Abstract
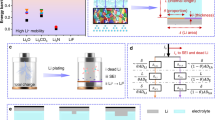
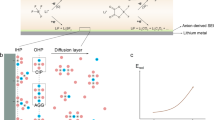
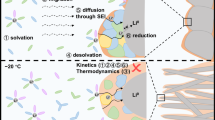
Current electrolyte design for Li metal anodes emphasizes fluorination as the pre-eminent guiding principle for high Coulombic efficiency (CE) based largely on perceived benefits of LiF in the solid–electrolyte interphase (SEI). However, the lack of experimental techniques that accurately quantify SEI compositional breakdown impedes rigorous scrutiny of other potentially key phases. Here we demonstrate a quantitative titration approach to reveal Li2O content in cycled Li anodes, enabling this previously titration-silent phase to be compared statistically with a wide range of other leading SEI constituents including LiF. Across diverse electrolytes, CE correlates most strongly with Li2O above other phases, reaching its highest values when Li2O particles order along the SEI, demonstrating integrated chemical–structural function. The beneficial role of Li2O was exploited to create entirely fluorine-free electrolytes that breach >99% CE, highlighting electrolyte/SEI oxygenation as an underexplored design strategy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are included within the article and its Supplementary Information files. Source data are provided with this paper.
References
Electrochemical Energy Storage Technical Team Roadmap (US DOE Vehicle Technologies Office, 2017).
Schmuch, R., Wagner, R., Hörpel, G., Placke, T. & Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267–278 (2018).
Batteries: 2021 Annual Progress Report (US DOE Vehicle Technologies Office, 2022).
Xiao, J. et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020).
Hobold, G. M. et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021).
Hobold, G. M. & Gallant, B. M. Quantifying capacity loss mechanisms of Li metal anodes beyond inactive Li0. ACS Energy Lett. 7, 3458–3466 (2022).
Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
Meng, Y. S., Srinivasan, V. & Xu, K. Designing better electrolytes. Science 378, eabq3750 (2022).
Wu, H., Jia, H., Wang, C., Zhang, J. G. & Xu, W. Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv. Energy Mater. 11, 2003092 (2020).
Peled, E. & Menkin, S. Review—SEI: past, present and future. J. Electrochem. Soc. 164, A1703–A1719 (2017).
Yu, W., Yu, Z., Cui, Y. & Bao, Z. Degradation and speciation of Li salts during XPS analysis for battery research. ACS Energy Lett. 7, 3270–3275 (2022).
Dedryvère, R. et al. XPS identification of the organic and inorganic components of the electrode/electrolyte interface formed on a metallic cathode. J. Electrochem. Soc. 152, A689 (2005).
Schechter, A., Aurbach, D. & Cohen, H. X-ray photoelectron spectroscopy study of surface films formed on Li electrodes freshly prepared in alkyl carbonate solutions. Langmuir 15, 3334–3342 (1999).
Wang, C., Meng, Y. S. & Xu, K. Perspective—fluorinating interphases. J. Electrochem. Soc. 166, A5184–A5186 (2018).
Suo, L. et al. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl Acad. Sci. USA 115, 1156–1161 (2018).
Tan, J., Matz, J., Dong, P., Shen, J. & Ye, M. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 11, 2100046 (2021).
Li, T., Zhang, X.-Q., Shi, P. & Zhang, Q. Fluorinated solid–electrolyte interphase in high-voltage lithium metal batteries. Joule 3, 2647–2661 (2019).
Guo, R. & Gallant, B. M. Li2O solid electrolyte interphase: probing transport properties at the chemical potential of lithium. Chem. Mater. 32, 5525–5533 (2020).
Smeu, M. & Leung, K. Electron leakage through heterogeneous LiF on lithium–metal battery anodes. Phys. Chem. Chem. Phys. 23, 3214–3218 (2021).
Chen, Y. C., Ouyang, C. Y., Song, L. J. & Sun, Z. L. Electrical and lithium ion dynamics in three main components of solid electrolyte interphase from density functional theory study. J. Phys. Chem. C. 115, 7044–7049 (2011).
He, M., Guo, R., Hobold, G. M., Gao, H. & Gallant, B. M. The intrinsic behavior of lithium fluoride in solid electrolyte interphases on lithium. Proc. Natl Acad. Sci. USA 117, 73–79 (2020).
Huang, W., Wang, H., Boyle, D. T., Li, Y. & Cui, Y. Resolving nanoscopic and mesoscopic heterogeneity of fluorinated species in battery solid-electrolyte interphases by cryogenic electron microscopy. ACS Energy Lett. 5, 1128–1135 (2020).
Heiskanen, S. K., Kim, J. & Lucht, B. L. Generation and evolution of the solid electrolyte interphase of lithium-ion batteries. Joule 3, 2322–2333 (2019).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Zhao, Y. et al. Electrolyte engineering for highly inorganic solid electrolyte interphase in high-performance lithium metal batteries. Chem 9, 682–697 (2023).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Kim, M. S. et al. Suspension electrolyte with modified Li+ solvation environment for lithium metal batteries. Nat. Mater. 21, 445–454 (2022).
Liu, Y. et al. Solubility-mediated sustained release enabling nitrate additive in carbonate electrolytes for stable lithium metal anode. Nat. Commun. 9, 3656 (2018).
Deng, W. et al. Quantification of reversible and irreversible lithium in practical lithium-metal batteries. Nat. Energy 7, 1031–1041 (2022).
McShane, E. J. et al. Quantifying graphite solid–electrolyte interphase chemistry and its impact on fast charging. ACS Energy Lett. 7, 2734–2744 (2022).
Xiang, Y. et al. Quantitatively analyzing the failure processes of rechargeable Li metal batteries. Sci. Adv. 7, eabj3423 (2021).
von Holtum, B. et al. Accessing the primary solid–electrolyte interphase on lithium metal: a method for low-concentration compound analysis. ChemSusChem 16, e202201912 (2023).
Jaworowski, R. J., Potts, J. R. & Hobart, E. W. The determination of oxygen in lithium. Anal. Chem. 35, 1275–1279 (1963).
Sax, H. I. & Steinmetz, H. Determination of Oxygen in Lithium Metal (Oak Ridge National Laboratory, 1958).
Lu, J. et al. A lithium–oxygen battery based on lithium superoxide. Nature 529, 377–382 (2016).
Steinberg, K. et al. Imaging of nitrogen fixation at lithium solid electrolyte interphases via cryo-electron microscopy. Nat. Energy 8, 138–148 (2022).
Cao, X. et al. Effects of fluorinated solvents on electrolyte solvation structures and electrode/electrolyte interphases for lithium metal batteries. Proc. Natl Acad. Sci. USA 118, e2020357118 (2021).
Ren, X. et al. Role of inner solvation sheath within salt-solvent complexes in tailoring electrode/electrolyte interphases for lithium metal batteries. Proc. Natl Acad. Sci. USA 117, 28603–28613 (2020).
Fiedler, C., Luerssen, B., Rohnke, M., Sann, J. & Janek, J. XPS and SIMS analysis of solid electrolyte interphases on lithium formed by ether-based electrolytes. J. Electrochem. Soc. 164, A3742–A3749 (2017).
Aurbach, D., Ein‐Ely, Y. & Zaban, A. The surface chemistry of lithium electrodes in alkyl carbonate solutions. J. Electrochem. Soc. 141, L1–L3 (2019).
Leung, K., Soto, F., Hankins, K., Balbuena, P. B. & Harrison, K. L. Stability of solid electrolyte interphase components on lithium metal and reactive anode material surfaces. J. Phys. Chem. C. 120, 6302–6313 (2016).
Michan, A. L. et al. Fluoroethylene carbonate and vinylene carbonate reduction: understanding lithium-ion battery electrolyte additives and solid electrolyte interphase formation. Chem. Mater. 28, 8149–8159 (2016).
Jaumann, T. et al. Role of 1,3-dioxolane and LiNO3 addition on the long term stability of nanostructured silicon/carbon anodes for rechargeable lithium batteries. J. Electrochem. Soc. 163, A557–A564 (2016).
Corder, G. W. & Foreman, D. I. in Nonparametric Statistics for Non-Statisticians 122–154 (John Wiley & Sons, 2011).
Huang, Y. et al. Eco-friendly electrolytes via a robust bond design for high-energy Li metal batteries. Energy Environ. Sci. 15, 4349–4361 (2022).
Liu, S. et al. An inorganic-rich solid electrolyte interphase for advanced lithium-metal batteries in carbonate electrolytes. Angew. Chem. 60, 3661–3671 (2021).
Qiu, F. et al. A concentrated ternary‐salts electrolyte for high reversible Li metal battery with slight excess Li. Adv. Energy Mater. 9, 1803372 (2018).
Wang, Q. et al. Interface chemistry of an amide electrolyte for highly reversible lithium metal batteries. Nat. Commun. 11, 4188 (2020).
Yuan, X., Liu, B., Mecklenburg, M. & Li, Y. Ultrafast deposition of faceted lithium polyhedra by outpacing SEI formation. Nature 620, 86–91 (2023).
Peled, E., Golodnitsky, D. & Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 144, L208 (1997).
Li, Y. et al. Correlating structure and function of battery interphases at atomic resolution using cryoelectron microscopy. Joule 2, 2167–2177 (2018).
Cao, X., Jia, H., Xu, W. & Zhang, J.-G. Review—localized high-concentration electrolytes for lithium batteries. J. Electrochem. Soc. 168, 010522 (2021).
Chen, S. et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018).
Shkrob, I. A., Marin, T. W., Zhu, Y. & Abraham, D. P. Why bis(fluorosulfonyl)imide is a ‘magic anion’ for electrochemistry. J. Phys. Chem. C. 118, 19661–19671 (2014).
Moon, J. et al. Non-fluorinated non-solvating cosolvent enabling superior performance of lithium metal negative electrode battery. Nat. Commun. 13, 4538 (2022).
Ko, S. et al. Electrode potential influences the reversibility of lithium-metal anodes. Nat. Energy 7, 1217–1224 (2022).
Yu, Z. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 5, 526–533 (2020).
Kim, S. C. et al. Data-driven electrolyte design for lithium metal anodes. Proc. Natl Acad. Sci. USA 120, e2214357120 (2023).
Fan, X. et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 4, 174–185 (2018).
Sayahpour, B. et al. Quantitative analysis of sodium metal deposition and interphase in Na metal batteries. Energy Environ. Sci. 17, 1216–1228 (2024).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J. G. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2017).
Acknowledgements
This work made use of the Massachusetts Institute of Technology (MIT) Materials Research Science and Engineering Center Shared Experimental Facilities, supported by the National Science Foundation under award number DMR-14-19807. This work also made use of the MIT Department of Chemistry Instrumentation Facility. Titration method development was supported in part by an Electrochemical Society–Toyota Young Investigator Award (B.M.G. and K.S.). The Cryo-HRTEM work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under award number DE-SC0022955 (Y.L. and C.W.). We also thank T. Buonassisi, MIT, for helpful discussions regarding the statistical analysis presented herein.
Author information
Authors and Affiliations
Contributions
G.M.H. and B.M.G. conceived the idea and designed the experiments. G.M.H. carried out experiments and measurements. C.W. and Y.L. co-designed Cryo-HRTEM experiments and performed imaging. K.S. assisted with method development. G.M.H. and B.M.G. analysed the data and prepared the initial draft of the paper. All authors discussed the results and contributed to the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Xinyong Tao, Atsuo Yamada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–7, Figs. 1–46 and Tables 1–11.
Source data
Source Data Fig. 1
Source data of all samples used for statistical analysis.
Source Data Fig. 2
Source data of all samples used for statistical analysis.
Source Data Fig. 3
Source data of all samples used for statistical analysis.
Source Data Fig. 4
Integrated NMR data of all samples.
Source Data Fig. 5
Source data of all samples used for statistical analysis.
Source Data Fig. 6
Source data of all samples used for statistical analysis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hobold, G.M., Wang, C., Steinberg, K. et al. High lithium oxide prevalence in the lithium solid–electrolyte interphase for high Coulombic efficiency. Nat Energy (2024). https://doi.org/10.1038/s41560-024-01494-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41560-024-01494-x
This article is cited by
-
Hidden potential of lithium oxide
Nature Energy (2024)