-up period were made using analysis of variance models for continuous variables and chi-square tests for categorical variables.
As the different event-driven LAI initiation strategies followed by patients varied over time and depended on baseline characteristics and time-varying confounders that can affect both treatment decisions and outcomes, a dynamic MSM was used to estimate and compare outcomes across LAI initiation strategies in order to generate an unbiased estimate of the effect of each event-driven LAI initiation strategy on the study outcomes29,30,47,48. The authors followed the approach described in Neugebauer et al.29, which accounts for baseline and time-varying confounding due to change in disease severity over time affecting both treatment decisions and outcomes. Patients who did not initiate LAIs during the follow-up period were retained in the sample and censored at the end of their follow-up. Based on this approach, each outcome model includes MSM weights created to balance patient cohorts based on confounders occurring prior to initiating LAIs (inverse probability of treatment weight; IPTW) and based on the probability of being censored during the follow-up period (inverse probability of censoring weight; IPCW).
Patient IPTWs were calculated based on the estimated probability of initiating an LAI, and patient IPCWs were calculated based on the estimated probability of being uncensored at each 3-month interval of the follow-up period (Supplementary Fig. 1). For each patient, the final MSM weight was calculated as the cumulative product between treatment and censoring weights at each interval, over the entire follow-up period. The IPTW and IPCW models included baseline covariates (age, sex, race, state, year of the index date, type of healthcare plan [one indicator for fee-for-service and one indicator for dual Medicaid/Medicare coverage], Quan-CCI, number of unique mental health conditions, type of schizophrenia disorder [paranoid schizophrenia, schizoaffective disorder, or unspecified schizophrenia], suicide-related and violent behavior–related diagnoses, all-cause inpatient costs, all-cause ER costs, all-cause mental-health institute costs, all-cause home care costs, all-cause pharmacy costs, and use of mental health-related agents [anxiolytics, antidepressants, and mood stabilizers]) and time-varying covariates (number of schizophrenia-related inpatient admissions, ER visits, and outpatient visits [in prior interval] and cumulative number of schizophrenia-related inpatient admissions and ER visits [in all prior intervals]).
Clinical and cost outcomes were compared between event-driven LAI initiation strategies using doubly robust MSM-weighted generalized estimating equations models, additionally adjusting for baseline home care costs and the presence of a diagnosis for developmental disabilities during the baseline period. A Poisson distribution was used to calculate rate ratios for count outcomes. A normal distribution was used to calculate mean differences for continuous outcomes. To account for the overdispersion of count variables and non-normal distribution of cost variables, non-parametric bootstrap procedures were used to generate 95% confidence intervals and 2-sided p-values.
To assess whether results are similar across demographic subgroups, the comparison of clinical outcomes was replicated among the following demographic subgroups identified in the database: young adults 18–35 years old (to approximate a population of patients recently diagnosed with schizophrenia), patients >35 years old, males, females, whites/Caucasians, and Blacks. MSM weights were re-estimated for each subgroup.
Data availability
The source data that support the findings of this study were obtained from the individual states pursuant to a data use agreement with each state. Therefore, access to the source data is available only through requests made directly to the corresponding states and not to the authors of this study, being subject to each state’s requirements for data access.
Code availability
All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC). The SAS programs are proprietary materials of Analysis Group, Inc.; therefore, restrictions apply to the access of these codes, which cannot be made available publicly.
References
Kahn, R. S. et al. Schizophrenia. Nat Rev Dis Primers 1, 15067 (2015).
Keepers, G. A. et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 177, 868–872 (2020).
Florida Agency for Health Care Administration. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults (2019–2020).
Kreyenbuhl, J., Buchanan, R. W., Dickerson, F. B. & Dixon, L. B. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull 36, 94–103 (2010).
Mental Health Clinical Advisory Group. Mental health care guide for licensed practitioners and mental health professionals (Oregon Health Authority, 2019).
Osser, D. N., Roudsari, M. J. & Manschreck, T. The psychopharmacology algorithm project at the Harvard South Shore Program: an update on schizophrenia. Harv. Rev. Psychiatry 21, 18–40 (2013).
Llorca, P. M. et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry 13, 340 (2013).
Kane, J. M., Kishimoto, T. & Correll, C. U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J. Clin. Epidemiol. 66, S37–S41 (2013).
Haddad, P. M., Brain, C. & Scott, J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat. Outcome Meas. 5, 43–62 (2014).
Higashi, K. et al. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther. Adv. Psychopharmacol. 3, 200–218 (2013).
Brissos, S., Veguilla, M. R., Taylor, D. & Balanza-Martinez, V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther. Adv. Psychopharmacol. 4, 198–219 (2014).
MacEwan, J. P. et al. Patterns of adherence to oral atypical antipsychotics among patients diagnosed with schizophrenia. J. Manag. Care Spec. Pharm. 22, 1349–1361 (2016).
Offord, S., Wong, B., Mirski, D., Baker, R. A. & Lin, J. Healthcare resource usage of schizophrenia patients initiating long-acting injectable antipsychotics vs oral. J. Med. Econ. 16, 231–239 (2013).
Kishimoto, T., Hagi, K., Kurokawa, S., Kane, J. M. & Correll, C. U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 8, 387–404 (2021).
El Khoury, A., Patel, C., Huang, A., Wang, L. & Bashyal, R. Transitioning from oral risperidone or paliperidone to once-monthly paliperidone palmitate: a real-world analysis among Veterans Health Administration patients with schizophrenia who have had at least one prior hospitalization. Curr. Med. Res. Opin. 35, 2159–2168 (2019).
Joshi, K. et al. Real-world adherence and economic outcomes associated with paliperidone palmitate versus oral atypical antipsychotics in schizophrenia patients with substance-related disorders using Medicaid benefits. J. Comp. Eff. Res. 7, 121–133 (2018).
Correll, C. U. et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J. Clin. Psychiatry 77, 1–24 (2016).
Pilon, D. et al. Treatment patterns, health care resource utilization, and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin. Ther. 39, 1972–1985 (2017).
Besenius, C., Clark-Carter, D. & Nolan, P. Health professionals’ attitudes to depot injection antipsychotic medication: a systematic review. J. Psychiatr. Ment. Health Nurs. 17, 452–462 (2010).
Heres, S., Hamann, J., Kissling, W. & Leucht, S. Attitudes of psychiatrists toward antipsychotic depot medication. J Clin Psychiatry 67, 1948–1953 (2006).
Kirschner, M., Theodoridou, A., Fusar-Poli, P., Kaiser, S. & Jager, M. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode of psychosis. Ther. Adv. Psychopharmacol. 3, 89–99 (2013).
Correll, C. U. et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia 8, 1–10 (2022).
American Association of Community Psychiatrists (AACP). Clinical Tips Series: Long Acting Antipsychotic Medications (2017).
Florida Agency for Health Care Administration. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults (2017–2018).
Brown, B., Turkoz, I., Mancevski, B. & Mathews, M. Evaluation of paliperidone palmitate long-acting injectable antipsychotic therapy as an early treatment option in patients with schizophrenia. Early Interv. Psychiatry 14, 428–438 (2020).
Hargarter, L. et al. Once-monthly paliperidone palmitate in recently diagnosed and chronic non-acute patients with schizophrenia. Expert Opin. Pharmacother. 17, 1043–1053 (2016).
Subotnik, K. L. et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72, 822–829 (2015).
Vega, D., Acosta, F. J. & Saavedra, P. Nonadherence after hospital discharge in patients with schizophrenia or schizoaffective disorder: a six-month naturalistic follow-up study. Compr. Psychiatry 108, 152240 (2021).
Neugebauer, R., Fireman, B., Roy, J. A., O’Connor, P. J. & Selby, J. V. Dynamic marginal structural modeling to evaluate the comparative effectiveness of more or less aggressive treatment intensification strategies in adults with type 2 diabetes. Pharmacoepidemiol. Drug Saf. 21, 99–113 (2012).
Hernan, M. A., Lanoy, E., Costagliola, D. & Robins, J. M. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin. Pharmacol. Toxicol. 98, 237–242 (2006).
Robins, J., Orellana, L. & Rotnitzky, A. Estimation and extrapolation of optimal treatment and testing strategies. Stat. Med. 27, 4678–4721 (2008).
Baser, O., Xie, L., Pesa, J. & Durkin, M. Healthcare utilization and costs of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J. Med. Econ. 18, 357–365 (2015).
Kamstra, R., Pilon, D., Lefebvre, P., Emond, B. & Joshi, K. Treatment patterns and Medicaid spending in comorbid schizophrenia populations: once-monthly paliperidone palmitate versus oral atypical antipsychotics. Curr. Med. Res. Opin. 34, 1377–1388 (2018).
Lafeuille, M. H. et al. Comparison of rehospitalization rates and associated costs among patients with schizophrenia receiving paliperidone palmitate or oral antipsychotics. Am. J. Health Syst. Pharm. 72, 378–389 (2015).
Patel, C. et al. Risk of subsequent relapses and corresponding healthcare costs among recently-relapsed Medicaid patients with schizophrenia: a real-world retrospective cohort study. Curr. Med. Res. Opin. 37, 665–674 (2021).
Pilon, D. et al. Short-term rehospitalizations in young adults with schizophrenia treated with once-monthly paliperidone palmitate or oral atypical antipsychotics: a retrospective analysis. Curr. Med. Res. Opin. 35, 41–49 (2019).
Pilon, D. et al. Adherence, healthcare resource utilization and Medicaid spending associated with once-monthly paliperidone palmitate versus oral atypical antipsychotic treatment among adults recently diagnosed with schizophrenia. BMC Psychiatry 17, 207 (2017).
Patel, C. et al. National and regional description of healthcare measures among adult Medicaid beneficiaries with schizophrenia within the United States. J. Med. Econ. 25, 792–807 (2022).
Ostuzzi, G. et al. Factors associated with first- versus second-generation long-acting antipsychotics prescribed under ordinary clinical practice in Italy. PLoS ONE 13, e0201371 (2018).
Tipirneni, R. & Ayanian, J. Spillover benefits of medicaid expansion for older adults with low incomes. JAMA Health Forum 3, e221389 (2022).
Dencker, S. J. & Axelsson, R. Optimising the use of depot antipsychotics. CNS Drugs 6, 367–381 (1996).
Fayek, M., Flowers, C., Signorelli, D. & Simpson, G. Psychopharmacology: underuse of evidence-based treatments in psychiatry. Psychiatr. Serv. 54, 1453–1456 (2003).
Lin, D. et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs 35, 469–481 (2021).
Tiihonen, J. et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29823 patients with schizophrenia. JAMA Psychiatry 74, 686–693 (2017).
Parellada, E. et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J. Psychopharmacol. 19, 5–14 (2005).
Munday, J. et al. Early initiation of long-acting injectable antipsychotic treatment is associated with lower hospitalization rates and healthcare costs in patients with schizophrenia: real-world evidence from US claims data. Curr. Med. Res. Opin. 35, 1231–1239 (2019).
Hernan, M. A., Brumback, B. & Robins, J. M. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11, 561–570 (2000).
Hernan, M. A., Brumback, B. A. & Robins, J. M. Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Stat. Med. 21, 1689–1709 (2002).
Acknowledgements
This study was supported by Janssen Scientific Affairs, LLC. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication. Medical writing assistance was provided by Flora Chik, PhD, an employee of Analysis Group, Inc. Part of the material in this manuscript was presented at the US Psychiatric and Mental Health Congress 2021, held in San Antonio, TX, October 29–November 1, 2021 and at the Academy of Managed Care Pharmacy (AMCP) annual meeting, held in Chicago, IL, March 29–April 1, 2022.
Author information
Authors and Affiliations
Contributions
B.E., M.H.L., L.M., I.G., and P.L. contributed to study conception and design, collection, and assembly of data, and data analysis and interpretation. C.U.C., C.B., C.P., D.L., and P.M. contributed to study conception and design, as well as data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Corresponding author
Ethics declarations
Competing interests
B.E., M.H.L., L.M., I.G., and P.L. are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. C.B., C.P., D.L., and P.M. are employees of Janssen Scientific Affairs, LLC and stockholders of Johnson & Johnson. C.U.C. has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Cardio Diagnostics, Compass, Damitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Pharmabrain, Recordati, Relmada, Reviva, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Mindpax, and LB Pharma.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Correll, C.U., Benson, C., Emond, B. et al. Comparison of clinical outcomes in patients with schizophrenia following different long-acting injectable event-driven initiation strategies. Schizophr 9, 9 (2023). https://doi.org/10.1038/s41537-023-00334-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-023-00334-3