Abstract
Renewable electricity powered electrochemical CO2 reduction (CO2R) offers a valuable method to close the carbon cycle and reduce our overreliance on fossil fuels. However, high purity CO2 is usually required as feedstock, which potentially decreases the feasibility and economic viability of the process. Direct conversion of flue gas is an attractive option but is challenging due to the low CO2 concentration and the presence of O2 impurities. As a result, up to 99% of the applied current can be lost towards the undesired oxygen reduction reaction (ORR). Here, we show that acidic electrolyte can significantly suppress ORR on Cu, enabling generation of multicarbon products from simulated flue gas. Using a composite Cu and carbon supported single-atom Ni tandem electrocatalyst, we achieved a multicarbon Faradaic efficiency of 46.5% at 200 mA cm-2, which is ~20 times higher than bare Cu under alkaline conditions. We also demonstrate stable performance for 24 h with a multicarbon product full-cell energy efficiency of 14.6%. Strikingly, this result is comparable to previously reported acidic CO2R systems using pure CO2. Our findings demonstrate a potential pathway towards designing efficient electrolyzers for direct conversion of flue gas to value-added chemicals and fuels.
Similar content being viewed by others
Introduction
Rapidly rising anthropogenic CO2 emissions have led to serious concerns over global warming and climate change issues1,2. There is therefore an urgent need to develop new technologies that can efficiently capture, store and utilize CO23. To this end, renewable electricity powered electrochemical CO2 reduction (CO2R) offers a valuable method to close the carbon cycle and reduce modern society’s overreliance on fossil fuels4,5,6,7,8,9,10,11,12. By transforming waste CO2 into value-added chemicals and fuels, this technology can also help establish a sustainable pathway towards net-zero chemical production and store renewable energy in the form of chemical bonds13,14,15,16,17,18. In particular, the production of multicarbon (C2+) molecules such as ethylene and ethanol are attractive due to their large market size and high carbon footprint2,19.
While the majority of literature reports use pure CO2 as feedstock, it is essential to note that the cost of purifying CO2 from flue gas can amount to $70–$100 per ton20,21,22. Depending on the product of interest, this can constitute a substantial portion of operating costs (~30% for ethylene)9,20. There is therefore interest in developing CO2R electrolyzers that can directly convert the CO2 in flue gas streams to the desired products. However, this is challenging because typical flue gas contains relatively low concentrations of CO2 (~15% v/v) and non-negligible amounts of O2 (≥3% v/v)20. This inevitably results in CO2 mass transport limitations, which promotes the undesired hydrogen evolution reaction (HER)23,24. More importantly, the presence of O2 impurities can result in up to 99% of applied current being lost to the much more thermodynamically favorable oxygen reduction reaction (ORR)20,25,26,27.
To address this issue, Wang and co-workers coated their cobalt phthalocyanine catalysts using a selectively permeable polymer25 with a CO2/O2 selectivity of ~20. As a result, they achieved a 75.9% Faradaic efficiency (FE) for CO production when a CO2 feedstock containing 5% O2 was employed. In another study, Sinton and co-workers employed ionomer coatings that selectively slowed down O2 mass transport20. This enabled C2+ products to be generated with a FE of 68% under high pressure (10 bar) conditions with flue gas containing O2 (4% v/v) and CO2 (15% v/v). Although these selective mass transport control strategies have been relatively successful, it is important to explore other avenues that could be simple yet effective for enabling direct conversion of CO2 in flue gas.
In this work, we discovered that (1) choice of electrolyte and (2) catalyst design can be used to enable oxygen-tolerant production of C2+ products in simulated flue gas. Specifically, we found that the use of acidic electrolyte results in a significant suppression of ORR activity as compared to alkaline electrolyte. This was demonstrated to be the case for both a pure Cu catalyst and a carbon-supported single-atom Ni catalyst. Density functional theory (DFT) simulations suggest that this is due to an increase in the free energy change of the rate-determining step for ORR on each of these catalysts in acidic media. We then created a composite system by coating the single-atom Ni onto Cu, creating a tandem electrocatalyst which was able to facilitate the direct conversion of CO2 in simulated flue gas to C2+ products with an FE of 46.5% at 200 mA cm−2 in acidic electrolyte. Under these conditions, we achieved up to 24 h of stable operation with a 14.6% full-cell energy efficiency (EE) towards C2+ products.
Results
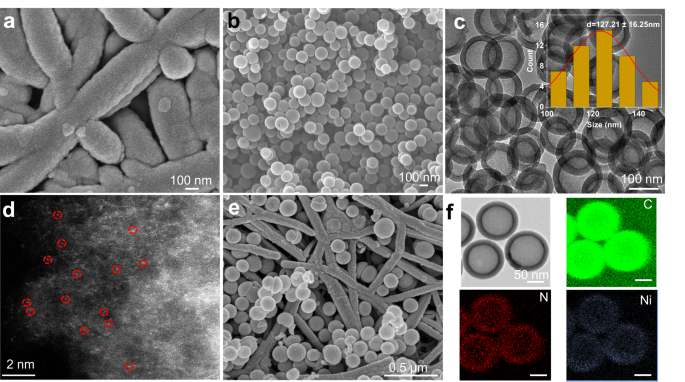
Our first objective in this work was to design a catalyst capable of generating C2+ products with high selectivity in acidic media. The catalyst testing and optimization process was to be initially conducted using pure CO2 feedstock, before proceeding on to our simulated flue gas experiments. Thus, we began by using magnetron sputtering to deposit 200 nm thick Cu films onto porous hydrophobic polytetrafluoroethylene (PTFE) substrates for use as CO2R gas diffusion electrodes. Representative top-down scanning electron microscopy (SEM) images (Fig. 1a and Fig. S1) show an open web-like morphology of interconnected PTFE fibers coated conformally with the Cu catalyst. These catalysts were then characterized using X-ray diffraction (XRD), where we observed Cu (111) to be the dominant facet (Fig. S2). This was found to be consistent with our Pb underpotential deposition (UPD) experiments (Fig. S3). X-ray photoelectron spectroscopy (XPS) measurements (Fig. S4) showed the expected peaks in the Cu 2p narrow scans, with the presence of Cu oxides due to inevitable oxidation of the surface from exposure to ambient air28 (Fig. S5).
a SEM images of Cu sputtered onto porous hydrophobic PTFE gas diffusion substrates. b SEM and (c) TEM images of Ni–N4, consisting of Ni single-atoms hosted on a carbon support. d AC-HAADF-STEM image of Ni–N4. e SEM images of the Cu PTFE/Ni–N4 composite catalyst. f HAADF-STEM and corresponding EDS mapping images of the Ni–N4 catalyst.
CO2R was then carried out with these Cu PTFE catalysts in acidic electrolyte (0.05 M H2SO4 and 0.5 M K2SO4) at constant cathodic current densities of 100, 200, 400, and 600 mA cm−2. These experiments were performed using a custom-made gas diffusion electrochemical flow cell system, with a similar design to that previously reported in the literature29. The results in Fig. S6 show that a substantial amount of hydrogen evolution reaction (HER) occurs. For instance, at 600 mA cm−2 we observe a HER FE of 59.2% and a C2+ FE of only 17.9% (Table S1-2). The HER FE tends to decrease with the current density and was lowest at 100 mA cm−2 with a value of 14.3%. However, the C2+ FE was only 53.3% which is lower than state-of-the-art acidic CO2R systems reported in the literature30,31,32,33,34,35,36,37,38,39.
Compared to alkaline and neutral media, the C2+ product FE tends to be lower in acidic electrolyte. This has been proposed to be due to an enhanced HER activity, less facile C–C coupling kinetics and a weakened *CO binding energy32,36,40,41. This can in principle, be remedied using tandem catalysis, whereby a CO-generating catalyst is located in close proximity to Cu. Cu active sites are thus exposed to a mix feed of CO2/CO, which promotes the formation of C2+ products. Although we previously reported that acidic conditions are not so favorable for tandem catalysis40, some improvements in the C2+ FE were still observed over the pure Cu base case. Hence, we opted to apply this strategy to increase the C2+ product FE of our Cu PTFE catalysts.
In this work, our CO-generating catalyst of choice was a single-atom Ni catalyst42,43,44,45,46,47, consisting of Ni–N4 active sites hosted on a carbon support (see supporting information for full synthesis details). Hence, we termed this as Ni–N4 based on its operating active site for electrochemical CO2 to CO conversion. Figure 1b–d and Fig. S7 are representative SEM and high-resolution transmission electron microscopy (HR-TEM) images of Ni–N4 with a nanosphere morphology. Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) shows dense bright spots corresponding to Ni single-atoms that are dispersed homogeneously throughout the carbon support (Fig. 1d). Energy-dispersive X-ray spectroscopy (EDS) mapping reveals uniform distribution of the elements C, N, and Ni (Fig. 1f).
XRD patterns (Fig. S8) of the Ni–N4 electrocatalysts also indicate the absence of any metallic Ni phases, with only peaks corresponding to graphitic carbon. The Ni content is also quantified to be ~0.81 wt% using inductively coupled plasma atomic emission spectrometry (ICP-OES). The XPS survey spectra of Ni–N4 shows the expected peaks associated with C, N, and Ni. In the Ni 2p narrow scan (Fig. S9), the binding energy of Ni 2p3/2 at 854.6 eV is in the range between Ni0 (853.0 eV) and Ni2+ (855.7 eV), indicating a weakly oxidated state of Ni species in Ni–N448. The narrow scan N 1s XPS spectra of Ni–N4 can be fitted into four characteristic peaks, among which is the pyridinic N species that can act as coordinating sites for the single Ni metal atoms49,50.
The coordination environment of the Ni species was also investigated using X-ray absorption fine spectroscopy (XAFS). In Fig. S10, the X-ray absorption near-edge structure (XANES) spectrum of the Ni K-edge of Ni–N4 is shown, along with standard data for Ni foil and NiO as references. The XANES spectrum of Ni–N4 falls between that of Ni foil and NiO, indicating that the valence state of the isolated Ni atoms ranges between the metallic (Ni0) and oxidized (Ni2+) states51,52. The extended X-ray absorption fine structure (EXAFS) spectra of Ni–N4 was also analyzed using a Fourier transform (FT) k3-weighted χ(k) function. The results show a significant peak at 1.31 Å, which is indicative of Ni–N coordination. In comparison, Ni foil exhibits the distinctive Ni–Ni pair at 2.15 Å, while the Ni-O interaction in NiO is evident around 1.65 Å53. In addition, the least-squares EXAFs curve fitting shows that Ni atoms are coordinated with four N in the first shell, indicating that the Ni species in the catalyst exists predominantly as Ni–N4 (Fig. S10).
CO2R was then carried out with the Ni–N4 catalyst using the same flow cell system and electrolyte (0.05 M H2SO4 and 0.5 M K2SO4). From the results (Fig. S11 and Table S3), we found that these catalysts yielded a high CO FE (>90%) in an applied current density range of 100–400 mA cm−2. This value increases gradually with higher current density, with a CO FE of ~99% at 400 mA cm−2. Hence, these results demonstrate that our Ni–N4 catalysts can effectively perform the role of CO2-to-CO conversion for tandem catalysis.
Next, we built the composite catalyst (Fig. 2) by first spray coating a layer of Ni–N4 catalysts (loading 0.25 mg cm−2) onto the surface of Cu PTFE to obtain Cu PTFE/Ni–N4-1. In the second step, 5 nm of Cu was sputter deposited followed by another layer of Ni–N4 (loading 0.125 mg cm−2) to form Cu PTFE/Ni–N4-2. This second step was repeated another time, which yields the final Cu PTFE/Ni–N4 composite catalyst. Figure 1e and Fig. S12 show representative SEM images and Fig. S13 and Fig. S14 show the XRD patterns and XPS spectra of the Cu PTFE/Ni–N4 composite catalyst. The Raman spectra (Fig. S15) of Cu PTFE/Ni–N4 are consistent with that of Ni–N4, indicating that the coating process does not directly alter the properties of Ni–N4. The double-layer capacitance of the catalyst was also determined using cyclic voltammetry (Fig. S16) and as expected, we found Cu PTFE/Ni–N4 to have the largest value, indicating a higher electrochemically active surface area. Pb UPD experiments (Fig. S17) showed similar results to bare Cu PTFE, which indicates that the addition of the Ni–N4 layer does not directly alter the properties of Cu. From electrochemical impedance spectroscopy (EIS) measurements (Fig. S18), we also found that Cu PTFE/Ni–N4 exhibits a significantly lower charge transfer resistance (Rct) value of 7.29 Ω compared to Ni–N4 (10.13 Ω) and Cu PTFE (8.91 Ω).
Cu PTFE is fabricated by sputtering 200 nm of Cu onto a porous hydrophobic PTFE substrate. A layer of Ni–N4 catalyst is then spray coated onto the Cu PTFE to yield Cu PTFE/Ni–Ni4-1. Cu PTFE/Ni–Ni4-2 is made by sputtering 5 nm of Cu, followed by spraying another layer of Ni–N4 catalyst. This step is repeated another time to obtain the final Cu PTFE/Ni–Ni4 composite catalyst.
CO2R in acidic media was then carried out with the Cu PTFE/Ni–N4 catalysts. The results at 200 mA cm−2 (Figs. S19, S20 and Table S4–7) show significant improvements in the C2+ FE with a value of 66.7% with the composite Cu PTFE/Ni–N4 catalyst as compared to only 29.5% with bare Cu PTFE (Fig. 3a). Up to a certain limit, the C2+ FE is also observed to increase with more layers of Cu and Ni–N4 (Fig. S21 and Table S8–11), and we postulate this to be due to a higher density of interfaces between Cu and Ni–N4 which are beneficial for tandem catalysis. Cu PTFE/Ni–N4 was also tested at current densities of 100, 400 and 600 mA cm−2, with the results shown in Fig. 3b. We found that the highest C2+ FE occurs at 400 mA cm−2, with a value of 82.4% and C2+ partial current density of 329.7 mA cm−2. From the results, ethylene and ethanol are the dominant C2+ products, with a FE of 44.2% and 29.6% respectively. Notably, we find that this performance is comparable to recently reported state-of-the-art acidic CO2R systems26,29,30,31,33.
CO2R FE results as a function of current density for: (a) Cu PTFE and (b) Cu PTFE/Ni–N4 in acidic electrolyte. c Potential resolved in situ Raman spectroscopy of Cu PTFE/Ni–N4 in acidic electrolyte during CO2R. d Correlation between the proportion of *COatop and FE towards C2+ products for Cu PTFE/Ni–N4 in acidic electrolyte. e, f Potential resolved in situ Raman spectroscopy of Cu PTFE. All the error bars represent standard deviation based on three independent samples.
We also tested the long-term stability of the composite catalyst (Fig. S22) at 200 mA cm−2 for 24 h and found no significant deterioration in performance over this period. The full cell operating voltage was also measured to be 6.5 V (Tables S6–7), which means that our system has a C2+ full-cell EE of 14.5 %. In addition, XRD (Fig. S13), XPS (Fig. S23), and SEM (Fig. S24) characterization of Cu PTFE/Ni–N4 was also carried out after the stability measurement, and we find no significant differences in the catalyst as compared to the pristine case. Cu 2p3/2 XPS fitting curves revealed that the Cu surface was mainly in the oxidized state in Cu PTFE/Ni–N454,55 both before and after CO2R (Fig. S14 and Fig. S23) and is consistent with our Raman spectroscopy results (Fig. S5). This is due to oxidation of the Cu surface in ambient air and reduces to its metallic state upon application of a cathodic potential during CO2 reduction56,57.
In situ X-ray absorption spectroscopy (XAS) experiments were also performed on Cu PTFE/Ni–N4 to investigate the changes of Cu valence state and structure during CO2R (Fig. 3d and Figs. S25, S26). As soon as a cell voltage of –2.0 V vs Ag/AgCl was applied, immediate reduction of Cu oxides to metallic Cu was observed. Extended X-ray absorption fine structure (EXAFS) analysis showed that the Cu first shell coordination switched from CuO (Cu–O bond at ∼1.5 Å) to Cu–Cu (∼2.2 Å) upon application of the cathodic electrochemical potential. Hence, these results show that metallic Cu serves as the active site for CO2R and is consistent with previous results in the literature28,56,57.
To understand why Cu PTFE/Ni–N4 performs better than bare Cu PTFE, we performed in situ Raman spectroscopy measurements using a custom-made electrochemical flow cell (Fig. S27). Upon application of a potential, Raman peaks located at 1,750-2,100 cm–1 (C ≡ O stretching of *CO) and 2700-3,100 cm–1 (C–H vibration) appear58,59. Based on previous work by Waegele and co-workers60, we can classify three *CO adsorption modes according to: (1) hollow-adsorbed CO at ∼1780 cm–1 (*CO hollow), (2) bridge CO at ∼1940 cm−1 (*CObridge) and (3) low-frequency band linear CO at ∼2070 cm−1 (COatop). A contour map of the stretching vibrations of *CO and C-H on Cu PTFE/Ni–N4 and Cu PTFE are shown in Fig. 3c and Fig. 3e, respectively.
With Cu PTFE/Ni–N4, we note the presence of peaks related to –CH3 and –CH2 at around 2700-3,100 cm–1. As for *CO, we observed *CObridge in the region of 1750-2,100 cm–1, *COatop centered at 1973 and 2074 cm−1 and the absence of *COhollow. At the lower overpotentials, absorbed CO mainly exists as *CObridge (Fig. 3d). However, at the larger overpotentials, the proportion of *COatop increases at the expense of *CObridge. For instance, at the most negative potential (−2.05 V vs Ag/AgCl), *CObridge is no longer observed and *COatop becomes the only adsorbed CO species. We postulate that at the higher overpotentials, CO generation by Ni–N4 becomes accelerated, which increases the CO tandem supply to the Cu sites. These findings are consistent with results by Bao and co-workers, where they found that a higher CO pressure in the feed leads to an increased *CO coverage and proportion of *COatop on Cu58. In Fig. 3d, the highest proportion of COatop was identified to be at −2.05 V vs Ag/AgCl. On the other hand, the optimal voltage for peak C2+ FE was identified to be at −1.72 V vs Ag/AgCl. Previous work by Li et al.59 found a volcano relationship between the C2+ FE and the COatop to CObridge ratio. In our case, we postulate that a similar situation could be occurring, with an optimal COatop to CObridge ratio occurring at −1.72 V vs Ag/AgCl, resulting in peak C2+ FE at this potential.
On the other hand, the in situ Raman spectroscopy results are quite different with bare Cu PTFE. For bare Cu PTFE, we do not observe peaks related to –CH2 (Fig. 3e), which contrasts with our results with Cu PTFE/Ni–N4 (Fig. S28 and Table S12). We also note the absence of *CObridge and the presence of *COhollow instead (Fig. S29 and Table S13). The proportion of *COatop also decreases with increasing overpotentials, which is opposite to that with Cu PTFE/Ni–N4. Hence without a tandem supply of CO, the *CO coverage and proportion of *COatop on Cu is lower, which then leads to a decrease in the C2+ product FE (Fig. 3f). Based on these results, we reason that tandem catalysis works to increase the proportion of *COatop towards a more optimal COatop to CObridge ratio, resulting in a higher FE towards C2+ products59.
In addition, we conducted in situ Raman spectroscopy tests with Cu PTFE/Ni–N4 to obtain an indication of the local pH of the electrode based on the HCO3− and CO32− peaks61. In the voltage range of −1.09 to −2.05 V vs. Ag/AgCl, we did not observe the presence of HCO3- or CO32- peaks in the Raman spectrum (Fig. S30a). This absence indicates that the local pH of the electrode remains acidic, since a neutral or alkaline pH is necessary for HCO3- or CO32- to be present. Based on acid-base equilibria, we therefore deduced that the local pH should be a value of 4.5 or lower62. To verify these results, we repeated the same experiments, except that 1 M KOH was added to shift the bulk pH from a value of 1.7 to 2.2. In this case, we began to observe the HCO3− and CO32− peaks in the Raman spectra (Fig. S30b). This indicates that this electrolyte can no longer maintain the local pH in an acidic range under CO2R conditions.
Having established an effective electrocatalyst for CO2R in acidic media, our next goal was to uncover the conditions which are beneficial towards enabling the direct conversion of CO2 in flue gas. Since the thermodynamic potential of ORR is >1 V positive compared to CO2R, the presence of O2 impurities is the primary challenge that must be overcome20. We first performed ORR experiments by introducing pure O2 into the gas chamber and electrolyte, using the same electrochemical cell for CO2R experiments. With this, the ORR activity of Cu PTFE, Ni–N4 and Cu PTFE/Ni–N4 was evaluated in both alkaline (1 M KOH) and acidic media (0.05 M H2SO4 + 0.5 M K2SO4). For all three catalysts, the results (Fig. 4a and Fig. S31) indicate that ORR activity becomes significantly suppressed under acidic conditions. For example, with Cu PTFE/Ni–N4 at a potential of 0.41 V vs RHE, the ORR current density decreases from −0.91 mA cm−2 in alkaline electrolyte to −0.17 mA cm−2 in acidic electrolyte.
a LSV curves in pure O2 saturated acidic and alkaline electrolyte. b Free energy diagrams of ORR on Ni–N4 and Cu PTFE at 1.23 V vs. SHE. The highlighted steps show that the free energy change of the rate-determining step is increased under acidic conditions. c Product FE for Cu PTFE in 1 M KOH and Cu PTFE/Ni–N4 in 0.05 M H2SO4 + 1.5 M Cs2SO4 under different current densities. d Comparison of the C2+ product full-cell EE and FE with previously reported acidic CO2R systems31,32,33,34,35. We note that these systems run on pure CO2 in contrast with our system which runs on simulated flue gas. e Full-cell voltage and C2+ product FE of Cu PTFE/Ni–N4 at −200 mA cm−2 using simulated flue gas over a 24 h testing period. All the error bars represent standard deviation based on three independent samples.
Next, we performed DFT simulations to understand the suppression of ORR activity under acidic conditions. Specifically, we investigated the different stages in the ORR reaction pathway (*OOH, *O, and *OH) on two structures: Cu (111), and Ni–N4 (Figs. S34, S35). For both Ni–N4 and Cu (111), the first step involving O2 to *OOH has the largest uphill free energy change in the entire reaction pathway. Based on these calculations, we find that the free energy change of these steps becomes larger under acidic conditions. For instance, they increase from 1.21 eV to 1.38 eV and 0.87 eV to 1.04 eV for Ni–N4 and Cu (111) respectively under acidic conditions (Fig. 4b), which are consistent with our experimental observations.
Encouraged by these findings, we performed CO2R experiments using simulated flue gas (3% O2 v/v, 15% CO2 v/v and N2 balance) with both Cu PTFE/Ni–N4 and Cu PTFE in acidic and alkaline electrolyte. Because ORR yields water as the dominant product, its quantification is challenging since aqueous electrolyte is employed. Hence for all cases, we attribute the missing FE entirely towards ORR. With Cu PTFE, we find that the generation of C2+ products is significantly suppressed in alkaline electrolyte, with a maximum FE of only 2.3% at 200 mA cm−2, along with a 40.5% FE towards ORR (Fig. 4c and Tables S14, 15).
Once acidic electrolyte is employed, the C2+ product FE of Cu PTFE increases substantially by ~2.5 times to a value of 5.7% at 200 mA cm−2, together with a decrease in the ORR FE to 22.8% (Fig. S36 and Tables S16-17). Similar results are also observed with the Cu PTFE/Ni–N4 composite catalyst, where ORR is suppressed and C2+ product formation is promoted in acidic electrolyte. For instance, in alkaline electrolyte, the C2+ product FE is 16.1% with an ORR FE of 48.2% at 300 mA cm−2 (Fig. S37 Table S18-19). When acidic electrolyte is employed, the C2+ product FE rises to 29.1% and the ORR FE decreases to 39.9% at 300 mA cm−2 (Fig. S38 and Table S20-24). However, we also note that the HER FE increases slightly in acidic media. For instance, the HER FE for Cu PTFE/Ni–N4 was found to be 20.6% in alkaline electrolyte (Fig. S37) and 30.6% in acidic electrolyte (Fig. S39) at a current density of 200 mA cm−2. This is consistent with our DFT simulations (Figs. S32, S33), where we found that HER is indeed more facile under acidic conditions63,64,65.
To further improve the C2+ FE, we first replaced the 0.5 M K2SO4 in the electrolyte with 0.5 M Cs2SO4, since Cs+ ions are known in the literature to be better than K+ ions at promoting C-C coupling66,67. Employing this new acidic electrolyte formulation (Figs. S39, S40 and Tables S25–32), we observed an increase in the C2+ product FE to 38.9% on Cu PTFE/Ni–N4, with a C2+ full-cell EE of 10.2% at 200 mA cm−2. We then sought to further enhance the C2+ product FE and full-cell EE by increasing the Cs+ concentration of the electrolyte. This is because previous studies have indicated that an increased cation concentration can suppress HER in acidic systems32. Furthermore, a higher salt concentration would serve to improve the conductivity of the electrolyte and enhance the full-cell EE. Hence, by increasing the Cs+ concentration from 1 M to 3 M (0.05 M H2SO4 + 1.5 M Cs2SO4), we observed a further increase in the C2+ product FE to 46.5% and a decrease in the ORR FE to 21.0% with Cu PTFE/Ni–N4 at 200 mA cm−2 (Fig. 4c, Fig. S41 and Tables S33–40). Notably, this C2+ product FE is ~20 times higher than Cu PTFE in alkaline electrolyte, which exemplifies the success of our electrolyte optimization and catalyst design strategies.
We also found that Cu PTFE/Ni–N4 exhibits a C2+ EE of 14.6% at 200 mA cm−2 (Fig. S42), which was calculated based on the non-IR compensated full-cell operating voltage. Strikingly, this result is comparable to previously reported acidic systems in the literature that employ pure CO2 (Fig. 4d). Finally, we operated the system for 24 h at 200 mA cm−2 with this simulated flue gas feed, where we observed a stable full-cell operating voltage and no significant changes in the FE towards C2+ products over this extended testing period (Fig. 4e and Table S41).
An in situ Raman spectroscopy study of Cu PTFE/Ni–N4 was also performed in 0.05 M H2SO4 + 1.5 M Cs2SO4 electrolyte with the simulated flue gas feedstock. As shown in Fig. S43a, the peaks at 970 and 1040 cm–1 represent the O–O stretching vibration of *O268 and *OH species69 respectively, which are ORR intermediates. Further decreasing the potential causes the Raman peak around 1040 cm–1 to slightly weaken, while the Raman peak at 970 cm–1 remains relatively unchanged regardless of the applied potential. As for the *CO population, we surprisingly only observed the presence of COatop (Fig. S43b), which differs from the results with pure CO2 feedstock (Fig. 4c). Since ORR occurs simultaneously on the same catalyst surface, we postulate that the absence of CObridge with simulated flue gas could be due to the competitive adsorption of ORR intermediates.
Discussion
In this work, our goal was to enable direct conversion of CO2 in simulated flue gas to C2+ products through a combination of electrolyte selection and catalyst design. We first began by developing a Cu PTFE/Ni–N4 composite catalyst for CO2R in an acidic electrolyte. In this catalyst, Ni–N4 serves to convert CO2 to CO, which then transfers over to Cu active sites and boosts their C2+ product FE. Optimization of the Ni–N4 and Cu layers in this composite resulted in a catalyst that achieved a total C2+ FE of 82.3% at 400 mA cm−2 with pure CO2 feedstock. We then tested these catalysts for ORR, where we found significant suppression of activity in acidic media for both Cu and Ni–N4. This was supported by our DFT simulations where we found increases in the free energy change of the rate-determining steps for ORR on Cu and Ni–N4 in acidic media. Encouraged by these results, we employed the Cu PTFE/Ni–N4 catalyst for direct conversion of CO2 in simulated flue gas, where we obtained a total C2+ FE of 46.5% at a current density of 200 mA cm−2 with an acidic electrolyte. Notably, this C2+ FE is ~20 times higher than bare Cu PTFE with a value of only 2.3% under alkaline conditions. We also showcase stable performance for 24 h with a multicarbon (C2+) full-cell EE of 14.6%. This result is comparable to previously reported acidic CO2R systems that employ pure CO2. Importantly, our results demonstrate a potential pathway towards the design of efficient electrolyzers for direct conversion of CO2 in flue gas, using simple yet effective electrolyte and catalyst design strategies.
Methods
Chemicals
2,4-dihydroxybenzoic acid (≥99.9%), hexamethylenetetramine (≥99.9%), Pluronic P123 (≥99.0%), sodium oleate (≥99.0%), potassium sulfate (≥99.0%), cesium sulfate (≥99.0%), perchloric acid (70%), lead (II) perchlorate trihydrate (98%), potassium hydroxide (≥85%), sulfuric acid (95.0-98.0%), and nickel (II) acetylacetonate (≥99.9%) were purchased from Sigma-Aldrich. The hydrophobic porous polytetrafluoroethylene (PTFE) substrates with 0.45 μm pore size were purchased from Beijing Zhongxingweiye Instrument Co., Ltd. Deionized water (18.2 MΩ) from an OmniaPure UltraPure Water System (Stakpure GmbH) was used for all the experiments. The Cu (99.999%) target was obtained from Kurt J. Lesker Company. Carbon dioxide (99.9%), nitrogen (99.99%), and oxygen gas (99.99%) were obtained from Air Liquide Singapore Pte. Ltd. Nafion 117 proton exchange membrane, Fumasep FAA-3-PK-130 anion exchange membrane and Ti screen mesh were purchased from Fuel Cell Store. The electrochemical flow cell and Ag/AgCl (3 M KCl) reference electrodes were purchased from Tianjin Aida Hengsheng Technology Development Co. The standard calibration gas mixtures for calibrating the gas chromatography system were obtained from Air Liquide Singapore Pte. Ltd. All the chemicals used in this work were of analytical grade and used without further purification.
Materials characterization
Powder X-ray diffraction pattern (PXRD) was conducted on a Rigaku D/max 2500Pc X-ray powder diffractometer with monochromatized Cu Kα radiation (λ = 1.5418 Å). Scanning electron microscopy (SEM) was performed using a JEOL JSM-7610F field scanning electron microscope. Transmission electron microscopy (TEM) was carried out using a JEOL JEM-2100F field emission electron microscope working at 200 kV. Single atoms were characterized and identified using high-angle annular dark-field scanning transmission electron microscopy (HAADF STEM) with a FEI Themis Z scanning/transmission electron microscope operated at 300 kV, equipped with a probe spherical aberration corrector. X-ray photoelectron spectroscopy (XPS) was collected on a Kratos AXIS Supra+ spectrometer equipped with a monochromatized Al Kα X-ray source and a concentric hemispherical analyser. The survey scan was carried out using an emission current of 15 mA, pass energy of 160 eV and step size of 1 eV. The narrow scan was performed using an emission current of 15 mA, pass energy of 20 eV and step size of 0.1 eV. Ex-situ XAS experiments were performed at the XAFCA beamline of the Singapore Synchrotron Light Source. In situ XAS measurements were performed at the Australian Synchrotron Facility at ANSTO. CO2 reduction gas products were analyzed using an Agilent 8600 gas chromatography system equipped with a thermal conductivity detector and a flame ionization detector. Liquid products were quantified with high-performance liquid chromatography (Agilent 1260 Infinity II HPLC). The HPLC was equipped with a refractive index and UV detector. The eluent used was 1 mM H2SO4, with Aminex HPX-87H columns from Bio-Rad laboratories.
Preparation of Ni–N4 catalysts
The first step involved the synthesis of hollow polymer spheres, which was based on a previously reported method with some modifications. In a typical procedure, 90 mg 2,4-dihydroxybenzoic acid and 184.8 mg hexamethylenetetramine were dissolved in 60 ml deionized water. To this solution, 20 ml of another solution containing 30 mg Pluronic P123 and 72.96 mg sodium oleate was added under slow stirring. After slowly stirring for 10 min, the mixed solution was transferred into a 100 ml Teflon-lined stainless-steel autoclave and heated to a temperature of 160 °C for 2 h in a heating oven. After the reaction was complete, the autoclave was left to cool to room temperature. The hollow polymer spheres were then collected by centrifugation, washed three times with deionized water and ethanol and finally dried at 60 °C in a vacuum oven. 0.2 g of the hollow polymer spheres was then dispersed in 5 ml of ethanol. To this solution, 5 mL of another ethanol solution containing 7.0 mg Ni(acac)2 was added under stirring. The resulting mixture was then stirred at 80 °C until all the ethanol was evaporated. After that, the resulting mixture and 4.0 g dicyandiamide were separately placed in two alumina combustion boat located at the down-stream and up-stream direction in a tube furnace, respectively. The tube furnace was heated to 900 °C with a heating rate of 5 °C/min under flowing nitrogen gas (10 mL/min) and held at that temperature for two hours. After cooling to room temperature, the Ni–N4 catalysts were obtained.
Preparation of Cu PTFE
This was prepared by using a magnetron sputtering system (Cello Ohmiker-30CSL) to coat the porous hydrophobic PTFE membrane with 200 nm of Cu. A radio frequency (RF) power supply was used, and the sputtering power was controlled such that the total deposition rate was fixed at 2 Å/s.
Preparation of Cu PTFE/Ni–N4 composite catalysts
To prepare the composite catalysts, 8 mg of Ni–N4 catalyst was added into a mixed solution of 1.92 mL isopropanol and 80 μL Nafion solution. The resulting mixed solution was then ultrasonically treated for 2 h to form a homogeneous ink. After that, the Ni–N4 catalyst ink (1 ml) was sprayed onto the Cu PTFE (4 cm by 4 cm) using an air brush (loading 0.25 mg cm−2) and this electrode was named Cu PTFE/Ni–N4-1. Cu PTFE/Ni–N4-2 is prepared by sputtering of 5 nm of Cu on Cu PTFE/Ni–N4-1, followed by spraying 0.5 ml of the Ni–N4 catalyst ink (loading 0.125 mg cm−2). Cu PTFE/Ni–N4 is produced by magnetron sputtering of another 5 nm of Cu onto Cu PTFE/Ni–N4-2, followed by spraying 0.5 ml of the Ni–N4 catalyst ink (loading 0.125 mg cm−2). Cu PTFE/Ni–N4-4 is prepared by sputtering of 5 nm of Cu onto Cu PTFE/Ni–N4, followed by spraying 0.5 ml of the Ni–N4 catalyst ink (loading 0.125 mg cm−2).
In situ Raman spectroscopy
In situ Raman spectroscopy was carried out with a Horiba LabRam Odyssey Nano Raman Spectrometer system. Measurements were performed using a custom-made in situ electrochemical flow cell, with a gas chamber at the backside of the gas diffusion electrode for continuous CO2 flow. An Olympus N2667700 water immersion objective was dipped into the electrolyte to collect the Raman spectra. IrOx coated Ti mesh were used as the counter electrode in acidic electrolyte. The Ag/AgCl (3 M KCl) reference electrode was used for all experiments.
Electrochemical measurements
All electrochemical measurements were carried out using an Autolab PGSTAT204 potentiostat. CO2R experiments were conducted in a gas diffusion electrode electrochemical flow cell system with an electrode exposed area of 1 cm2. High-purity CO2 gas flowed at a rate of 30 sccm behind the cathode GDL controlled by a mass flow controller (MC–2000SCCM-D/5 M, Alicat Scientific). The flow cells were assembled with IrOx/Ti mesh as the anode, Ag/AgCl as the reference electrode, and a Nafion exchange membrane (Nafion 117; size: 2.5 cm by 2.5 cm; thickness: 0.18 mm) to separate the cathode and anode chambers. The IrOx/Ti mesh electrode was prepared using a dip coating and thermal decomposition method, according to methods described by Luc et al.70. The Nafion proton exchange membrane was activated in 5 wt.% H2SO4 at 80 °C for 2 h before use.
CO2R with simulated flue gas (3% O2 v/v, 15% CO2 v/v and N2 balance) was performed using the same electrochemical cell. For these experiments, the missing FE was assumed to be entirely attributed to ORR, due to the inability to quantify the amount of product (H2O) generated. To evaluate the ORR activity of our catalysts, we flowed pure O2 into the gas chamber as well as the electrolyte of the electrochemical cell. These ORR tests were conducted in either 1 M KOH (alkaline) or 0.05 M H2SO4 + 0.5 M K2SO4 (acidic) electrolyte. To saturate the electrolyte with O2, the electrolyte was bubbled with O2 for 20 min prior to each experiment. Linear sweep voltammetry (LSV) tests were conducted at a sweep rate of 5 mV s−1. Before the actual LSV measurement, CV cycling was conducted to obtain stable curves.
For gas product quantification, 1 mL of the gas exiting the electrochemical cell was injected into the gas chromatograph using a gas-tight syringe. The Faradaic efficiency (FE) of the gas products were calculated based on the following equation:
where N is the number of electrons transferred, F is the Faraday constant, v is the gas flow rate, c is the concentration of the detected gas product in ppm, i is the total current and Vm is the unit molar volume of gas. The gas flow rate was measured at the outlet of the electrochemical cell using a bubble flow meter.
FE of liquid products was determined as below:
where n is the moles of liquid product in the cathodic compartment, N is the electron transfer number, F = 96,485 C mol−1, t is the reaction time, J is the recorded current.
The energy efficiency (EE) for the formation of C2+ products is calculated as follows:
where Vfullcell is the full cell voltage applied in the experiment (without ohmic loss correction) and FEi is the measured Faradaic efficiency of each product. \({E}_{i}^{0}\) is the standard reduction potential of each product (ethylene: 0.08 V) (ethanol: 0.09 V) (acetate: -0.26 V) (propanol: 0.21 V).
Density functional theory simulations
The projected augmented wave (PAW) approach71 and the generalized gradient approximation (GGA) of Perdew, Burke and Ernzerhof (PBE)72 exchange-correlation functional (widely accepted for catalysis calculations) were employed in the Vienna ab initio Simulation Package (VASP)73 to perform all the plane wave density functional theory (DFT) simulations. To simulate the properties of the actual bulk material and surface, we employed 4 × 4 × 1 Cu (111) slab in a vacuum. The top two layers of Cu (111) were allowed to move freely due to their interaction with the adsorbates, while the remaining layers were held fixed in their optimized crystalline positions. We employed a graphene supercell with surface periodicity of 4 × 4 graphene supercell to simulate adjacent regions of Ni–N4. A vacuum distance of 18 Å was introduced in the z-direction. We used a cut-off energy of 400 eV for the plane wave basis sets and a 4 × 4 × 1 Г centered Monkhorst-Pack mesh for k-point sampling in the first Brillouin zone. The convergence criteria for energy and forces were set at 1 × 10−5 eV and 0.01 eV Å−1, respectively74. The computational hydrogen electrode was utilized to obtain free energies for each state as done in ref. 75. We calculated the reaction free energies of oxygen reduction reaction (ORR) on Cu (111) and Ni–N4 surfaces, considering their dependence on the potential. For evaluating the H2O dissociation energy barrier, the transitional state was located using the Nudged Elastic Band method. The free energy of adsorbed H (ΔGH) on surfaces is expressed as Eq. (4):
where ΔEH is the hydrogen adsorption energy, ΔEZPE and ΔS are the zero-point energy difference and the entropy difference between the adsorbed state and the gas phase, respectively, and T is the system temperature (298.15 K).
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon request.
References
Davidson, D. J. Exnovating for a renewable energy transition. Nat. Energy 4, 254–256 (2019).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2016).
Fan, L. et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C 2+ products. Sci. Adv. 6, eaay3111 (2020).
Gao, D., Arán-Ais, R. M., Jeon, H. S. & Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019).
Ross, M. B. et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2, 648–658 (2019).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Stephens, I. E. L. et al. 2022 roadmap on low temperature electrochemical CO2 reduction. J. Phys. Energy 4, 042003 (2022).
Qiao, J., Liu, Y., Hong, F. & Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014).
Masel, R. I. et al. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 16, 118–128 (2021).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Hori, Y. In Modern Aspects of Electrochemistry Vol. 42 (eds. Vayenas, C. G., White, R. E. & Gamboa-Aldeco, M. E.) 89–189 (Springer New York, 2008).
Higgins, D., Hahn, C., Xiang, C., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 4, 317–324 (2019).
Tian, Y. et al. Membrane-free electrocatalysis of CO2 to C2 on CuO/CeO2 nanocomposites. Front. Chem. 10, 915759 (2022).
Xu, Y. et al. Oxygen-tolerant electroproduction of C2 products from simulated flue gas. Energy Environ. Sci. 13, 554–561 (2020).
Service, R. F. Cost of carbon capture drops, but does anyone want it? Science 354, 1362–1363 (2016).
Ho, M. T., Allinson, G. W. & Wiley, D. E. Factors affecting the cost of capture for Australian lignite coal fired power plants. Energy Procedia 1, 763–770 (2009).
Lum, Y., Yue, B., Lobaccaro, P., Bell, A. T. & Ager, J. W. Optimizing C-C coupling on oxide-derived copper catalysts for electrochemical CO2 reduction. J. Phys. Chem. C 121, 14191–14203 (2017).
Möller, T. et al. The product selectivity zones in gas diffusion electrodes during the electrocatalytic reduction of CO2. Energy Environ. Sci. 14, 5995–6006 (2021).
Lu, X. et al. A bio-inspired O2-tolerant catalytic CO2 reduction electrode. Sci. Bull. (Beijing) 64, 1890–1895 (2019).
He, M. et al. Oxygen induced promotion of electrochemical reduction of CO2 via co-electrolysis. Nat. Commun. 11, 3844 (2020).
Li, P. et al. Acid–base interaction enhancing oxygen tolerance in electrocatalytic carbon dioxide reduction. Angew. Chem. Int. Ed. 59, 10918–10923 (2020).
Lum, Y., & Ager, J. W. Stability of Residual Oxides in Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction Investigated with 18 O Labeling. Angew Chem Int Ed Engl. 57, 551–554 (2018).
Liu, K., Smith, W. A. & Burdyny, T. Introductory guide to assembling and operating gas diffusion electrodes for electrochemical CO2 reduction. ACS Energy Lett. 4, 639–643 (2019).
Fan, M. et al. Cationic-group-functionalized electrocatalysts enable stable acidic CO2 electrolysis. Nat. Catal. https://doi.org/10.1038/s41929-023-01003-5 (2023).
Cao, Y. et al. Surface hydroxide promotes CO2 electrolysis to ethylene in acidic conditions. Nat. Commun. 14, 2387 (2023).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
O’Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Zhao, Y. et al. Conversion of CO2 to multicarbon products in strong acid by controlling the catalyst microenvironment. Nat. Synth. 2, 403–412 (2023).
Ozden, A. et al. Energy- and carbon-efficient CO2/CO electrolysis to multicarbon products via asymmetric ion migration–adsorption. Nat. Energy 8, 179–190 (2023).
Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Luo, T. et al. Tandem catalysis on adjacent active motifs of copper grain boundary for efficient CO2 electroreduction toward C2 products. J. Energy Chem. 70, 219–223 (2022).
Zhu, Y. et al. Tandem catalysis in electrochemical CO2 reduction reaction. Nano Res. 14, 4471–4486 (2021).
Meng, D.-L. et al. Highly selective tandem electroreduction of CO2 to ethylene over atomically isolated nickel-nitrogen site/copper nanoparticle catalysts. Angew. Chem. Int. Ed. 60, 25485 (2021).
Ling, N. et al. Acidic media impedes tandem catalysis reaction pathways in electrochemical CO2 reduction. Angew. Chem. Int. Ed. 62, e202308782 (2023).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Zheng, T. et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 3, 265–278 (2019).
Jiang, K. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018).
Zheng, Y. et al. Poly-phenylenediamine-derived atomically dispersed Ni sites for the electroreduction of CO2 to CO. Inorg. Chem. Front. 6, 1729–1734 (2019).
Koshy, D. M. et al. Understanding the origin of highly selective CO2 electroreduction to CO on Ni,N-doped carbon. Catalysts. Angew. Chem. Int. Ed. 59, 4043 (2020).
Sun, Y. et al. Highly selective CO2 electroreduction to CO by the synergy between Ni–N–C and encapsulated Ni nanoparticles. Dalton Trans. 52, 928–935 (2023).
Hou, Y. et al. Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity. Appl. Catal. B. 271, 118929 (2020).
Jiao, L. et al. Single-atom electrocatalysts from multivariate metal–organic frameworks for highly selective reduction of CO2 at low pressures. Angew. Chem. Int. Ed. 59, 20589–20595 (2020).
Gong, Y.-N. et al. Regulating the coordination environment of MOF-templated single-atom nickel electrocatalysts for boosting CO2 reduction. Angew. Chem. Int. Ed. 59, 2705–2709 (2020).
Zhang, T. et al. Atomically dispersed Nickel(I) on an Alloy-encapsulated Nitrogen-doped Carbon Nanotube Array for High-performance Electrochemical CO2 reduction reaction. Angew. Chem. Int. Ed. 59, 12055–12061 (2020).
Zhao, C. et al. Ionic exchange of metal–organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 139, 8078–8081 (2017).
Cheng, Y. et al. Atomically dispersed transition metals on carbon nanotubes with ultrahigh loading for selective electrochemical carbon dioxide reduction. Adv. Mater. 30, 1706287 (2018).
Yang, H. et al. Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities. Nat. Commun. 11, 593 (2020).
Su, X. et al. Complementary Operando Spectroscopy identification of in-situ generated metastable charge-asymmetry Cu2-CuN3 clusters for CO2 reduction to ethanol. Nat. Commun. 13, 1322 (2022).
Ghodselahi, T., Vesaghi, M. A., Shafiekhani, A., Baghizadeh, A. & Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 255, 2730–2734 (2008).
Mandal, L. et al. Investigating the role of copper oxide in electrochemical CO2 reduction in real time. ACS Appl. Mater. Interfaces 10, 8574–8584 (2018).
Garza, A. J., Bell, A. T. & Head-Gordon, M. Is subsurface oxygen necessary for the electrochemical reduction of CO2 on copper? J. Phys. Chem. Lett. 9, 601–606 (2018).
Wei, P. et al. Coverage-driven selectivity switch from ethylene to acetate in high-rate CO2/CO electrolysis. Nat. Nanotechnol. 18, 299–306 (2023).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
Gunathunge, C. M., Li, J., Li, X. & Waegele, M. M. Surface-adsorbed CO as an infrared probe of electrocatalytic interfaces. ACS Catal. 10, 11700–11711 (2020).
Lu, X. et al. In situ observation of the pH gradient near the gas diffusion electrode of CO2 reduction in alkaline electrolyte. J. Am. Chem. Soc. 142, 15438–15444 (2020).
Brannon Andersen, C. Understanding carbonate equilibria by measuring alkalinity in experimental and natural systems. J. Geosci. Educ. 50, 357–362 (2002).
Zhong, W. et al. RhSe 2: a superior 3D electrocatalyst with multiple active facets for hydrogen evolution reaction in both acid and alkaline solutions. Adv. Mater. 33, 2007894 (2021).
Yang, C. et al. Ni‐activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions. Adv. Energy Mater. 10, 2002260 (2020).
Kumar, A. et al. Moving beyond bimetallic-alloy to single-atom dimer atomic-interface for all-pH hydrogen evolution. Nat. Commun. 12, 6766 (2021).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Singh, M. R., Kwon, Y., Lum, Y., Ager, J. W. & Bell, A. T. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016).
Dong, J.-C. et al. Direct in situ raman spectroscopic evidence of oxygen reduction reaction intermediates at high-index Pt (hkl) Surfaces. J. Am. Chem. Soc. 142, 715–719 (2020).
Wei, J. et al. Probing the oxygen reduction reaction intermediates and dynamic active site structures of molecular and pyrolyzed Fe–N–C electrocatalysts by in situ Raman spectroscopy. ACS Catal. 12, 7811–7820 (2022).
Luc, W. Rosen, J. & Jiao, F. An Ir-based anode for a practical CO2 electrolyzer. Catalysis Today 288, 79–84 (2017).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Wang, M. et al. Co-doped Ni3N nanosheets with electron redistribution as bifunctional electrocatalysts for efficient water splitting. J. Phys. Chem. Lett. 12, 1581–1587 (2021).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Acknowledgements
Y.L. and Jia Z. acknowledge support and funding from the A*STAR (Agency for Science, Technology, and Research) under its LCERFI program (Award No: U2102d2002). Y.L. acknowledges support and funding from the NRF Fellowship (Award No: NRF-NRFF14-2022-0003). We acknowledge Dr. Bernt Johannessen and the use of the Australian Synchrotron Facility at the Australian Nuclear Science and Technology Organisation (ANSTO) for the collection of the in situ XAS data used in this work. We acknowledge the use of the XAFCA beamline of the Singapore Synchrotron Light Source (SSLS) for the collection of the ex-situ XAS data used in this work.
Author information
Authors and Affiliations
Contributions
Y.L. supervised the project. Y.L. and M.W. conceived the idea and designed the experiments. M.W. and B.W. carried out all the experimental work. M.W. and Jia Z. performed and supervised the computational work, respectively. M.W. and Jiguang Z. performed the in situ Raman spectroscopy experiments and catalyst synthesis. M.W., B.W., and Q.Y. carried out the in situ XAS experiments. S.X. carried out the ex-situ XAS experiments. B.W. prepared the IrOx-coated Ti mesh electrodes and Ni single atom catalysts. M.Z. carried out the XPS measurements. Jiguang Z. carried out the XRD measurements and analysis. N.L. prepared the Cu PTFE catalysts. Z.M. and W.R.L. contributed to data analysis and manuscript editing. Y.L. and M.W. co-wrote the manuscript. All authors discussed the results and assisted during the manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, M., Wang, B., Zhang, J. et al. Acidic media enables oxygen-tolerant electrosynthesis of multicarbon products from simulated flue gas. Nat Commun 15, 1218 (2024). https://doi.org/10.1038/s41467-024-45527-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45527-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.