Abstract
Anti-Kasha’s process in organic luminogens has attracted many attentions since its discovery. However, only limited examples of anti-Kasha’s rule have been reported and anti-Kasha triplet energy transfer (ET) is even less-touched. Benefiting from anti-Kasha’s rule, this work provided an efficient strategy to realize excitation wavelength dependent (Ex-De) afterglow in a host-guest system. The host has almost imperceptible RTP upon 365 nm excitation and guest is totally RTP inactive, while the doping host-guest system exhibits Ex-De afterglow with improved quantum yields. Anti-Kasha triplet ET process is realized from the higher excited triplet state T2 of host to the lowest excited singlet state S1 of the aggregated/unimolecular guest. ET efficiency in the doping system could be tuned by simply changing its processing methods to guide host and guest to adopt denser or looser intermolecular packing. The strategy of anti-Kasha triplet ET endows the host-guest doping system with multiple stimuli-responsive properties, including Ex-De afterglow, mechano-, and thermal-triggered afterglow behaviors. The corresponding applications of these properties are also realized in multiple information anti-counterfeiting and display.
Similar content being viewed by others
Introduction
Organic room temperature phosphorescence (RTP) systems with long lifetime afterglow have been deeply investigated and widely applied in various areas, including organic optoelectronics, chemical biomedicine, optical sensing, and information encryption1,2,3. Excellent phosphorescence has been realized using various strategies through boosting intersystem crossing (ISC) from excited singlet state (S1) to triplet states (Tn) and meanwhile inhibiting nonradiative decays, including heavy atom effect4,5,6, El-Sayed’s rule7,8, crystallization engineering9,10,11,12,13, recombination of charge-separated states14,15,16,17, energy transfer (ET)18,19, hydrogen-bonding interaction20,21,22,23, through-space conjugation24,25,26, polymer matrix assistance27,28,29,30,31,32,33, resonance activation34,35, multicomponent combination36,37,38,39,40,41,42, and etc. The emission behaviors of organic RTP materials are sensitive to temperature, oxygen, light, or humidity, so that they can be regulated by external stimuli, and have versatile potential applications. However, RTP systems with multiple stimuli-responsive behaviors are still less developed, and their single function could not meet complicated practical needs43.
Tunable RTP emission, particularly excitation wavelength dependent (Ex-De) afterglow, provides an opportunity to explore multiple stimuli-responsive materials with potential prospects in optical sensing44,45,46,47,48,49. Ex-De luminescence is mainly attributed to the emission from different photophysical processes that requires controlled expressions of excitation dynamics. At present, it is mainly divided into three categories: emission from different excited states in single-component systems50,51,52,53,54,55,56, emission from different components in multicomponent systems57,58,59,60,61,62, and emission from different aggregated states in cluster systems63,64,65,66,67,68,69,70,71,72,73,74. The most practical strategy to achieve tunable Ex-De luminescence is utilizing multicomponent systems with the advantages in facile preparation, conveniently tunable process and wide applicability.
Among small molecular multicomponent systems, Ex-De afterglow is less explored. In 2021, Huang’s group reported several ultralong organic host/guest phosphorescence materials with dynamic lifetime-tuning properties, which was obtained by introducing different hosts with tuning triplet energy levels in order to boost ISC and ET processes, and Ex-De phosphorescence was demonstrated61. However, considerable efforts usually focus on ET of the lowest excited state but seldom on higher excited state. If the utilization of higher excited state energy could be realized, Ex-De afterglow based on unusual anti-Kasha’s rule would be feasible in multicomponent systems. Anti-Kasha’s rule suggests that photophysical and photochemical processes in condensed phase can be carried out in higher excited state of a given multiplicity75,76. However, most excitons are unstable in higher excited state and they easily transfer to the lowest excited state through internal conversion (IC), therefore, it is difficult to detect the process of anti-Kasha’s rule. Up to now, only limited examples of anti-Kasha’s rule have been reported77,78 and ET in higher excited state is even less-touched79,80. The higher triplet states are more susceptible to environmental quenching and non-radiative interference, hence detectable anti-Kasha emission is only limited in a low-temperature and inert environment. If grievous quenching of higher excited state excitions could be overcome and multiple radiative processes in anti-Kasha system were reasonably utilized, it will be feasible to achieve ET from higher triplet state T2 to construct new Ex-De afterglow systems.
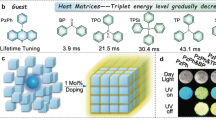
Herein, this work provided an efficient way to realize Ex-De afterglow by ET process in a host-guest system using a simple doping strategy. The ET process is accomplished from the higher excited triplet state T2 of the host to the lowest excited singlet state S1 of the aggregated/unimolecular guest (Fig. 1a). The host has almost imperceptible RTP upon 365 nm excitation and the guest is totally RTP inactive (Fig. 1b), while the doping host-guest system exhibits Ex-De afterglow with improved quantum yields (Figs. 1b and S1). ET efficiency in the doping system could be tuned by changing intermolecular packing degree depending on different doping treatments. The strategy of anti-Kasha triplet ET endows the doping system with multiple stimuli-responsive properties, including Ex-De afterglow, mechano-, and thermal-triggered afterglow behaviors. Based on these features, corresponding applications of these properties are also explored in multiple information anti-counterfeiting and display.
a The high-energy excitons (represented by ‘car’) from T2 state of host can transfer to S1 state of guest (represented by ‘side road’) through anti-Kasha triplet ET or to its own T1 state (represented by ‘main road’) through IC. The transition between inefficient and efficient ET process can be achieved by employing distinct processing methods, resulting in multiple variation of emission behaviors. b Images of host, guest, DS-a, DS-g and DS-m with doping ratio of 1:1000 before and after 365/254 nm UV irradiation under ambient conditions.
Results
Preparations of host, guest, and doping systems
Both the commercially available 9(10H)-acridone and diphenylsulfone were purified by two-step recrystallization process with different solvent conditions. The chemical structure of two compounds was verified with NMR (Figs. S23–S26) and their high purity was proved by high performance liquid chromatography (HPLC) (Figs. S2 and S3). Thermogravimetric analysis of diphenylsulfone and 9(10H)-acridone revealed their high thermal stability with the decomposition temperature more than 200 and 300 °C (Figs. S4 and S5), respectively, which ensured a solid foundation for its applications. Molecules 9(10H)-acridone and diphenylsulfone were then ultilized as guest and host to construct the host-guest doping system, respectively. After mixing the solution of guest and host at certain mass ratio, the solvent was then removed by rotary evaporation. Finally, the as-prepared doping powders DS-a with the mass ratios of 1:100, 1:1000 and 1:10000 were obtained. A control group of the doping samples was also prepared with mass ratios of 1:1000 upon thermal melting-cooling (DS-m) or mechanical grinding (DS-g) treatment.
Photophysical properties of host and guest
As shown in Fig. 2a, the UV–vis spectra of guest in 2-MeTHF exhibited two vibration absorption bands around 250–310 and 320–400 nm, which are attributed to locally excited states. Powder of guest showed similar absorption bands, but the range is broader and extends to 480 nm. The 77 K photoluminescence spectra of guest solution had two emissions located at 390–467 and 467–650 nm, which belong to fluorescence and phosphorescence, respectively (Fig. S6a). Interestingly, the fluorescence of guest gradually weakened after the addition of poor solvent n-hexane (Fig. S7), indicating the effect of aggregation caused quenching (ACQ), which could also be confirmed by the comparison of fluorescence quantum yields of its THF solution (16.6%) and powder (0.9%). Though the emission of guest powder was weak at room temperature, its specific emission could be observed clearly at low temperature. Compared to its solution, guest powder exhibited red-shifted fluorescence around 426–542 nm and phosphorescence around 530-720 nm at 77 K (Fig. S6a). Due to definite ACQ characteristics, the guest should be competent for a candidate to act as an energy acceptor when dispersed in the host matrix.
Host has shorter absorption and fluorescence emission wavelength because of weak conjugate structure. The absorption and emission spectra of host in both unimolecular state (solution) and aggregated state (solid) were then investigated. As shown in Fig. 2b, unimolecular host displays a vibrational absorption band between 240 and 285 nm, and the tail absorption is further extended to 350 nm once the molecules aggregate. In addition, a wide and weak absorption band occurs between 350 and 450 nm, which may derive from the absorption of intermolecular charge transfer. At 77 K, unimolecular host has the fluorescence band from 270 to 335 nm and the phosphorescence band from 348 to 520 nm (Fig. S6b). The corresponding phosphorescence lifetime exhibited double exponential decay, which implied the existence of two phosphorescence emission processes from double triplet excited states (Fig. S8 and Table S2).
Surprisingly, the powder of host has two types of afterglows at low temperatures (Fig. 2c). The time-dependent spectra, emission decay curves and dual-exponential emission in solution of host combine to show that the shorter wavelength emission at 425 nm originates from the higher triplet state T2 (752.1 ms at 77 K and 149.1 ms at 298 K, Figs. S9a and S9b), while the longer wavelength emission at 480 nm from the low-lying T1 state (2360.7 ms at 77 K and 326.3 ms at 298 K, Figs. S9c and S9d), which suggests an anti-Kasha behavior. This property is similar to that of previous reported molecules51,81,82, which also generated phosphorescence from T2 and T1 in the aggregated state. However, the phosphorescence quantum yield of host powder at room temperature is only 0.48% (Table S1), indicating that most of the excitons are lost through non-radiative process. The pathway for the non-radiative decay is likely through the strong ISC between singlet and triplet state; the triplet excitons are vulnerable to thermal motion, collision, and they are easily deactivated through quenching by air. Given the dual phosphorescence property of host, it is possible to construct a doping system by triplet-singlet ET to improve the exciton utilization and solve aggregation quenching. Stimuli-responsive luminescence of doping system can be also achieved through regulating host-guest packings.
Photophysical properties of the host-guest doping system
The photophysical properties of DS-a 1:1000 were then studied in detail (Figs. 3 and S10b). According to its excitation-afterglow profiles (Fig. S11), the excitation wavelengths of 295 nm and 374 nm were chosen to ensure the integrity of the absorption spectrum and PL spectrum of host. As shown in Fig. 3a, the delayed spectrum of DS-a 1:1000 upon 374 nm excitation shows multiple emission peaks, which is likely a combination of multiple emissions from different excited states. The afterglow emission range and wavelength of DS-a 1:1000 combined together overlapped well with the superposition of prompt fluorescence of guest in the unimolecular state (solution) and aggregated state (powder) at 77 K (nor delayed emission of guest in unimolecular state), indicating that they are correlated. To our surprise, an unusual phenomenon occurred when DS-a 1:1000 was exposed to 295 nm UV light. As shown in Fig. S10b, DS-a 1:1000 emits obvious prompt PL between 300 and 390 nm and long wavelength delayed emission between 390 and 750 nm after 295 nm excitation. The emission around 300-390 nm is prompt fluorescence because its lifetime is only 0.2 ns. The 295 nm-excited delayed PL spectrum of DS-a 1:1000 also shows multiple emission peaks, which is similar to that of 374 nm-excited, except that a new generated red-shifted emission peak appears at 490 nm. Depending on similar wavelengths and peak shapes and the delayed PL spectrum upon 295 nm excitation matches well with the prompt fluorescence of unimolecular/aggregated guest and T1 phosphorescence of aggregated host (Fig. 3b). Though similar phenomenon has been rarely reported67,68,72, important clues could be confirmed by the decay curves and the corresponding emission lifetimes (Figs. S12, S9b and S9d). The newborn emission at 490 nm possesses a long lifetime of ~380 ms upon the 295 nm excitation, which differs from ~135 ms short lifetime of the emissions upon 374 nm excitation, revealing the existence of another afterglow (T1 of the aggregated host, Fig. S9d). As a result, the delayed PL spectrum shows abnormal red-shift emission with the shorter excitation wavelength, and their color difference can be distinguished even by naked eyes, indicating impressive Ex-De afterglow characteristic (Fig. 1b).
To further support our surmise on the composition of the delayed PL spectrum, more experiments were carried out. Upon 374 nm excitation, normalized delayed spectra show that the proportion of emissions at 400 and 424 nm significantly decreases with the increase of the doping ratio of DS-a, while the 472 nm emission increases (Fig. 3c and S10). This trend makes sense as the aggregation of guest is enhanced with the increase of doping ratio and the combined sources from aggregated and unimolecular guest also become more significant. Furthermore, the peak shape of delayed PL spectra of DS-a 1:1000 after 374 nm excitation shows no significant variation with different delayed times (Fig. S13) and the lifetimes at different wavelengths are essentially the same (Table S3), confirming that the afterglow should only come from the same photophysical process (ET from T2 in host to S1 in guest, discussed below) but different excited states. These experiments prove that the afterglow of DS-a upon 374 nm excitation can be deemed as delayed fluorescence derived from S1 of both aggregated and unimolecular guest.
To figure out what causes the difference between the emissions upon the excitation of 295 nm and 374 nm, we tried to scrutinize related clues. On the basis of the absorption characteristics of host powder, we deduced that the whole process from excitation to emission is accompanied by ET. In Fig. 4a, the prompt emission of host powder partially overlaps with the absorption of guest solution and guest powder (shaded area). In contrast, the 323 nm emission of host powder overlaps less with the absorption of the guest solution, thus the fluorescence emission of aggregated host retains in doping system. The 360–400 nm absorption of guest solution and 360–460 nm absorption of guest powder are exactly close to T2 of host. The sufficient overlap indicates that ET from T2 of host to guest is practicable. To the best of our knowledge, ET from higher energy levels is rarely reported in previous literature. Moreover, the absorption of guest exactly covers T2 and involves less with T1, which will effectively reduce the influence from T1 phosphorescence of host. The involvement of T2 in ET is also demonstrated by the similar lifetimes of 400–460 nm emission of DS-a and that of T2 emission of host powder. As shown in Fig. S14 and Table S3, lifetime of T2 ( ~ 150 ms) is very close to that of 400-460 nm emission of DS-a with different doping ratios, which strongly proves that the energy is transferred from T2 of host to guest. The prompt PL spectra of DS-a with different doping ratios under excitation of 295 nm indicates that the afterglow proportion increased significantly with the increase of doping ratio (Fig. S15), especially ~ 460 nm emission, suggesting that the increase of doping ratio not only improve the ET efficiency, but also increase the emission proportion from S1 of aggregated guest. Ample evidence indicates that the ET process does exist consistently and plays a key role in this host-guest system.
a Normalized absorption of the powder and the solution of guest, and normalized steady-state and delayed PL spectra of host powder; dash area is the overlap of prompt emission of host powder and absorption of guest powder and guest solution. Normalized delayed PL spectra of DS-a, DS-m and DS-g with a doping ratio of 1:1000 under b 295 nm and c 374 nm excitation.
Although we confirm that ET of T2 leads to the long-lifetime delayed fluorescence of guest in DS-a, it is still difficult to explain why the 295 nm excitation can selectively induce T1 phosphorescence of host. Based on current research, we propose a hypothesis that the generation of T1 phosphorescence is related to the degree of light absorption by host. That means that the process depends on the number of the excitons produced. When the excitation wavelength is shorter than 295 nm, aggregated host absorbs enough energy and singlet excitons can be fully generated. These singlet excitons return to the ground state by fluorescence or non-radiative transition and reach T2 state by ISC process; in this process, sufficient T2 excitons not only cause significant fluorescence of guest through ET, but also transfer to T1 through IC process accompanying with T1 phosphorescence of host. When DS-a is excited with the wavelength longer than 350 nm, less excitons are produced due to weak absorption of aggregated host, resulting in insufficient T2 excitons. Therefore, T2 excitons preferentially transfer energy to guest and few excitons return to T1 state, probably resulting in phosphorescence silence of T1. To prove this hypothesis, we need to design new authentication experiments. It is well known that ET requires not only the overlap of absorption range of energy acceptor and emission range of energy donor, but also short intermolecular spacing. To improve the utilization efficiency of triplet excitons and reduce the intermolecular distance, mixed host and guest were thermal-melted and then cooled to prepare thermal crystallization sample (DS-m 1:1000). Thermal crystallization would provide compact packing and rigid environment to prevent oxygen and moisture from quenching the triplet excitons. As a result, DS-m 1:1000 exhibit more significant afterglow compared to relative loose DS-a 1:1000, as verified by the increase of afterglow proportions (Fig. S16). DS-m 1:1000 does not exhibit Ex-De afterglow (Figs. S17 and 1b), but its delayed spectra are composed of the delayed fluorescence from S1 from the aggregated and unimolecular guest. Their delayed fluorescence is nearly the same irrespective of 295 nm or 374 nm excitation (Fig. 4b, c), reflecting that ET efficiency is largely improved and IC process from T2 to T1 is almost suppressed. On the contrary, when host and guest were mixed and mechanically ground to obtain DS-g 1:1000, the proportion of >480 nm emission after 295 nm excitation increases (Figs. 4b and S18), indicating the ET process is weakened owing to long intermolecular distances and weak intermolecular interactions. The emission intensity ratio of 472 nm to 400 nm of DS-g 1:1000 after 374 nm excitation is lower than that of DS-a 1:1000 or DS-m 1:1000 (Fig. 4c), which should relate to the low utilization efficiency of aggregated guest under loose packing environment. The loose molecular packing of DS-g 1:1000 is also not conducive to the utilization of triplet excitons, resulting in the significantly low quantum yield of DS-g 1:1000 after 374 nm excitation (Table S1). Powder XRD patterns of different doping samples and single crystal of host were then measured and showed in Fig. S19. All the doping samples have well-ordered crystalline structure with multiple similar diffraction peaks from 2θ = 5°−25° with those of the single crystal of host, suggesting that the crystalline morphology in host is basically maintained after the doping of guest. Apart from relative intensity of characteristic peaks, the diffraction patterns of all doping samples resemble each other, which probably derive from subtle discrepancies of stacking density, dispersion of guest, etc. These subtle discrepancies impact the energy transfer efficiency and ultimately endow the doping samples doping method-dependent afterglow characteristic. The above evidences prove our hypothesis and fully explain the mechanism of Ex-De afterglow, paving a way for material design and theoretical improvement.
Mechanism of the Ex-De afterglow of the host-guest system
According to the above experimental evidences, we proposed anti-Kasha triplet energy transfer principles. As shown in Fig. 5a, when the host-guest system is excited at 295 nm, host absorbs enough energy to generate abundant excitons. Some excitons are consumed in the form of prompt fluorescence, but the residual reaches T2 through the ISC process. T2 excitons can undergo ET process from T2 of host to S1 of guest and the arrival S1 excitons are responsible for the delayed fluorescence. The other T2 excitons transform into T1 excitons through IC process, and subsequently produce T1 phosphorescence of host. However, not enough excitons are produced due to weak absorption of host under 374 nm excitation, resulting in the low production of T2 excitons. Since S1 of guest is closer to T2 of host, the triplet-singlet ET efficiency should be higher than the T2-T1 IC efficiency. Preferential ET leads to consumption of most of the T2 excitons, so only the delayed fluorescence of guest is observed after 374 nm excitation (Fig. 5b). When the doping system was prepared by thermal melting-cooling (DS-m) or grinding (DS-g), intermolecular spacing and environment should be diverse from those in DS-a, which led to an increase or decrease of ET efficiency (Figs. S20 and S21), respectively. Thus, the delayed spectra of this host-guest doping system can be tuned through different stimuli: DS-m shows single afterglow, while DS-g or DS-a exhibits Ex-De afterglow after 295/374 nm excitation (Fig. 1). Due to the increase of exciton utilization after doping, the quantum yield of the doping systems has a significant performance improvement compared to that of the pure host or guest (Table S1), achieving synergetic reinforcing effect.
Benzophenone is a commonly used host and has a similar structure with diphenylsulfone. For comparison, a new host-guest doping system DS-BP based on benzophenone was constructed through liquid mixing (Fig. S22a). However, no obvious afterglow of DS-BP could be observed by naked eyes. The absorption spectrum of aggregated benzophenone has no separated absorption band compared with that of diphenylsulfone. The similarity between the prompt and delayed PL of benzophenone powder at room temperature or 77 K, as well as the decay tendencies demonstrate that its emission essentially originates from phosphorescence (Figs. S22b and S22c). The same lifetimes at different wavelengths prove that the delayed PL comes from a single emission state of T1. As shown in Figs. S22d and S22e, the delayed PL of DS-BP around 600 nm can be attributed to T1 of guest, while the band between 400 and 550 nm is derived from T1 of benzophenone, which can be proved by their different lifetimes and spectral contours. Unlike the phenomena in DS-a/DS-m/DS-g, the delayed PL of DS-BP has no characteristic of prompt fluorescence of unimolecular guest, which probably point to the mismatch between T1 of aggregated benzophenone and absorption of unimolecular guest (Fig. S22f). These results further verify the rationality of anti-Kasha triplet energy transfer and Ex-De afterglow behavior in DS-a.
Applications of host-guest doping system
Since the host and guest can be mixed via facile grinding, solution evaporation or heating, the doping systems is of great potential to serve as security label, anti-counterfeiting/display ink or thermal printing paper (Fig. 6). Based on the force-triggered RTP effect of the doping system, prototype of security label was prepared. Powders of guest and host (1:100) were manually shaken to ensure a certain degree of uniformity. Then the mixture was cast on the glass substrate and sealed with scotch tape in order to avoid material leakage. The as-prepared label could not emit RTP after 365 nm excitation. When scraping on the scotch tape with a metal spatula or something hard, the blue RTP of the ground region was switched on and formed strong contrast (Fig. 6a).
By mixing of the host and guest in liquid form, anti-counterfeiting/display ink could be designed (Fig. 6b). Filter paper was dip-coated with ethanol solution of guest (0.5 mg/mL) and naturally dried. The whole guest-loaded paper was dark after turning off the UV light by virtue of the absence of RTP activity. When ethanol solution of host (20 mg/mL) was drawn on this filter paper with a Chinese brush to allow to evaporate, host and guest mixed together to form DS-a. After turning off 365 and 254 nm excitation light, the letters with bluish and greenish RTP emission could be observed clearly, respectively. This dynamic RTP phenomenon would promote the effect of anti-counterfeiting. Following the same principle, host and guest could also be pre-mixed in solution to prepare display inks and used for inkjet printing.
Through thermal mixing of host and guest, a thermal printing paper was designed as followed. Weighing paper was cut into a proper size and then soaked in ethanol solution of guest (0.5 mg/mL) to prepare the guest-loaded paper. After the solvent evaporated, fine powder of host was gently smeared on one side of the guest-loaded paper. When the thermal paper passed through the print head of thermal printing machine, guest and host would partially blend together to form DS-m under the action of heating due to the low melting point of host. As shown in Fig. 6c, two sky-blue emissive Chinese characters (meaning ‘Shenzhen’) could be observed by naked eyes upon 365 or 254 nm excitation, corresponding to the formation of DS-m. Different kinds of letters or characters could also be printed according to specific requirements. Utilizing this doping system, multiple anti-counterfeiting/display applications could be successfully achieved with the unique stimuli-responsive RTP characteristic.
Discussion
In summary, we have successfully developed an organic host-guest doping system, in which 9(10H)-acridone acted as guest and diphenylsulfone as host to turn-on afterglow under 365 nm excitation via facile grinding, solution evaporation or heating. This doping system possessed several characteristic features: (1) Ex-De afterglow, which is a very useful property for non-invasive tuning the emission of materials and rarely observed among two-component small molecule doping systems. (2) Anti-Kasha’s rule, ET process from higher excited triplet state T2 of host to the lowest excited singlet state S1 of the aggregated/unimolecular guest plays a crucial role in the Ex-De afterglow behaviors, which is even rarer. (3) Multiple processing methods, the Ex-De afterglow and RTP turn-on phenomenon could be achieved through grinding, writing, painting, or thermal printing. Thanks to these features, we successfully realized applications in multiple information anti-counterfeiting and display. This work opened up a way for the preparation of two-component small molecule Ex-De RTP materials.
Methods
Materials
9(10H)-Acridone (98%), diphenyl sulfone (99%), and benzophenone (99%) were purchased from Energy Chemical Company. Solvents (AR) were purchased from Xilong Chemical Co., Ltd.
Instruments and methods
All photophysical measurements, including steady or delay spectra, decay curve, and quantum yield, were carried out on Edinburgh Instruments FLS1000 with different accessories. 1H and 13C NMR spectra were recorded on a VNMRS 400 NMR spectrometer (Varian, USA). Sample heating was performed on a microcomputer temp-controlled heating board (JF-966A, JFTOOLS, China). TGA analysis was performed on a NETZSCH SA409PC thermogravimeter. Photos were taken by Nikon D7100 or HUAWEI P20. High-performance liquid chromatogram (HPLC) curves were recorded using an Agilent 1260 Infinity II(QA&QC-HPLC-19) by DAICEL CHIRAL TECHNOLOGIES(CHINA) CO., LTD. Thermal printing was performed on a thermal printing machine (DL-581PS, Deli, China).
Purification of host
The high purity host was obtained after recrystallizing from its ethanol and then acetone/H2O solution.
Purification of guest
The high purity guest was obtained after recrystallizing from its acetic acid and then methanol solution.
Purification of benzophenone
Benzophenone was obtained after recrystallizing from its petroleum ether solution.
Data availability
All relevant data are included in this Article and its Supplementary Information files. Data is available from the authors upon request. Source data are provided in this paper.
References
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Gao, H. & Ma, X. Recent progress on pure organic room temperature phosphorescent polymers. Aggregate 2, e38 (2021).
Kenry, Chen, C. & Liu, B. Enhancing the performance of pure organic room-temperature phosphorescent luminophores. Nat. Commun. 10, 2111 (2019).
Bolton, O., Lee, K., Kim, H. J., Lin, K. Y. & Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 3, 205–210 (2011).
Cai, S. et al. Enhancing ultralong organic phosphorescence by effective π-type halogen bonding. Adv. Funct. Mater. 28, 1705045 (2018).
She, P. et al. Controlling organic room temperature phosphorescence through external heavy‐atom effect for white light emission and luminescence printing. Adv. Opt. Mater. 8, 1901437 (2019).
Zhao, W. et al. Rational molecular design for achieving persistent and efficient pure organic room-temperature phosphorescence. Chem 1, 592–602 (2016).
Wu, Z. et al. Persistent room temperature phosphorescence from triarylboranes: a combined experimental and theoretical study. Angew. Chem. Int. Ed. 59, 17137–17144 (2020).
Yuan, W. Z. et al. Crystallization-induced phosphorescence of pure organic luminogens at room temperature. J. Phys. Chem. C. 114, 6090–6099 (2010).
Gong, Y. et al. Room temperature phosphorescence from natural products: crystallization matters. Sci. China Chem. 56, 1178–1182 (2013).
Hamzehpoor, E. & Perepichka, D. F. Crystal engineering of room temperature phosphorescence in organic solids. Angew. Chem. Int. Ed. 59, 9977–9981 (2020).
Zheng, S., Zhu, T., Wang, Y., Yang, T. & Yuan, W. Z. Accessing tunable afterglows from highly twisted nonaromatic organic AIEgens via effective through-space conjugation. Angew. Chem. Int. Ed. 59, 10018–10022 (2020).
Ye, W. et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 20, 1539–1544 (2021).
Kabe, R. & Adachi, C. Organic long persistent luminescence. Nature 550, 384–387 (2017).
Alam, P. et al. Two are better than one: a design principle for ultralong-persistent luminescence of pure organics. Adv. Mater. 32, 2001026 (2020).
Lin, Z., Kabe, R., Nishimura, N., Jinnai, K. & Adachi, C. Organic long-persistent luminescence from a flexible and transparent doped polymer. Adv. Mater. 30, 1803713 (2018).
Alam, P. et al. Organic long-persistent luminescence from a single-component aggregate. J. Am. Chem. Soc. 144, 3050–3062 (2022).
Zhao, W. et al. Boosting the efficiency of organic persistent room-temperature phosphorescence by intramolecular triplet-triplet energy transfer. Nat. Commun. 10, 1595 (2019).
Zhou, Z. et al. Energy transfer mediated enhancement of room‐temperature phosphorescence of carbon dots embedded in matrixes. Adv. Opt. Mater. 10, 2100704 (2021).
Zheng, H., Cao, P., Wang, Y., Lu, X. & Wu, P. Ultralong room-temperature phosphorescence from boric acid. Angew. Chem. Int. Ed. 60, 9500–9506 (2021).
Zhang, T. et al. Hydrogen bonding boosted the persistent room temperature phosphorescence of pure organic compounds for multiple applications. J. Mater. Chem. C. 7, 9095–9101 (2019).
Zhu, Y. et al. Ultralong polymeric room temperature phosphorescence materials fabricated by multiple hydrogen bondings resistant to temperature and humidity. Adv. Opt. Mater. 9, 2100782 (2021).
Zhou, B. & Yan, D. Hydrogen-bonded two-component ionic crystals showing enhanced long-lived room-temperature phosphorescence via TADF-assisted förster resonance energy transfer. Adv. Funct. Mater. 29, 1807599 (2019).
Yu, J. et al. Purely organic room-temperature phosphorescence endowing fast intersystem crossing from through-space spin-orbit coupling. JACS Au. 1, 1694–1699 (2021).
Zhang, T., Zhao, Z., Ma, H., Zhang, Y. & Yuan, W. Z. Polymorphic pure organic luminogens with through-space conjugation and persistent room-temperature phosphorescence. Chem. Asian J. 14, 884–889 (2019).
Zheng, X. et al. Space conjugation induced white light and room-temperature phosphorescence from simple organic small molecules: single-component WLED driven by both UV and blue chips. Mater. Chem. Front. 5, 6960–6968 (2021).
Yuan, S. et al. Effectively unlocking the potential molecular room temperature phosphorescence of pure carbazole derivatives. Adv. Opt. Mater. 10, 2200090 (2022).
Yue, L. et al. Organic phosphor doped thermoplastics with ultralong and memorable room temperature phosphorescence different from crystals. Chem. Eng. J. 433, 2200090 (2022).
Huang, W., Fu, C., Liang, Z., Zhou, K. & He, Z. Strong circularly-polarized room-temperature phosphorescence from a feasibly separable scaffold of Bidibenzo[b,d]furan with locked axial chirality. Angew. Chem. Int. Ed. 61, e202202977 (2022).
Zhang, G., Palmer, G. M., Dewhirst, M. W. & Fraser, C. L. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat. Mater. 8, 747–751 (2009).
Liu, X. et al. An electroactive pure organic room-temperature phosphorescence polymer based on a donor-oxygen-acceptor geometry. Angew. Chem. Int. Ed. 60, 2455–2463 (2021).
Ma, X., Xu, C., Wang, J. & Tian, H. Amorphous pure organic polymers for heavy-atom-free efficient room-temperature phosphorescence emission. Angew. Chem. Int. Ed. 57, 10854–10858 (2018).
Wu, B. et al. Ultralong and high‐efficiency room temperature phosphorescence of organic‐phosphors‐doped polymer films enhanced by 3D network. Adv. Opt. Mater. 8, 2001192 (2020).
Tao, Y. et al. Resonance-activated spin-flipping for efficient organic ultralong room-temperature phosphorescence. Adv. Mater. 30, 1803856 (2018).
Tao, Y. et al. Resonance-induced stimuli-responsive capacity modulation of organic ultralong room temperature phosphorescence. J. Am. Chem. Soc. 144, 6946–6953 (2022).
Zhang, Z. et al. Highly efficient and stable deep-blue room temperature phosphorescence via through-space conjugation. Chem. Eng. J. 442, 136179 (2022).
Han, X. et al. Dual guests synergistically tune the phosphorescence properties of doped systems through chemical interactions with bases. ACS Mater. Lett. 4, 1764–1773 (2022).
Zhang, X. et al. Highly efficient and persistent room temperature phosphorescence from cluster exciton enables ultrasensitive off-on VOC sensing. Matter 5, 3499 (2022).
Singh, M. et al. Recent advances of cocrystals with room temperature phosphorescence. Adv. Opt. Mater. 9, 2002197 (2021).
Xu, X. et al. Guest-activated quaternary ammonium salt hosts emit room temperature phosphorescence. Chem. Commun. 58, 11143–11146 (2022).
Zhang, X. et al. Ultralong UV/mechano-excited room temperature phosphorescence from purely organic cluster excitons. Nat. Commun. 10, 5161 (2019).
Guo, S. et al. Recent progress in pure organic room temperature phosphorescence of small molecular host–guest systems. ACS Mater. Lett. 3, 379–397 (2021).
Yang, J., Fang, M. & Li, Z. Stimulus-responsive room temperature phosphorescence materials: internal mechanism, design strategy, and potential application. Acc. Mater. Res. 2, 644–654 (2021).
Guo, J., Yang, C. & Zhao, Y. Long-lived organic room-temperature phosphorescence from amorphous polymer systems. Acc. Chem. Res. 55, 1160–1170 (2022).
Zhang, H. & Tang, B. Z. Through-space interactions in clusteroluminescence. JACS Au. 1, 1805–1814 (2021).
Wang, Z. et al. Recent progress in organic color-tunable phosphorescent materials. J. Mater. Sci. Tech. 101, 264–284 (2022).
Chen, X., Wang, Y., Zhang, Y. & Yuan, W. Clustering-triggered emission of nonconventional luminophores. Prog. Chem. 31, 1560–1575 (2019).
Shen, Y., An, Z., Liu, H., Yang, B. & Zhang, Y. Excitation-dependent multicolour luminescence of organic materials: internal mechanism and potential applications. Angew. Chem. Int. Ed. 62, e202214483 (2023).
Zheng, X. et al. Excitation- & concentration-dependent color-tunable ultra-long phosphorescence of isophthalonitriles doped polymers. Chem. Eng. J. 469, 143929 (2023).
Wang, M. et al. Excitation-dependent organic phosphors exhibiting different luminescence colors for information anti-counterfeiting. Chem. Eng. J. 429, 132288 (2022).
She, P. et al. Single‐component molecular dual persistent room temperature phosphorescence from low‐ and high‐lying triplet states. Adv. Opt. Mater. 10, 2102706 (2022).
Previtali, A. et al. Tunable linear and nonlinear optical properties from room temperature phosphorescent cyclic triimidazole-pyrene bio-probe. Chem. Eur. J. 27, 16690–16700 (2021).
Lucenti, E. et al. Unravelling the intricate photophysical behavior of 3-(pyridin-2-yl)triimidazotriazine AIE and RTP polymorphs. Chem. Sci. 11, 7599–7608 (2020).
Ma, X. et al. A color-tunable single molecule white light emitter with high luminescence efficiency and ultra-long room temperature phosphorescence. J. Mater. Chem. C. 9, 727–735 (2021).
Khisamov, R. M., Ryadun, A. A., Sukhikh, T. S. & Konchenko, S. N. Excitation wavelength-dependent room-temperature phosphorescence: unusual properties of novel phosphinoamines. Mol. Syst. Des. Eng. 6, 1056–1065 (2021).
Yang, H. et al. Multicolour fluorescence based on excitation-dependent electron transfer processes in o-carborane dyads. Angew. Chem. Int. Ed. 61, e202115551 (2022).
Wang, D. et al. Excitation-dependent triplet-singlet intensity from organic host-guest materials: tunable color, white-light emission, and room-temperature phosphorescence. J. Phys. Chem. Lett. 12, 1814–1821 (2021).
Zhu, Y. et al. Dual-mode white light emissions from a single copolymer with an ultralong phosphorescence lifetime. ACS Appl. Polym. Mater. 4, 5638–5647 (2022).
Kuila, S., Garain, S., Bandi, S. & George, S. J. All‐organic, temporally pure white afterglow in amorphous films using complementary blue and greenish‐yellow ultralong room temperature phosphors. Adv. Funct. Mater. 30, 2003693 (2020).
Liu, R., Jiang, T., Liu, D. & Ma, X. A facile and green strategy to obtain organic room-temperature phosphorescence from natural lignin. Sci. China Chem. 65, 1100–1104 (2022).
Xie, Z. et al. Wide-range lifetime-tunable and responsive ultralong organic phosphorescent multi-host/guest system. Nat. Commun. 12, 3522 (2021).
Li, D., Yang, J., Fang, M., Tang, B. Z. & Li, Z. Stimulus-responsive room temperature phosphorescence materials with full-color tunability from pure organic amorphous polymers. Sci. Adv. 8, eabl8392 (2022).
Wang, Y. L. et al. Hydrogen bonding-induced oxygen clusters and long-lived room temperature phosphorescence from amorphous polyols. Chin. Chem. Lett. 34, 107684 (2023).
Xu, L. et al. Maltose: a natural disaccharide with aggregation-induced emission and room temperature phosphorescence and its multiple applications. Dyes Pigments 201, 110259 (2022).
Xiao, G., Ma, Y. J., Fang, X. & Yan, D. Quadruple anticounterfeiting encryption: anion-modulated forward and reverse excitation-dependent multicolor afterglow in two-component ionic crystals. ACS Appl. Mater. Interfaces 14, 30246–30255 (2022).
Dou, X. et al. Color-Tunable, Excitation-Dependent, and Time-Dependent Afterglows from Pure Organic Amorphous Polymers. Adv. Mater. 32, 2004768 (2020).
Wang, Z. et al. Color-Tunable Polymeric Long-Persistent Luminescence Based on Polyphosphazenes. Adv. Mater. 32, 1907355 (2020).
Su, Y. et al. Excitation-Dependent Long-Life Luminescent Polymeric Systems under Ambient Conditions. Angew. Chem. Int. Ed. 59, 9967–9971 (2020).
Wang, Z. et al. Four-in-one stimulus-responsive long-lived luminescent systems based on pyrene-doped amorphous polymers. Angew. Chem. Int. Ed. 61, e202203254 (2022).
Wang, Y. et al. Nonconventional luminophores with unprecedented efficiencies and color-tunable afterglows. Mater. Horiz. 7, 2105–2112 (2020).
Zhou, Q. et al. Tunable photoluminescence properties of cotton fiber with gradually changing crystallinity. Front. Chem. 10, 805252 (2022).
Bian, L. et al. Color-tunable ultralong organic phosphorescence materials for visual UV-light detection. Sci. China Chem. 63, 1443–1448 (2020).
Kausar, F., Yang, T., Zhao, Z., Zhang, Y. & Yuan, W. Z. Clustering-triggered emission of nonaromatic polymers with multitype heteroatoms and effective hydrogen bonding. Chem. Res. Chin. U. 37, 177–182 (2021).
Li, H. et al. Single-component color-tunable circularly polarized organic afterglow through chiral clusterization. Nat. Commun. 13, 429 (2022).
Kasha, M. & MCGLYNN, S. P. Molecular electronic spectroscopy. Annu. Rev. Phys. Chem. 7, 403–424 (1956).
Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Farady Soc. 9, 14–19 (1950).
Demchenko, A. P., Tomin, V. I. & Chou, P. T. Breaking the kasha rule for more efficient photochemistry. Chem. Rev. 117, 13353–13381 (2017).
Zhang, Q.-S. et al. High-temperature and dynamic RGB (Red-Green-Blue) long-persistent luminescence in an anti-kasha organic compound. Angew. Chem. Int. Ed. 61, e202205556 (2022).
Gong, Y. et al. Multiwavelength anti-kasha’s rule emission on self-assembly of azulene-functionalized persulfurated arene. J. Phys. Chem. C. 123, 22511–22518 (2019).
Veys, K. & Escudero, D. Anti-kasha fluorescence in molecular entities: central role of electron-vibrational coupling. Acc. Chem. Res. 55, 2698–2707 (2022).
Zhou, C. et al. Ternary emission of fluorescence and dual phosphorescence at room temperature: a single-molecule white light emitter based on pure organic aza-aromatic material. Adv. Funct. Mater. 28, 1802407 (2018).
He, Z. et al. White light emission from a single organic molecule with dual phosphorescence at room temperature. Nat. Commun. 8, 416 (2017).
Acknowledgements
This work was supported by the National Nature Science Foundation of China (21905177 and 21975061) (G.X. and Z.H.) and the Natural Science Foundation of Guangdong Province (2019KZDXM008 and 2021A1515010192) (B.S.L.) and Shenzhen Fundamental Research Program (JCYJ20210324094607021 and JCYJ20190806142403535) (B.S.L. and Z.H.).
Author information
Authors and Affiliations
Contributions
Conceptualization: B.S.L., G.H., and Z.H. Methodology: G.H., Z.H., and B.S.L. Investigation: W.H. and W.X. Visualization: W.X., W.H., and J.L. Funding acquisition: G.H., B.S.L., and Z.H. Project administration: G.H. and Z.H. Supervision: B.S.L. and B.Z.T. Writing (original draft): W.H. and W.X. Writing (review and editing): G.H., B.S.L., Z.H., and B.Z.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, W., Huang, W., Li, J. et al. Anti-Kasha triplet energy transfer and excitation wavelength dependent persistent luminescence from host-guest doping systems. Nat Commun 14, 8098 (2023). https://doi.org/10.1038/s41467-023-43687-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43687-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.