Abstract
Pulmonary rehabilitation (PR) improves functional capacity, health-related quality of life (HRQoL) in COPD patients, and maintenance programmes are relevant in preserving those improvements. However, little is known about the structure of maintenance programmes after PR. We performed a systematic review and meta-analysis of experimental and quasi-experimental studies evaluating individuals with COPD admitted to a maintenance PR programme, delivered after an initial PR programme. We reported functional capacity evaluation (6-minute-walking-test), HRQoL, dyspnoea and symptom control. Searches were performed on the 11th April 2021 using MEDLINE, Embase, EBSCO, CINAHL, Web of Science and Cochrane Library. We extracted summary-level data from trial publications and used a random-effects model, predicting that severe heterogeneity was detected. The protocol was registered in PROSPERO (CRD42021247724). Fifteen studies were included in the meta-analysis, with 1151 participants. Maintenance programmes were associated with a pooled mean increase of 27.08 meters in 6mWT (CI: 10.39 to 43.77; I2 = 93%; p < 0.0001), being better in supervised, long (>12 month) home-based programmes; and having a potential MD of -4.20 pts in SGRQ (CI: -4.49 to -3.91; I2 = 0%; p = 0.74). Regarding dyspnoea and exacerbations, we found a nonsignificant trend for improvement after maintenance PR programmes. Severe COPD patients showed smaller improvements in programmes up to a year. Overall, the strength of the underlying evidence was moderate. Despite limitations of risk of bias and heterogeneity, our results support that home-based, supervised, long-term maintenance PR programmes may significantly improve functional capacity in COPD patients and HRQoL.
Similar content being viewed by others
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable and treatable disease, and it is the third cause of death worldwide1. COPD patients often present a decline in functional capacity (FC) over time and persistent symptoms such as dyspnoea, low exercise tolerance and fatigue, leading to poor health-related quality of life and an increased risk for exacerbations and death1.
Pulmonary Rehabilitation (PR) is one of the most cost-effective treatments for COPD1. Its standard core elements are physical exercise training, patient-directed education, smoking cessation support, disease self-management and behaviour change1,2,3,4. However, improvements in the functional capacity of COPD patients last less than one year1,2,4.
Functional capacity tends to decrease over time, mainly if the patient does not change his behaviour concerning physical activity1. While initial studies showed a difference at six months favouring supervised maintenance exercises5,6,7, the effect seems not to be sustained at 12 months. A more recent meta-analysis suggested that maintenance PR programmes might confer long-term efficacy (12 months) on functional capacity in patients with COPD8, with a mean difference in the six-minute walk test (6mWT) of 27 meters. The 6mWT is a predictor of morbidity and mortality9 among COPD patients, it is a simple clinical exercise test for the objective evaluation of functional exercise capacity improvement in COPD, which measures the distance a patient can quickly walk on a flat hard surface in 6 minutes.
It is well known that PR programmes include outpatient or inpatient (centre-based), community and home-based types. Most PR programmes are centre-based. Participants are prescribed personalized and structured programmes3, usually lasting eight to twelve weeks1,2. Still, such programmes vary significantly depending on healthcare systems and local policies2,10. According to the consensus of PR, to maintain the benefits of this intervention, health behaviour change is crucial1,2,4.
Malaguti et al. 11, in a recent Cochrane review that specifically included papers on supervised maintenance programs, states that these programmes have an impact on functional exercise capacity. Particularly, the optimal duration to achieve relevant benefits, the role of the supervision and setting of maintenance PR programmes is still unclear12. Thus, little is known about what components should constitute a maintenance programme8.
This systematic review and meta-analysis aimed to synthesise’ evidence of interventional studies, including maintenance PR programmes on COPD patients, evaluate its impact on functional capacity and other disease-related secondary outcomes, and determine the components of those programmes that lead to better long term outcomes.
Methods
This systematic review and meta-analysis followed the Cochrane Handbook recommendations13. Our study protocol has been prospectively registered and published in the PROSPERO database, CRD42021247724.
Search strategy and selection criteria
The primary outcome of this study was the functional capacity measured by the 6mWT, and we selected experimental and quasi-experimental studies evaluating individuals with COPD submitted to a maintenance PR programme, delivered after an initial PR programme and reporting an evaluation of functional capacity measured by the 6mWT14,15. Quasi-experimental studies were considered in this systematic review because we aimed to acknowledge the components of the intervention.
Secondary outcomes considered for inclusion were dyspnoea, measured by the modified Medical Research Council scale (mMRC);16 exacerbations, measured by hospital admissions during the maintenance rehabilitation programme;17 and health-related quality of life (HRQoL), measured by the St George’s Respiratory Questionnaire (SGRQ)18, the EuroQoL519 or SF-3620.
We excluded non-interventional studies and studies without an initial PR programme. In addition, studies in which the initial PR programme included only physical exercise or only education were also excluded because they are not actual PR programmes according to the accepted definition of PR2,4. We did not exclude studies based on language.
The search was performed on the 11th April 2021 in the Scopus database (MEDLINE and Embase), EBSCO, the Cumulative Index to Nursing and Allied Health Literature [CINAHL] Complete, Web of Science and Cochrane Library. In addition, we reviewed the references of the included literature and correlated systematic reviews. See “Supplementary Information 1 – Search strategy”.
Two researchers (LS, TM) conducted the study selection independently, using the RYYAN QCRI app (available at: https://www.rayyan.ai/), and all disagreements were resolved by consensus or through consultation with a third investigator (MP) in the review team. Duplicated and irrelevant studies were rejected first by examining titles and abstracts. After that, full texts of potentially eligible studies were obtained and reviewed according to inclusion and exclusion criteria. When the full text was not available, we contacted the authors to obtain raw data, and if the authors did not answer in two weeks, the study was excluded from the selection.
Data analysis
For selected articles, two researchers (LS and TM) extracted the following study characteristics: study design, country of study, year of publication, sample size and participant characteristics [such as age, sex and severity of COPD assessed by percentage (%) of predicted Forced Expiratory Volume in the 1st second (FEV1)], duration of the previous/initial PR, setting/design and duration of the maintenance PR programme, supervision and follow-up. Estimates of the association between the intervention parameters (PR programmes) and the study outcomes were measured as mean and standard deviation (SD) for all continuous outcomes. Missing SD was calculated as recommended in the 16.1.3.2 chapter of Cochrane Handbook for Systematic Reviews of Interventions13. Studies that do not report group means or MD were not included in the meta-analysis. Agreement between researchers was calculated with kappa statistics13.
The meta-analysis was conducted using RevMan software v.5.421. Due to the expected heterogeneity, we applied a random-effect model. For continuous outcome measures, data are presented as the mean and SD, and the effect size was estimated by the mean difference (MD) with 95% confidence intervals (CI). Missing SDs were arithmetically calculated13. Studies not reporting group means or MD were not included in the meta-analysis. Data not accessed through meta-analyses was summarised and described in the text.
In addition, there was, as we expected, heterogeneity due to the lack of evidence concerning the structure of maintenance programmes. Therefore, we performed sensitivity (leave-one-out) and subgroup analyses defined as a priori. We compared the effectiveness of the interventions between the duration of the initial PR programme, the severity of COPD (assessed by % of predicted FEV1, type of professional supervision and setting, risk of bias and publication year2,4,11. The guidelines recommend that these programmes last between eight and twelve weeks, therefore, we wanted to evaluate if the effects of a maintenance PR program were different in a twelve, eight or less than eight weeks programme2,4
The duration of the maintenance program and supervision by a health professional are aspects that vary substantially between existing programs and settings. We also wanted to adjust for the severity of COPD, according to % of predicted FEV1, and, finally, if the studies published before the 2013 guidelines2 had different results. The methodological quality of the primary studies was also a reason for the analysis.
We used R22,23,24,25 to perform a mixed-effects meta-regression to analyze the continuous variables in consideration of the presence of “residual heterogeneity”26. In addition, subgroup analysis was performed for all the categorical variables.
Assessment of the risk of bias
Two independent researchers (LS and TM) assessed the quality of the evidence for the collected outcomes of interest using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system13. We appraised each study’s components, including selection, performance, detection, attrition, reporting, and other biases. For each study, either for the grading of each component and for the global study rating, we assigned categories of risk of bias: low, unclear, and high. The global grading involved taking an average of all individual components13. As blinding of allocation to patient and PR therapist was not possible, studies without any other source of bias were considered as being at the lowest risk possible for this type of intervention. We assessed publication bias with a funnel plot created in RevMan software v.5.421.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
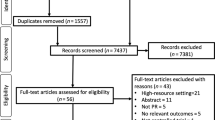
The electronic database searches identified 3890 records, from which we removed 1298 duplicate records. From the remaining 2592 references, 2557 were excluded by title and abstract. This resulted in 35 records for the full-text review. After this, we excluded six records for failing to meet the inclusion criteria (three studies did not report an evaluation of functional capacity measured by the 6mWT. Two of them reported the endurance shuttle walk test27,28 and found no differences between groups in this test, and one study evaluated physical activity29. Three studies did not have an initial PR programme or the programme only included physical exercise, three were not a PR intervention, and one study was still ongoing30) (Fig. 1). We were unable to contact the authors of 5 records. Six citations reported duplicated data, referring to the same studies. Finally, 17 studies were kept after careful inspection. The k value was 0,85, indicating an almost perfect agreement13.
Risk of bias in included studies
The risk of bias full details may be found in “Supplementary Information 3 - Quality Assessment and Risk of Bias Table”. All studies had no blinding of participants and personnel, which was due to the nature of the intervention itself. Four studies presented a lack of blinding as the only source of bias, representing the best bias-free design possible for this kind of intervention. In addition, more than half of the included studies did not refer to the blinding of the outcome assessment, which is an important step that may overcome the difficulty upon blinding the intervention (Fig. 2).
The funnel plot analysis detected no relevant publication bias for all outcomes (see “Supplementary Information 4 – Complete meta-analysis data”).
Description of studies
This review included 17 studies,14 of which were randomised controlled trials31,32,33,34,35,36,37,38,39,40,41,42,43,44, and 3 were quasi-experimental trial (two studies had no control group45,46, and one was not randomized47). Detailed information on included studies is available in “Supplementary Information 2 - Complete data of selected studies”.
The publication year varies from 200231,32 to 202140. The mean participants’ ages ranged between 62 and 69 years old, and the mean percentage of predicted FEV1 was between 34 and 66%.
The studies included 1222 participants, all receiving an initial PR programme, varying between three39 and twelve weeks33,34,36,38 duration. Among these, 1151 participants were randomly assigned to either a control or an intervention group. Forty participants were allocated to one of the groups, though not randomly47.
The intervention group had access to a maintenance PR programme, varying between 231 and 3636 months. The presence of a healthcare provider supervision was variable (Table 1); in five studies, the intervention was always supervised31,33,43,47. In the other five, there was no supervision32,34,39,40,42, in six studies some of the sessions were supervised35,37,38,41,44.
In six studies, intervention was assessed through a home-based programme31,34,39,41,43, three took place in a community centre33,37,47, four studies used programmes that incorporated both home-based and community-based sessions32,35,38,44, and two studies assessed telerehabilitation40,42.
Five studies showed a significant improvement in functional capacity31,35,36,43,47 after the maintenance programme. In general, they used strategies for measuring programme adherence through daily exercise records performed by patients36. Within this group, three studies included home-based supervised programmes31,36,43.
In five studies36,40,42,43,47, education for self-management was provided. Only one study controlled the exercise intensity by the patient’s maximum heart rate and symptoms43. Pedometers were used in two studies38,39, and an experiment with music during exercise training and walks was explored in one study31.
One study36 included structured healthcare provider supervision regarding long-term programme transitions, and another study43 modified the intervention over time, reducing the number of sessions supervised in stages and alternating with telephone contacts.
Meta-analysis and meta-regression
This review included 15 studies in the meta-analysis31,32,33,34,35,36,37,38,39,40,41,42,43,44,47, two of which were excluded45,46 because they lacked a control group. The two studies with more weight were Wetering et al. and Brooks et al. 32,36. We analysed the changes after the initial PR until the end of the maintenance and calculated the MD between these two moments.
Primary outcome – Functional capacity measured with the 6mWT
Most studies showed an effect favouring maintenance PR programmes over usual care, of which five studies showed high certainty evidence (Fig. 3). The pooled estimate for the maintenance group is a MD of 27 meters (CI: 10.4 to 43.8; I2 = 93%; p < 0.0001) (Fig. 3), which lies between the minimal important difference confidence interval that ranges between 25 and 3314.
Considering the severe heterogeneity detected across studies (I2 = 93%), we performed a sensitivity analysis with the leave-one-out method. Only one study showed an effect favouring usual care over maintenance PR programmes with high certainty evidence32. Although it revealed a slight reduction of heterogeneity to I2 = 81%, no significant changes were found in the pooled estimate. Supplementary Information 4 summarises the main findings of clinical outcomes from selected studies.
Nine studies33,34,37,38,39,40,41,42,44 showed low certainty evidence of the effect, in which 439,40,41,44 the effect favoured the maintenance PR group over usual care. These studies included interventions with health technologies and telerehabilitation and were mainly unsupervised.
Subgroup and sensitivity analyses are available in detail in “Supplementary Information 4 - Complete meta-analysis data”.
The sub-analysis according to maintenance PR programme duration found a difference in the 6mWT favouring maintenance compared to the usual care group in programmes with more than 12 months36,41 with a MD of 14 meters with high certainty evidence and a significative reduction of heterogeneity (CI 12.8 to 15.0; I2 = 0%; p < 0.38). However, although programmes with eight weeks favoured maintenance PR programmes over usual care, they had high heterogeneity. Shorter programmes had low certainty evidence and high heterogeneity, although they also favoured maintenance PR programmes over usual care.
Subgroup analysis according to the initial PR programme duration revealed a difference in the 6mWT favouring maintenance compared to the usual care in studies with the initial PR programme of 12 weeks33,34,36,38 with high certainty evidence and a significative reduction of heterogeneity (MD of 14 meters; CI 12.8 to 15.0; I2 = 0%; p = 0.63). Studies with eight weeks of initial PR programmes35,40,41,42,43,44 also revealed a difference in the 6mWT favouring maintenance compared to the usual care (MD of 38 meters; CI 15.9 to 61.6; I2 = 60%; p = 0.03) but with marked heterogeneity across studies. One study32 has both inpatients in a 6-week PR programme and outpatients in an 8-week programme. Two studies31,37 have no data about the duration of the PR initial programme, and we excluded them from this subanalysis.
Regarding supervision by healthcare professionals, subgroup analysis revealed a difference in the 6mWT favouring maintenance compared to the usual care in studies with supervised intervention with high certainty evidence, although there was no significant reduction of heterogeneity (MD of 77 meters; CI 19.2 to 134.1.0; I2 = 90%; p < 0.00001). Programmes in which some of the sessions were supervised and others were unsupervised35,36,37,38,41,44 the results in the 6mWT favoured maintenance PR programmes compared to the usual care, with high certainty evidence and no heterogeneity detected across studies. (MD of 14 meters; CI 12.8 to 15.0; I2 = 0%; p = 0.51).
Considering COPD severity, studies that include people with all degrees of severity32,34,37,40,41,43,47 the results in the 6mWT favoured maintenance PR programmes compared to the usual care, with high certainty evidence and no heterogeneity detected across studies. (MD of 14 meters; CI 12.8 to 15.0; I2 = 0%; p = 0.44). Notwithstanding, although studies that enrolled moderate to severe COPD patients also favoured maintenance PR programmes compared to the usual care, they had low certainty evidence and high heterogeneity.
According to the setting of the maintenance PR programme (Fig. 4), home-based maintenance programmes31,34,36,39,41,43, the 6mWT favoured maintenance PR programmes compared to the usual care, with high certainty evidence but significant heterogeneity detected across studies. (MD of 51 meters; CI 13.6 to 87.4; I2 = 92%; p < 0.00001). Even though other settings, such as community-based, telerehabilitation or the combination between home and community-based, favoured maintenance PR programmes compared to the usual care, they had low certainty evidence and relevant heterogeneity.
According to the studies’ risk of bias subgroup analysis, studies with lower risk36,37,39,42 showed results in the 6mWT favouring maintenance PR programmes compared to the usual care, with high certainty evidence and a significant reduction of the detected heterogeneity across studies (MD of 14 meters; CI 12.8 to 15.0; I2 = 0%; p = 0.78).
Subgroup analysis according to publication year revealed no relevant findings.
In meta-regression, as we observe in Fig. 5, supervision, setting, and programme duration are heterogeneity moderators (p = 0.05), and, when adjusted to programme duration and setting, supervised programmes have a mean increment of 89 meters in MD between groups when compared to unsupervised programmes (p < 0.001). In the same metaregression, home-based programmes have a mean increment of 57 meters between groups compared to centre-based programmes (p < 0.01).
The meta-regression has shown that when adjusting to the setting and duration of the maintenance PR programme, supervised programmes when compared to unsupervised programmes, had a mean increment of 89 meters in MD between groups (p < 0.001), and home-based programmes had a mean increment of 57 meters (p < 0.01) in MD between groups when adjusted to both supervision and duration of the maintenance PR programme. Furthermore, when adjusting to the severity of the disease using the FEV1, supervised PR programmes had a mean increment of 73 meters (p < 0,01) compared to unsupervised programmes.
Secondary outcome - Health-related quality of life
Among the primary studies that evaluated the HRQoL, we found two studies42,46 using the EQ5D and two others using the SF-36 form40,41 to monitor the HRQoL. None revealed significant changes in HRQoL between groups. In addition, four studies evaluated HRQoL with the SGRQ35,36,38,42.
Overall, the studies showed little to no difference between maintenance and usual care groups concerning the SGRQ, and high heterogeneity was detected. We performed a sensitivity analysis using the leave one out method. Therefore, we exclude the studies on the risk of bias. (See Supplementary Information 4) After removing Spencer et al. 35, heterogeneity is not detectable, and the SGRQ results in favour maintenance PR programmes compared to the usual care, with high certainty evidence and a significant reduction of the detected heterogeneity across studies (MD of −4 pts; CI −4.5 to −3.9; I2 = 0%; p = 0.57) (Fig. 6). The same trend was found when considering only studies with a low risk of bias36,42 (MD −4 pts; CI −4.5 to −3.9; I2 = 0%; p = 0.74), achieving the minimal important change to general COPD patients of four points18.
Secondary outcome - Dyspnoea
The studies showed little to no difference between maintenance and usual care groups concerning the mMRC, and high heterogeneity was detected (MD −1.2 pts; CI −2.5 to 0.1; I2 = 99%; p < 0.0001)34,36,43 On sensitivity analysis, we observed that the one study43, although with the more pronounced potential benefit, is the one most contributing to heterogeneity. As there are only three studies with data, no subgroup analyses were performed.
Secondary outcome - Symptoms control
The studies showed little to no difference between maintenance and usual care groups concerning symptom control, using the Chronic Respiratory Disease Questionnaire33,37,39,40,41.
Secondary outcome - Exacerbations
Exacerbations were monitored in five studies32,33,35,40,43. The mean number of exacerbations in the maintenance programmes was lower than in the control group but without significant estimates (Intervention: 17.8 exacerbations; Control: 18.8 exacerbations).
Discussion
This review has synthesised the available evidence of the long‐term effects and the structure of maintenance PR programmes in COPD patients compared to usual care. Our results confirm that such programmes significantly increased functional capacity. This improvement was more pronounced in supervised PR programmes, in those with longer duration (over 12 months), in a home-based setting, and after initial programmes with more than eight weeks.
Our results are aligned with recent meta-analyses11,48, showing that maintenance PR programmes confer long-term efficacy on functional capacity in COPD patients compared to usual care.
We found that different types of follow-up had different effects on patients. High certainty evidence suggests that more extended programmes (>12 months) seem superior to shorter ones compared to usual care. This comes with no surprise, as exercise training is known to play a central role in PR, and maintaining that training is one of the most critical factors2,4. However, continuing more extended PR programmes is often difficult due to a lack of human resources and, in home-based programmes, the need for healthcare professionals to visit the patient’s home. Additionally, the costs of delivering such maintenance programmes have not been well documented across different models. The impact of maintenance programmes on other healthcare costs (e.g. hospitalisation, primary care visits) is unknown and should be the topic of further studies.
We could also demonstrate that home-based programmes are associated with better maintenance of patients’ functional capacity compared to usual care. This is not unexpected, as a Cochrane Review48 comparing PR or maintenance rehabilitation in COPD patients showed that telerehabilitation improved exercise capacity in maintenance programmes. Additionally, rehabilitation at home has been shown to improve long-term HRQoL11.
While home-based settings are convenient for the patient, they may lack the equipment or floor space. In addition, it may be challenging to determine the progress within a therapy session. Nevertheless, home-based maintenance PR programmes provide an adequate setting for promoting pedometer-based physical activity, diaries and guidebooks49, which have demonstrated, using positive feedback, increasing the number of steps-per-day performed by patients50, which can be ways to achieve better physical activity levels in COPD patients following PR51. Furthermore, such feedback mechanisms are essential to motivation, which plays a central role in behaviour change.
The cornerstone of maintenance programme success might be engaging COPD patients in therapeutic adherence, considering they are time-consuming and need a stable commitment to the plan49. For that reason, supervision by healthcare professionals seems important to achieve better results, either in centre-based or home-based interventions.
Maintenance PR programmes included in the meta‐analysis differed in several aspects, including clinical setting, duration and composition. However, our work demonstrates the superiority of supervised training during the programme compared to usual care, rather than other modalities, having supervised programmes have a mean increment of 62 meters in MD between groups compared to unsupervised programmes (Supplementary Information 4), following other studies8,11. In addition, home-based PR programmes seem to have better outcomes with structured schedules, including health professional visits and regular phone calls between them38,39,41,43.
Supervised PR maintenance programmes are effective in the early stages to better tailor exercise training to the patient and thereby increase programme compliance2,3,10, and can progressively be replaced by non-supervised sessions, maintaining a good impact on functional capacity and decreasing health system burdens. This “stepwise approach” in time, with tighter supervision in the early stages of the programme, gradual increase of more spaced visits and telephone calls, and unsupervised strategies in later stages, might lead to long-lasting changes in behaviour and support patients’ autonomy and responsibility for their health status while optimizing resources. This is an important finding regarding programme feasibility and resources needed to implement it35,36,37,38,41,44.
Our review could not find evidence of the ideal core components of maintenance PR programmes, such as the type of intervention and its specific components or the appropriate duration/format/setting according to each specific profile of COPD patient. However, the effect of different components of rehabilitation in COPD, such as contributions of educational activities and psychological support to exercise training, would be relevant to physicians and allied healthcare professionals who prescribe rehabilitation and policymakers who allocate the resources.
As patients with COPD are concerned mainly with treating symptoms1, HRQoL should be considered an additional critical outcome in PR. The present meta‐analysis reconfirms the previous findings52 that PR effectively relieves dyspnoea and fatigue and improves patients’ control over the disease.
Of particular relevance, our findings showed that more severe patients do not seem to improve symptom control with shorter (<6 months) maintenance programmes. These results are aligned with Salman et al. 53, which demonstrated that severe COPD rehabilitation groups did significantly better than control groups only when the rehabilitation programmes were six months or longer. Severe COPD patients have a fluctuating course of the disease, with frequent exacerbations and a vicious cycle of increasing dyspnoea, which leads to decreased exercise tolerance, depression, and social isolation, all predictors of poor HRQoL and high risk of mortality54. Furthermore, from a rehabilitation point of view, such patients present skeletal muscle wasting, which further contributes to muscle fatigue during exercise. This can lead patients to stop exercising even before reaching their aerobic capacity55. However, patients with severe COPD are the subgroup that shows the most improvements after PR56, which might mean that this subgroup of patients will benefit the most from long-term interventions. Therefore, despite the lack of suggestions for long-term rehabilitation schemes (>6 months) in severe COPD patients in current guidelines2, this subgroup should be actively offered home-based, supervised, long-term programmes, according to the best available evidence.
Owing to the paucity of data in included studies, we could not establish the ideal structure of PR, mainly regarding its specific components or even specific features/duration/setting to each profile of COPD patient.
This systematic review has limitations. The intervention was heterogeneous among included studies in terms of duration, type, setting or supervision. Most studies had an unclear and high risk of bias in their global quality rating, with some evidence of significant heterogeneity across studies, limiting the strength of evidence and the confidence of our findings. Given the objective of this systematic review, we understand that by excluding programmes that have not delivered a comprehensive PR programme as defined2,4 before the maintenance PR programme, we could have lost some relevant studies that could have reached different results. The same concerns other outcome measures such as the endurance shuttle walking test (ESWT) and the incremental shuttle walking test (ISWT). However, the ESWT and the ISWT have been used in fewer studies and show little to no difference between maintenance and usual care11. Our pooled estimate using the 6mWT was the same as a previous review8.
However, this review has some strengths. It has been conducted with a robust methodology, following the Cochrane Handbook recommendations13, and our search was comprehensive. We also performed a GRADE-based assessment of the quality of evidence and found limited reasons to suspect publication bias according to the funnel plot (Supplementary Information 4).
Our results highlight the importance of PR maintenance programmes on COPD treatment, mainly when home-based, supervised and long-term, delivered after an initial PR intervention. COPD patients submitted to maintenance PR programmes seem to benefit from significant improvements in functional capacity and other clinical outcomes, ultimately reducing the risk for future exacerbations and death. Functional capacity seems to be improved after 12 months or more if both education and physical exercise are present in the patient’s daily routine. More long-term studies are needed to evaluate the potential benefit of maintenance PR programmes above the 12-month horizon, mainly in relevant clinical outcomes, such as clinical control and exacerbation risk. Finally, future guidelines and stakeholders should reinforce the importance and investment in maintenance PR programmes, as they play a crucial role in COPD treatment.
We recommend that supervised, home-based maintenance PR programmes lasting 12 months be routinely offered as an add-on to remaining treatment strategies since the programmes with this structure increase functional capacity in COPD patients. In addition, severe COPD patients should be offered more extended programmes (>12 months).
Regarding dyspnoea and exacerbations, we found a non-significant trend for improvement after maintenance PR programmes. Severe COPD patients showed smaller improvements in programmes for up to a year. Overall, the strength of the underlying evidence was moderate.
Data availability
Raw data were available from the cited articles. In addition, derived data supporting the findings of this study are available from the corresponding author (LS) on request.
References
GOLD. Global Strategy for Prevention, Diagnosis and Management of COPD. 177 (2022).
Spruit, M. A. et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 188, 1390–1413 (2013).
Clini, E., Holland, A. E., Pitta, F. & Troosters, T. Textbook of Pulmonary Rehabilitation. (SPRINGER).
Holland, A. E. et al. AMERICAN THORACIC SOCIETY Defining Modern Pulmonary Rehabilitation. 18, (2021).
Beauchamp, M. K., Evans, R. & Janaudis-ferreira, T. Systematic Review of Supervised Exercise Programs After Pulmonary Rehabilitation in Individuals With COPD. Chest 144, 1124–1133 (2013).
Jenkins, A. R. et al. Efficacy of supervised maintenance exercise following pulmonary rehabilitation on health care use: a systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 257–273 (2018).
Busby, A. K., Reese, R. L. & Simon, S. R. Pulmonary rehabilitation maintenance interventions: A systematic review. Am. J. Health Behav. 38, 321–330 (2014).
Imamura, S. et al. Long-term efficacy of pulmonary rehabilitation with home-based or low frequent maintenance programs in patients with chronic obstructive pulmonary disease: a meta-analysis. Ann. Palliat. Med. 9, 2606–2615 (2020).
Issues, S., Test, M. W. & Equipment, R. & Preparation, P. American Thoracic Society ATS Statement: Guidelines for the Six-Minute Walk. Test 166, 111–117 (2002).
Nici, L. Can we make it last? Maintaining benefits achieved with pulmonary rehabilitation. Lung 185, 241–242 (2007).
Malaguti, C., S, D. C., Janjua, S. & AE, H. Supervised maintenance programmes following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease (Review). https://doi.org/10.1002/14651858.CD013569.pub2.Copyright (2021).
Demeyer, H. et al. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest 146, 318–327 (2014).
Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions. (2019).
Holland, A. E. et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 44, 1428–1446 (2014).
Spencer, L., Alison, J. & McKeough, Z. Six-minute walk test as an outcome measure: are two six-minute walk tests necessary immediately after pulmonary rehabilitation and at three-month follow-up? Am. J. Phys. Med. Rehabil. 87, 224–228 (2008).
Hajiro, T. et al. Analysis of Clinical Methods Used to Evaluate Dyspnea. Am. J. Respir. Crit. Care Med. 158, 1185–1189 (1998).
Calverley, P. M. A. Minimal clinically important difference - Exacerbations of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2, 143–148 (2005).
Welling, J. B. A., Hartman, J. E., Ten Hacken, N. H. T., Klooster, K. & Slebos, D. J. The minimal important difference for the St George’s Respiratory Questionnaire in patients with severe COPD. Eur. Respir. J. 46, 1598–1604 (2015).
McNamara, R. J., McKeough, Z. J., Mo, L. R., Dallimore, J. T. & Dennis, S. M. Community-based exercise training for people with chronic respiratory and chronic cardiac disease: A mixed-methods evaluation. Int. J. COPD 11, 2839–2850 (2016).
Brigden, A. et al. Defining the minimally clinically important difference of the SF-36 physical function subscale for paediatric CFS/ME: Triangulation using three different methods. Health Qual. Life Outcomes 16, 1–7 (2018).
RevMan User guide. Welcome to RevMan 5. 4. 4, (2020).
RStudio Team. RStudio: Integrated Development for R. (2020).
R Core Team. R: A Language and environmental for statistical computing. (2021).
Viechtbauer, W. Conducting meta-analyses in R with the metafor. J. Stat. Softw. 36, 1–48 (2010).
Balduzzi, S., Rücker, G. & Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 22, 153–160 (2019).
Cooper, H. M., Valentine, J. C. & Hedges, L. V. Handbook of Research Synthesis and Meta-Analysis 2nd Edition. The Lancet vol. 389 (2017).
Wilson, A. M. et al. The effects of maintenance schedules following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomised controlled trial. BMJ Open 5, e005921 (2015).
Ringbaek, T., Brøndum, E., Martinez, G., Thøgersen, J. & Lange, P. Long-term Effects of 1-Year Maintenance Training on Physical Functioning and Health Status in Patients With COPD - A randomized controlled study. 47–52.
Saini, P. K. et al. Activity maintenance after pulmonary rehabilitation-first results of an online coaching program. Am. J. Respir. Crit. Care Med. 195 CC-, (2017).
Ovadya, D. et al. Remote Prescribed and Monitored Excercise Program After Pulmonary Rehabilitation in Individuals With Chronic Lung Disease.
Bauldoff, G. S., Hoffman, L. A., Zullo, T. G. & Sciurba, F. C. Exercise Maintenance Following Pulmonary Rehabilitation * Effect of Distractive Stimuli. Chest 122, 948–954 (2002).
Brooks, D., Krip, B., Mangovski-Alzamora, S. & Goldstein, R. S. The effect of postrehabilitation programmes among individuals with chronic obstructive pulmonary disease. Eur. Respir. J. 20, 20–29 (2002).
Román, M. et al. Efficacy of pulmonary rehabilitation in patients with moderate chronic obstructive pulmonary disease: a randomized controlled trial. BMC Fam. Pract. 14, 21–29 (2013).
Souza, Y. D. E. et al. Use of a Home-Based Manual as Part of a Pulmonary Rehabilitation Program. Respir. Care 63, 1485–1491 (2018).
Spencer, L. M., Alison, J. A. & Mckeough, Z. J. Mantaining benefits following pulmonary rehabilitation: a randomised controlled trial. Eur. Respir. J. 35, 571–577 (2010).
Wetering, C. R. Van, Hoogendoorn, M., Mol, S. J. M., Molken, M. P. M. H. R. & Schols, A. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax 7–13 https://doi.org/10.1136/thx.2009.118620 (2010).
Butler, S. J. et al. Randomized controlled trial of community-based, post-rehabilitation exercise in COPD. Respir. Med. 174, (2020).
Cruz, J., Brooks, D. & Marques, A. Walk2Bactive: A randomised controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron. Respir. Dis. 13, 57–66 (2016).
Moulin, M., Taube, K., Wegscheider, K., Behnke, M. & Bussche, H. van der. Home-Based Exercise Training as Maintenance after Outpatient Pulmonary Rehabilitation. RESPIRATION 77, 139–145 (2008).
Galdiz, J. B. et al. Telerehabilitation Programme as a Maintenance Strategy for COPD Patients: A 12-Month Randomized Clinical Trial. Arch. Bronconeumol. 57, 195–204 (2021).
Guell, M.-R. et al. Benefits of Long-Term Pulmonary Rehabilitation Maintenance Program in Patients with Severe Chronic Obstructive Pulmonary Disease - Three-Year Follow-up. Am. J. Respir. Crit. Care Med. 195, 622–629 (2017).
Jiménez-Reguera, B. et al. Development and Preliminary Evaluation of the Effects of an mHealth Web-Based Platform (HappyAir) on Adherence to a Maintenance Program After Pulmonary Rehabilitation in Patients With Chronic Obstructive Pulmonary Disease: Randomized Controlled Trial. JMIR MHEALTH UHEALTH 8, (2020).
Li, Y., Feng, J., Li, Y., Jia, W. & Qian, H. A new pulmonary rehabilitation maintenance strategy through home-visiting and phone contact in COPD. Patient Prefer. Adherence 97–104 (2018).
Ries, A. L., Kaplan, R. M., Myers, R. & Prewitt, L. M. Maintenance after Pulmonary Rehabilitation in Chronic Lung Disease. Am. J. Respir. Crit. Care Med. 167, 880–888 (2003).
Cockram, J., Cecins, N. & Jenkins, S. Maintaining exercise capacity and quality of life following pulmonary rehabilitation. RESPIROLOGY 11, 98–104 (2006).
Zanaboni, P., Hoaas, H., Lien, L. A., Hjalmarsen, A. & Wootton, R. Long-term exercise maintenance in COPD via telerehabilitation: a two-year pilot study. J. Telemed. Telecare 23, 74–82 (2017).
Moullec, G. & Ninot, G. An integrated programme after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: effect on emotional and functional dimensions of quality of life. Clin. Rehabil. 24, 122–136 (2010).
Cox, N. et al. Telerehabilitation for chronic respiratory disease (Review). https://doi.org/10.1002/14651858.CD013040.pub2.www.cochranelibrary.com (2021).
Pinto, D. et al. Maintaining Effects of Pulmonary Rehabilitation at Home in Chronic Obstructive Pulmonary Disease: A Systematic Literature Review. HOME Heal. CARE Manag. Pract. https://doi.org/10.1177/1084822321990376.
Armstrong, M. et al. Use of pedometers as a tool to promote daily physical activity levels in patients with COPD: a systematic review and meta-analysis. Eur. Respir. Rev. 28, (2019).
Legault, L. Encyclopedia of Personality and Individual Differences. Encycl. Personal. Individ. Differ. 1–9 https://doi.org/10.1007/978-3-319-28099-8 (2020).
McCarthy, B. & McDonald, C. F. Review: Pulmonary rehabilitation improves health-related QoL and exercise capacity in COPD. Ann. Intern. Med. 162, JC4 (2015).
Salman, G. F., Mosier, M. C., Beasley, B. W. & Calkins, D. R. Rehabilitation for patients with chronic obstructive pulmonary disease: Meta-analysis of randomized controlled trials. J. Gen. Intern. Med. 18, 213–221 (2003).
Ambrosino, N. & Simonds, A. The clinical management in extremely severe COPD. Respir. Med. 101, 1613–1624 (2007).
Gea, J., Pascual, S., Casadevall, C., Orozco-Levi, M. & Barreiro, E. Muscle dysfunction in chronic obstructive pulmonary disease: Update on causes and biological findings. J. Thorac. Dis. 7, E418–E438 (2015).
Spruit, M. A. et al. Differential response to pulmonary rehabilitation in COPD: Multidimensional profiling. Eur. Respir. J. 46, 1625–1635 (2015).
Author information
Authors and Affiliations
Contributions
L.S. and T.M. conceived the study. L.S., T.M., P.C. and M.P. wrote the initial protocol, L.S. and T.M. did the literature search and screened articles. L.S., T.M. and J.B.-E. wrote the initial manuscript, organised the data and constructed tables. L.S. did the statistical analysis. All authors provided crucial feedback on the study protocol and contributed important intellectual content to the manuscript, including revisions. All authors had access to all the data in the study, and L.S. had the final responsibility to submit for publication.
Corresponding author
Ethics declarations
Competing interests
T.M. is an editor for npj Primary Care Respiratory Medicine. T.M. was not involved in the journal’s review of, or decisions related to, this manuscript. The remaining authors do not declare any competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, L., Maricoto, T., Costa, P. et al. A meta-analysis on the structure of pulmonary rehabilitation maintenance programmes on COPD patients’ functional capacity. npj Prim. Care Respir. Med. 32, 38 (2022). https://doi.org/10.1038/s41533-022-00302-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-022-00302-x
This article is cited by
-
Our contribution to systematic review and meta-analysis in primary care respiratory medicine
npj Primary Care Respiratory Medicine (2023)