Abstract
Poly(A) tail is a hallmark of eukaryotic messenger RNA and its length plays an essential role in regulating mRNA metabolism. However, a comprehensive resource for plant poly(A) tail length has yet to be established. Here, we applied a poly(A)-enrichment-free, nanopore-based method to profile full-length RNA with poly(A) tail information in plants. Our atlas contains over 120 million polyadenylated mRNA molecules from seven different tissues of Arabidopsis, as well as the shoot tissue of maize, soybean and rice. In most tissues, the size of plant poly(A) tails shows peaks at approximately 20 and 45 nucleotides, while the poly(A) tails in pollen exhibit a distinct pattern with strong peaks centred at 55 and 80 nucleotides. Moreover, poly(A) tail length is regulated in a gene-specific manner—mRNAs with short half-lives in general have long poly(A) tails, while mRNAs with long half-lives are featured with relatively short poly(A) tails that peak at ~45 nucleotides. Across species, poly(A) tails in the nucleus are almost twice as long as in the cytoplasm. Our comprehensive dataset lays the groundwork for future functional and evolutionary studies on poly(A) tail length regulation in plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw sequencing data generated in this study were deposited in China National Center for Bioinformation with accession PRJCA007575 and in NCBI with accession PRJNA788163. A read_info file and a bam file for each FLEP-seq2 library were recorded in China National Center for Bioinformation with accession OMIX881. The bam file contains sequence and alignment information; the read-info file includes all the reads mapping to protein-coding regions and stored the corresponding gene, intron status and poly(A) tail length of each read. The median poly(A) lengths of genes in each library were recorded in Supplementary Table 4. Source data are provided with this paper.
Code availability
The jupyter notebook (jupter core v.4.6.1, python v.3.7.4) that contains examples and scripts for analysis and visualization was uploaded to GitHub (https://github.com/ZhaiLab-SUSTech/flep_seq2_polya_analysis).
References
Nicholson, A. L. & Pasquinelli, A. E. Tales of detailed poly(A) tails. Trends Cell Biol. 29, 191–200 (2019).
Passmore, L. A. & Coller, J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-021-00417-y (2021).
Eckmann, C. R., Rammelt, C. & Wahle, E. Control of poly(A) tail length. WIREs RNA 2, 348–361 (2011).
Kühn, U. & Wahle, E. Structure and function of poly(A) binding proteins. Biochim. Biophys. Acta 1678, 67–84 (2004).
Zhao, T. et al. Impact of poly(A)-tail G-content on Arabidopsis PAB binding and their role in enhancing translational efficiency. Genome Biol. 20, 189 (2019).
Goldstrohm, A. C. & Wickens, M. Multifunctional deadenylase complexes diversify mRNA control. Nat. Rev. Mol. Cell Biol. 9, 337–344 (2008).
Eisen, T. J. et al. The dynamics of cytoplasmic mRNA metabolism. Mol. Cell 77, 786–799 (2020).
Vi, S. L. et al. Target specificity among canonical nuclear poly(A) polymerases in plants modulates organ growth and pathogen response. Proc. Natl Acad. Sci. USA 110, 13994–13999 (2013).
Hunt, A. G. mRNA 3’ end formation in plants: novel connections to growth, development and environmental responses. WIREs RNA 11, e1575 (2020).
Subtelny, A. O., Eichhorn, S. W., Chen, G. R., Sive, H. & Bartel, D. P. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 508, 66–71 (2014).
Udagawa, T. et al. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol. Cell 47, 253–266 (2012).
Chang, H., Lim, J., Ha, M. & Kim, V. N. TAIL-seq: genome-wide determination of poly(A) tail length and 3′ end modifications. Mol. Cell 53, 1044–1052 (2014).
Lim, J., Lee, M., Son, A., Chang, H. & Kim, V. N. mTAIL-seq reveals dynamic poly(A) tail regulation in oocyte-to-embryo development. Genes Dev. 30, 1671–1682 (2016).
Harrison, P. F. et al. PAT-seq: a method to study the integration of 3′-UTR dynamics with gene expression in the eukaryotic transcriptome. RNA 21, 1502–1510 (2015).
Woo, Y. M. et al. TED-seq identifies the dynamics of poly(A) length during ER stress. Cell Rep. 24, 3630–3641 (2018).
Legnini, I., Alles, J., Karaiskos, N., Ayoub, S. & Rajewsky, N. FLAM-seq: full-length mRNA sequencing reveals principles of poly(A) tail length control. Nat. Methods 16, 879–886 (2019).
Liu, Y., Nie, H., Liu, H. & Lu, F. Poly(A) inclusive RNA isoform sequencing (PAIso-seq) reveals wide-spread non-adenosine residues within RNA poly(A) tails. Nat. Commun. 10, 5292 (2019).
Parker, M. T. et al. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m6A modification. eLife 9, e49658 (2020).
Workman, R. E. et al. Nanopore native RNA sequencing of a human poly(A) transcriptome. Nat. Methods 16, 1297–1305 (2019).
Parker, M. T. et al. Widespread premature transcription termination of Arabidopsis thaliana NLR genes by the spen protein FPA. eLife https://doi.org/10.7554/eLife.65537 (2021).
Long, Y., Jia, J., Mo, W., Jin, X. & Zhai, J. FLEP-seq: simultaneous detection of RNA polymerase II position, splicing status, polyadenylation site and poly(A) tail length at genome-wide scale by single-molecule nascent RNA sequencing. Nat. Protoc. 16, 4355–4381 (2021).
Jia, J. et al. Post-transcriptional splicing of nascent RNA contributes to widespread intron retention in plants. Nat. Plants 6, 780–788 (2020).
Scheer, H. et al. The TUTase URT1 connects decapping activators and prevents the accumulation of excessively deadenylated mRNAs to avoid siRNA biogenesis. Nat. Commun. 12, 1298 (2021).
Zuber, H. et al. Uridylation and PABP cooperate to repair mRNA deadenylated ends in Arabidopsis. Cell Rep. 14, 2707–2717 (2016).
Wu, X., Wang, J., Wu, X., Hong, Y. & Li, Q. Q. Heat shock responsive gene expression modulated by mRNA poly(A) tail length. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.01255 (2020).
Lima, S. A. et al. Short poly(A) tails are a conserved feature of highly expressed genes. Nat. Struct. Mol. Biol. 24, 1057–1063 (2017).
Schafer, I. B. et al. Molecular basis for poly(A) RNP architecture and recognition by the Pan2-Pan3 deadenylase. Cell 177, 1619–1631 (2019).
Yi, H. et al. PABP cooperates with the CCR4-NOT complex to promote mRNA deadenylation and block precocious decay. Mol. Cell 70, 1081–1088 (2018).
Webster, M. W. et al. mRNA deadenylation is coupled to translation rates by the differential activities of Ccr4-not nucleases. Mol. Cell 70, 1089–1100 (2018).
Drechsel, G. et al. Nonsense-mediated decay of alternative precursor mRNA splicing variants is a major determinant of the Arabidopsis steady state transcriptome. Plant Cell 25, 3726–3742 (2013).
Kervestin, S. & Jacobson, A. NMD: a multifaceted response to premature translational termination. Nat. Rev. Mol. Cell Biol. 13, 700–712 (2012).
Kurosaki, T., Popp, M. W. & Maquat, L. E. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat. Rev. Mol. Cell Biol. 20, 406–420 (2019).
Szabo, E. X. et al. Metabolic labeling of RNAs uncovers hidden features and dynamics of the Arabidopsis transcriptome. Plant Cell 32, 871–887 (2020).
Ylstra, B. & McCormick, S. Analysis of mRNA stabilities during pollen development and in BY2 cells. Plant J. 20, 101–108 (1999).
Bai, B. et al. Seed-stored mRNAs that are specifically associated to monosomes are translationally regulated during germination. Plant Physiol. 182, 378–392 (2020).
Hao, H., Li, Y., Hu, Y. & Lin, J. Inhibition of RNA and protein synthesis in pollen tube development of Pinus bungeana by actinomycin D and cycloheximide. New Phytol. 165, 721–729 (2005).
Chao, L. M. et al. Arabidopsis transcription factors SPL1 and SPL12 confer plant thermotolerance at reproductive stage. Mol. Plant 10, 735–748 (2017).
Shao, N., Duan, G. Y. & Bock, R. A mediator of singlet oxygen responses in Chlamydomonas reinhardtii and Arabidopsis identified by a luciferase-based genetic screen in algal cells. Plant Cell 25, 4209–4226 (2013).
Krause, M. et al. tailfindr: alignment-free poly(A) length measurement for Oxford Nanopore RNA and DNA sequencing. RNA 25, 1229–1241 (2019).
Chantarachot, T. & Bailey-Serres, J. Polysomes, stress granules, and processing bodies: a dynamic triumvirate controlling cytoplasmic mRNA fate and function. Plant Physiol. 176, 254–269 (2018).
Yu, S. & Kim, V. N. A tale of non-canonical tails: gene regulation by post-transcriptional RNA tailing. Nat. Rev. Mol. Cell Biol. 21, 542–556 (2020).
Belostotsky, D. A. Unexpected complexity of poly(A)-binding protein gene families in flowering plants: three conserved lineages that are at least 200 million years old and possible auto- and cross-regulation. Genetics 163, 311–319 (2003).
Zhang, H. et al. A comprehensive online database for exploring approximately 20,000 public Arabidopsis RNA-seq libraries. Mol. Plant 13, 1231–1233 (2020).
Mattijssen, S. et al. The isolated La-module of LARP1 mediates 3′ poly(A) protection and mRNA stabilization, dependent on its intrinsic PAM2 binding to PABPC1. RNA Biol. 18, 275–289 (2021).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Acknowledgements
The group of J.Z. is supported by the National Key R&D Programme of China Grant (2019YFA0903903); the Programme for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S172); the Shenzhen Sci-Tech Fund (KYTDPT20181011104005); the Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes (2019KSYS006); and the Stable Support Plan Programme of Shenzhen Natural Science Fund Grant (20200925153345004). J.J. is supported by the National Natural Science Foundation of China (32100444) and the Shenzhen Fundamental Research Programme (JCYJ20210324105202007).
Author information
Authors and Affiliations
Contributions
J.Z., J.J., W.L. and Y.P. designed the experiments. W.L., J.J., B.L., H.F., X.J., Y.S. and Y.L. performed the experiments. J.J., W.L., Y.Y., W.M. and H.Z. analysed the data. J.Z. oversaw the study. J.J., W.L. and J.Z. wrote the manuscript and all authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Dominique Gagliardi, Qingshun Quinn Li, Sureshkumar Balasubramanian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
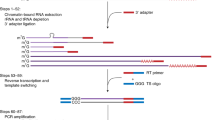
The schematic diagram of FLEP-seq and FLEP-seq2.
Extended Data Fig. 2 Comparison of FLEP-seq2 PacBio and Nanopore data.
a, The distribution of global poly(A) tail lengths of transcripts/reads in FELP-seq2 seedling Nanopore and PacBio libraries. b, the median poly(A) tail length of genes (5 nt bin) measured based on PacBio and Nanopore data. Only genes with at least 20 reads were used. c, The correlation of the median poly(A) tail lengths measured based on PacBio and Nanopore data. Only genes with at least 50 reads in both dataset were used. The two-tailed p value for testing non-correlation was indicated in the figure.
Extended Data Fig. 3
The poly(A) tail length distribution of total RNA and nuclear RNA in different species.
Extended Data Fig. 4 Comparison of the poly(A) tail length of intron-containing transcripts and fully spliced transcripts.
a, The comparison of median poly(A) tail lengths between fully spliced transcripts and intron-containing (with introns) transcripts in total RNA samples. Only transcripts spanning all annotated introns were used, and only genes with at least 20 fully spliced reads and 20 intron-containing reads were used. b, The comparison of median poly(A) tail lengths of intron-containing transcripts between nuclear and total RNA samples in different species. Only the transcripts spanning all annotated introns were used, and only genes with at least 20 intron-containing transcripts in both nuclear and total RNA libraries were used. N: gene number. The Pearson’s r values were labelled upper each figure. All the two-sided p-values for testing non-correlation were less than 2.2e-16.
Extended Data Fig. 5
Example of alternative-splicing isoforms showing differential poly(A) tail lengths.
Extended Data Fig. 6 Boxplot showing the distribution of Δ minor isoform ratio (minor/[minor+major]) between nuclear RNAs and total RNAs.
The sums of the number of minor and major isoform reads in both nuclear and total RNA samples are required to be more than 10. The p-values of one-sided Mann–Whitney U-tests are labelled in the figure. For the boxplots, the centre lines show the median, the box limits show the interquartile range and the whiskers extend to the furthest value within 1.5× the interquartile range from the quartiles. N: the number of isoforms.
Extended Data Fig. 7 The poly(A) tail lengths of minor isoforms with longer tail than major isoforms are highly consistent with the poly(A) tail of intron-containing transcripts.
The comparison of median poly(A) tail lengths between intron-containing transcripts with minor isoforms (upper) or major isoforms (below) in total RNA samples of different species. Only genes with at least 20 minor isoform reads, 20 major isoform reads and 20 intron-containing reads were used. The numbers of isoforms showing no differential, longer, and shorter poly(A) tails were separated with ‘:’ and labelled above each figure. N: the number of isoforms.
Extended Data Fig. 8 Tissue-specific and evolutionarily conserved regulation of poly(A) tail length in Arabidopsis.
a, The median poly(A) tail length correlation matrix of different samples. All the two-sided p-values for testing non-correlation were less than 2.2e-16. b, Heatmap plot showing the relative median poly(A) tail length of genes among different tissues. c, The number of genes identified to show differential poly(A) tail length distribution in different tissues (Real, bottom panel) and in random data (Control, upper panel). Inflo.: Inflorescence. r1: biological replicate 1; r2: biological replicate 2.
Extended Data Fig. 9 Internal and 3’ end non-A bases of Poly(A) tails.
a, The ratio of poly(A) tails with internal non-A bases or 3’ end non-A bases. Two biological replicates were used. Data are presented as mean values±SD. b, The length distribution of poly(A) tail with or without 3’ end non-A bases. 1 nt each bin.
Extended Data Fig. 10 Tissue-specific expression of Arabidopsis genes coding Poly(A) binding proteins (PAB).
The gene expression values (Fragments per kilobase per million mapped fragments, FPKM value) of PABs in public RNA-seq data are downloaded from http://ipf.sustech.edu.cn/pub/athrna/. N: the number of RNA-seq libraries.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Supplementary Tables
Supplementary Tables 1–7.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, J., Lu, W., Liu, B. et al. An atlas of plant full-length RNA reveals tissue-specific and monocots–dicots conserved regulation of poly(A) tail length. Nat. Plants 8, 1118–1126 (2022). https://doi.org/10.1038/s41477-022-01224-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01224-9
This article is cited by
-
Structured 3′ UTRs destabilize mRNAs in plants
Genome Biology (2024)
-
Remodeling of maternal mRNA through poly(A) tail orchestrates human oocyte-to-embryo transition
Nature Structural & Molecular Biology (2023)
-
Reference-free assembly of long-read transcriptome sequencing data with RNA-Bloom2
Nature Communications (2023)