Abstract
One-pot synthesis of heterocyclic aromatics with good optical properties from phenolic β-O-4 lignin segments is of high importance to meet high value added biorefinery demands. However, executing this process remains a huge challenge due to the incompatible reaction conditions of the depolymerization of lignin β-O-4 segments containing γ-OH functionalities and bioresource-based aggregation-induced emission luminogens (BioAIEgens) formation with the desired properties. In this work, benzannulation reactions starting from lignin β-O-4 moieties with 3-alkenylated indoles catalyzed by vanadium-based complexes have been successfully developed, affording a wide range of functionalized carbazoles with up to 92% yield. Experiments and density functional theory calculations suggest that the reaction pathway involves the selective cleavage of double C-O bonds/Diels-Alder cycloaddition/dehydrogenative aromatization. Photophysical investigations show that these carbazole products represent a class of BioAIEgens with twisted intramolecular charge transfer. Distinctions of emission behavior were revealed based on unique acceptor-donor-acceptor-type molecular conformations as well as molecular packings. This work features lignin β-O-4 motifs with γ-OH functionalities as renewable substrates, without the need to apply external oxidant/reductant systems. Here, we show a concise and sustainable route to functional carbazoles with AIE properties, building a bridge between lignin and BioAIE materials.
Similar content being viewed by others
Introduction
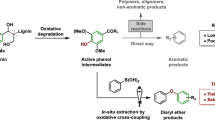
Both the damaging environmental impact and the increasing depletion of fossil resources have triggered strong interest in utilizing biomass for helping to satisfy the growing energy demand in a sustainable form as well as a source for basic chemicals1,2. As a high volume and fairly cheap renewable resource, lignin is a unique precursor for the production of aromatic chemicals3,4. With respect to structural moieties, β-O-4 (β-aryl ether) associated with γ-OH is the most abundant linkage in lignin, making up approximately 50–65% of all linkages, dependent on the lignin type (Fig. 1a)5. Effective depolymerization of this particular linkage could not only remove a considerable barrier in the degradation of lignin feedstocks, but also provide useful depolymerized molecules as scaffolds for potential applications. Thus, extensive efforts have been dedicated to deconstruct the β-O-4 motif to deliver C, H, O-containing products with additional hydrogen and oxygen donating agents. With the aim to functionalize lignin downstream products further, introduction of nitrogen to form nitrogen-containing aromatics is also emerging and increasingly brought into focus6,7,8. So far, most of the current strategies are limited to the employment of lignin-derived monophenols or modified β-O-4 dimers as starting materials to produce N-containing aromatics in the presence of a N-source through one or multiple steps9,10,11,12,13,14,15,16,17,18,19 (Fig. 1b, Routes 1–2). Additionally, hydrogenation and oxidation processes are unavoidable. As a challenge, the direct transformation of phenolic β-O-4 linkages to N-containing aromatics without external H or O source is still prominent. Building on recent progress, our group and that of Jiao independently developed methods for the preparation of various N-containing aromatics (benzylamines, pyrimidines, quinolines, quinoxalines and anilines) with organic amines or NaN3 as N-sources through controlled cleavage of the C-O bond in β-O-4 motifs and the construction of C-C/C-N bonds in a one-pot fashion (Fig. 1c, Route 3)20,21,22,23,24. However, these systems exhibit low efficiency and poor selectivity when meeting β-O-4 moieties containing γ-OH groups, leading to low yields of N-containing aromatics (<40%). The higher bond dissociation energies of β-O-4 linkage with γ-OH (69.2 kcal/mol)25 compared with β-O-4 without γ-OH (65.5 kcal/mol)26 leads to a variety of unwanted by-products, which is the main reason of lower yield. Moreover, the target molecule application is so far largely confined to the synthesis of medicinal intermediates, large volume application is prohibited by the need of further functionalization. Therefore, the development of a simple and efficient strategy for the production of N-containing aromatics would lead to new applications of lignin-based chemicals.
Bio-resource derived aggregation-induced emission luminogens (BioAIEgens, AIEgens or AIE-active nano agents obtained from natural resources including natural products or derivatives by modifying natural products)27,28,29,30 have raised considerable attention owing to their advantageous renewability, biodegradability, and biocompatibility, paving the way to potential applications in biomedical31,32,33, chemosensors/biosensors34,35, and optoelectronic devices36,37. As a natural biomass rich in aromatic and carbonyl subunits, lignin based building blocks display strong fluorescence and excellent self-assembly properties, which could make them serve as ideal precursors for the preparation of BioAIEgens33,38,39,40. However, its complex structure makes it difficult to controllably degrade, thereby limiting the application of lignin. Current studies are usually limited to macromolecular BioAIEgens directly coming from lignin itself or structurally modified lignin based macromolecules, resulting in the fact that the luminescence mechanism and structure-property relationships are still unclear due to the complicated and uncontrollable macromolecular structures. Therefore, the efficient and controllable conversion of lignin platform chemicals into value-added fine chemicals has become a hot research topic for utilization of lignin. Therefore, obtaining BioAIEgens with well-defined structures through lignin depolymerization would provide an alternative strategy for the design of lignin-derived BioAIEgens. Conventional state of the art design of AIEgens is based on restriction of intramolecular motion41. Moreover, the construction of electronic donor-acceptor (D-A) configuration is regarded as an effective approach to manipulate the properties of luminescent materials42,43. One type of D-A structured molecules, namely the D-A-D type, has attracted considerable interest44,45,46. In contrast, reports on A-D-A molecules as luminescent materials are relatively limited. In recent years, some reports suggest that A-D-A compounds can exhibit stronger charge transfer effects and light-harvesting capability compared to D-A-D structure, hence holding great potential in organic photovoltaics and biomedical applications47,48,49,50. BioAIEgen associated with A-D-A configuration may synchronously regulate the photophysical properties. Hence, developing a concise and sustainable route to construct BioAIEgens with A-D-A configuration would be a great achievement and highly desirable as potential phototheranostic agents.
Carbazole is an important N-containing heterocyclic organic compound with low ionization potential and a strong electron donor ability51, these features make them as an ideal electron donor to construct D-A structures52,53,54,55. Employing carbazole as a core moiety, introducing two electron acceptor groups (i.e., benzoyl) into a carbazole scaffold might provide an efficient way for the preparation of A-D-A-type AIEgens. Moreover, extensive synthetic strategies of the functionalized carbazoles focus on intermolecular cross-coupling reactions between C-H/C-X bonds (X = halo, N, O, C, etc)56, oxidative intramolecular C-H/C-H cross coupling of prefunctionalized diarylamines57, the construction of a benzene ring upon substituted indoles through transition metal catalysis58 or Brønsted acid catalysis59,60,61. However, most approaches suffer from the need of excess oxidants, multi-step reactions and the formation of non-renewable substrates, which raises atom economy and environmental concerns. Thus, a sustainable protocol for producing carbazoles synthesis is desirable.
In this work, we developed a sustainable route for the production of functionalized carbazoles through transformation of lignin β-O-4 segments. γ-OH groups of β-O-4 could be well tolerated. Furthermore, the establishment of the triangular A-D-A skeleton, based on the electron donor ability of carbazole and the corresponding electron acceptor behavior of benzoyl resulting from this unique synthetic methodology has successfully led to the desired twisted intramolecular charge transfer (TICT) AIE properties (Fig. 1d, Route 4).
Results and discussion
Reaction optimization
Initially, β-O-4 linkages containing a γ-OH group (1a), highly abundant in natural lignin, are selected as a probe reaction with alkenylated indole 2a catalyzed by vanadium associated with tridentate Schiff base ligands (V-based complexs)62. The results show that carbazole derivative 3a as a target product forms in 89% yield along with guaiacol (4a) in 99% yield (Supplementary Table 1, entry 1), demonstrating that carbazole formation occurs, associated with a selective C-O bond cleavage of the lignin model compound, C-C bond construction and dehydrogenation/aromatization in a one-pot fashion. Based on this exciting reactivity, the experiment parameters (catalyst loading, substrates ratio, and reaction temperature) are optimized (Supplementary Tables 1–3). Screening various solvents reveal that the reaction proceeds most efficiently in toluene, compared to N, N-dimethylformamide (DMF), CH3OH, CH3CN, tetrahydrofuran and H2O (Supplementary Table 1, entries 1–6). Additionally, the reaction is very sensitive to reaction temperature. Upon lowering the temperature, yield of 3a decreases significantly from 89% at 140 °C to 76% at 120 °C (Supplementary Table 1, entries 1, 7–8). In contrast, changing the catalyst to commercial VO(OEt)3 and 2,2’-bipyridine ligand, the carbazole 3a is formed only in 55% yield (Supplementary Table 1, entry 9). Notable, a blank experiment shows that without catalyst no product is formed (Supplementary Table 1, entry 10), illustrating the V-based catalyst indeed plays a crucial role.
Substrate scope
A broad spectrum of substrates with varying electronic and steric effects, and various functional groups on the aromatic ring of the lignin model compound 1 and alkenylated indoles 2 in the presence of V-based catalyst is explored, affording good to excellent yields of the corresponding carbazole derivatives 3. As shown in Fig. 2, lignin β-O-4 model substrates 1a and 1b bearing methoxy substituents on the aryl moieties associated with 2a as a reaction partner to generate the target carbazole products 3a and 3b lead to 91% and 86% isolated yield, along with 99% and 85% yield of guaiacol 4a, respectively (Fig. 2, entries 1–2). To our delight, the reaction also tolerates a hydroxyl group on the aryl ring of 1c, yielding 71% of 3c (Fig. 2, entry 3). Compound 1d bearing no functional group leads to a somewhat reduced yield of carbazole 3d (61%) and of phenol 4b (76%) (Fig. 2, entry 4), illustrating that methoxyl groups on the aryl ring of 1 execute a positive influence on the reaction efficiency. It is noteworthy that N-induced deconstruction of lignin β-O-4 linkages containing γ-OH group generally produces lower yields (<40%) of N-containing aromatics due to the complicated structure and higher steric hindrance20,21,22,23,24. With the catalytic system applied here, β-O-4 motifs with γ-OH 1a–1d are efficiently transformed to carbazole derivatives 3a–3d in high isolated yields (61–91%). In contrast, the reaction of a N-H free indole derivative ((E)-3-(1H-indol-3-yl)-1-phenylprop-2-en-1-one) with 1d is carried out, leading to ((E)-3-(1-(3-oxo-3-phenylpropyl)-1H-indol-3-yl)-1-phenylprop-2- en-1-one as main product in 21% yield due to some side reactions (Supplementary Fig. 1). In order to obtain high selectivity of carbazole products, N-protected indole derivatives (2a–2s) are used as substrates. For example, a benzyl substituent on the N-R moiety in 3-alkenylated is tolerated, leading to 86% yield of 3e (Fig. 2, entry 5). Additionally, electron-donating substituents such as –Me and -OCH3 on the aryl rings of the indole moieties apparently do not affect the reaction efficiency, since 3f–3h are obtained in 80–92% yield (Fig. 2, entries 6–8), whereas an electron-withdrawing Cl- group decreases the yield of product 3i to 75% (Fig. 2, entry 9). To further investigate the scope of this reaction, various substituents on the phenyl moiety of the alkenylated indoles 2 were applied. The substrates bearing-Me groups in meta- or para- position lead to 3k and 3l (83% and 84%) in higher yields compared with a Me group in ortho-position (3j, 53%) due to the steric hindrance in the latter case (Fig. 2, entries 10–12). No obvious electronic effect was detected for the substituted group on the aryl moiety of 2 in this transformation. P-Me, p-OCH3, p-F, p-Cl, p-CN and aryl substituted alkenylated indoles 2 proceed to deliver the expected products 3l–3r in 63–85% yield (Fig. 2, entries 11–18). Moreover, a tribenzene-alkene bearing substrate also react smoothly, giving the target product 3s in moderate yield (65%) (Fig. 2, entry 19). Notably, heterocyclic functionalities such as thiophene and furan groups also allow the formation of carbazole derivatives 3t and 3u in 95% and 87% yield (Fig. 2, entries 20–21). Beyond that, this process is also effective for a substrate containing an alkyl ring, obtaining target product 3v in 68% yield (Fig. 2, entry 22). Significantly, the phenol products 4a–4b, being valuable precursors of detergents, pesticides, antimicrobials and pharmaceutical drugs63, can be obtained in yields of 76–99%. All these results demonstrate that the system described in this work displays excellent tolerance to a broad substrate scope, not only providing access to carbazole derivatives in high isolated yields, but also achieving phenol derivatives in excellent yields, greatly increasing atom-economy.
Reaction conditions: 1 (0.4 mmol), 2 (0.2 mmol), V-complex catalyst (10 mol%), toluene (4 mL) in air, 140 °C, reaction time (t) = 20 h, yields of 3 and 4 are calculated based on the amounts of 2 and 1, respectively. aIsolated molar yield. bYields are determined by HPLC with an external standard method.
Mechanistic studies
With the aim to elucidate the mechanism of this cascade reaction, several control experiments are carried out. The initial and crucial step is the selective cleavage of double C-O bonds of the lignin β-O-4 with γ-OH motif with the assistance of the V- catalyst, determining the downstream reaction. Thus treatment of 1d alone under standard conditions yields 51% of enone (5a) and 60% of 4b (Equation 1, Fig. 3). Then the reaction of 5a with 2a affords the desired product 3d in 45% yield (Equation 2, Fig. 3), indicating that 5a is a key intermediate. Compared to 61% yield of 3d in Entry 4 Fig. 2 using 1d as a starting material, this lower yield is due to 5a being very unstable and an easily occurring side reactions at the rather high temperature of 140 °C64, in situ generated enone 5a from the lignin β-O-4 segment is more efficiently converted to carbazole. Aiming to verify the dehydrogenation ability of the V-catalyst, the same reaction as given in Equation 2 is performed in the absence of catalyst (Equation 3, Fig. 3). However, only 5% yield of 3d are obtained (Equation 3, Fig. 3). Similarly, the reactions of 5a with 2a with and without V-catalyst are also conducted under argon atmosphere (Equations 4 and 5, Fig. 3), showing that the target product 3d is formed in 37% yield with V-catalyst, compared with 3% yield of 3d in the absence of V-catalyst. These results show that the V-catalyst plays an important role for the subsequent intramolecular dehydrogenation. On the basis of these results, a plausible reaction pathway is proposed in Fig. 4: lignin β-O-4 model compounds initially undergo selective V-catalyzed cleavage of the C-O bond to release enones 5 and phenol co-products 4. Then 5 reacts with 2 via a Diels-Alder cycloaddition to form tetrahydrocarbazoles backbone 6, which subsequently generates the final product 3 through dehydro-aromatization. In the whole reaction the V-complex acts as a bifunctional catalyst for both the selective cleavage of the C-O bond of the lignin β-O-4 model compounds and for the dehydrogenation process.
To gain deeper insight into the detailed reaction mechanism of the vanadium-complex catalyzed selective C-O bond cleavage, density functional theory (DFT) calculations are performed. The calculated energy profile of the vanadium-catalyzed selective C-O bond cleavage of 1a is given in Fig. 5 and Supplementary Table 4 as well as Supplementary Data 1.
The reaction is proposed to be initiated by a ligand exchange between the V-catalyst CatCSS and the benzylic hydroxyl group of 1a to give intermediate IM1CSS and MeOH, which is agreement with previous work62. This process is found to occur via a four-membered transition state TS1CSS, with an energy barrier of 23.8 kcal/mol. Then, intermediate IM1CSS undergoes an intramolecular hydrogen transfer via transition state TS2OSS with an energy barrier of 23.9 kcal/mol relative to IM1CSS, leading to intermediate IM2OSS. The calculations show that the ensuing C(sp3)-O bond cleavage directly from intermediate IM2OSS takes place via transition state TS3OSS, which is 9.5 kcal/mol higher in energy than IM2OSS. Alternatively, it is found that a spin-crossover phenomenon from a singlet spin state to a triplet spin state can occur to yield the very stable ketone-coordinated V(III) IM2triplet, from which the C(sp3)-O bond cleavage via transition state TS3triplet is slightly more favored with an energy barrier of 21.3 kcal/mol relative to IM2triplet. Finally, the reaction is completed by a hydroxyl group elimination to deliver enone product 5a’. The results show that hydroxyl group elimination via a triplet spin state transition state TS4triplet is much higher in energy than that via singlet spin state transition state TS4CSS (11.4 versus 6.3 kcal/mol), showing that the spin-crossover phenomena from triplet spin state (IM3triplet) to singlet spin state (IM3CSS) has to occur prior to the hydroxyl group elimination. Taken all results together, the hydroxyl group elimination is found to be the rate-determining step of the overall catalytic cycle, with an energy barrier of 25.1 kcal/mol.
Investigation of optical properties
Additional to establishing a sustainable synthesis of carbazole derivatives their optical properties have been studied as well. It is interesting to note that the carbazole backbones of 3a–3v do possess a chemical structure of two benzoyl moieties as electronic acceptors on the meta positions of the electronic donor of carbazole, constituting a triangular A1-D-A2 configuration (Fig. 6a). The substrates involved herein have four changeable substituents on the A1-D-A2 skeleton, endowing most of them with AIE properties and distinct emission behavior (Supplementary Figs. 36–57). Represented by the non-substituted A-D-A skeleton of 3d (Fig. 6b, c), the PL intensity decreases immediately when water is added, accompanied by a red-shifted emission that is also observed in a solvent effect experiment with increased solvent polarity (Supplementary Fig. 58), indicating the existence of TICT property65,66. When fw increases up to 80%, the fluorescence revives, which is a typical feature of an AIE property. This might be because the restriction of intramolecular motion in the nanoaggregates leads to an intensified emission (Supplementary Fig. 59). For 3h and 3q, the photophysical properties in the aqueous mixtures show a similar trend as that of 3d, implying that they have TICT-AIE properties as well (Fig. 6d, Supplementary Figs. 43 and 52). In order to shed more light on these observations, DFT calculations were carried out (Fig. 6e), showing CT effects with an electron shift to the LUMO, which is located either on A1 or on A2, dependent on the substituents. 3d displays electrons localized on carbazole in the HOMO and an A1 localized LUMO, respectively. Location of the LUMO on A1 may be due to its unsymmetrical A1-D-A2 motif. For 3h, the substitution of two electron-donating methoxy groups on both D and A1 weakens the electron-acceptor ability of A1, with the LUMO not only localized on A1 but also slightly extending to A2. With regard to 3q, the LUMO mainly concentrates on A2, owing to the strong electron withdrawing ability of the –CN group. The variable substitutions not only cause varied electronic distributions and locations of HOMO and LUMO, but also lead to different energy differences (band gaps) between the frontier orbitals as exemplified for 3d (3.88 eV), 3h (3.66 eV), and 3q (3.65 eV), matching well with their luminescence colors. These results imply that the electronic distributions can be adjusted by altering the substituents on the A1-D-A2 skeleton, which can help to regulate the photophysical properties of the respective lignin-derived BioAIEgens.
a Molecular design of lignin-derived BioAIEgens; b PL spectra of 3d in acetonitrile/water (ACN/H2O) mixtures with different water fractions (fw), λex: 300 nm, concentration: 20 μM; c the plots of the αAIE and maximum emission wavelength versus the composition of the aqueous mixture of 3d, αAIE = I/I0, I0 = PL intensity in pure ACN, inset: fluorescence photographs taken under 365 nm UV irradiation; d plots of αAIE versus the composition of the aqueous mixture of 3h (I0 = PL intensity in pure ACN, λex: 311 nm) and 3q (I0 = PL intensity in pure THF, λex: 301 nm), inset: fluorescence photographs taken under 365 nm UV irradiation, concentration: 20 μM; e frontier molecular orbitals and their corresponding HOMO/LUMO energies of 3d, 3h, and 3q.
In addition to the fluorescence behavior in solution and aggregate states, the photophysical properties in the solid state have been further studied. With the aim of investigating the structure-property relationships of solid-state fluorescence materials, single crystals of 3p and 3q have been obtained for further analysis. Similar to the solution and aggregate states, the unsubstituted solid state of 3d still exhibits blue-violet fluorescence with a wavelength of 436 nm. 3p and 3q show a significant red-shift (3p: 493 nm; 3q: 511 nm) compared to 3d, probably due to the presence of electronically different substituents67,68, thus enhancing the characteristics of CT effects. With regard to the emission intensity, the solid-state quantum yield values of 3d, 3p, and 3q are 3.5%, 13.7% and 2.8%, respectively, and these trends are identical to the emission intensity reported in the solid-state emission spectra (Supplementary Fig. 60). In order to uncover the causes for the differences in emission intensity, the crystal structures of 3p and 3q have been analyzed in detail (Fig. 7 and Supplementary Table 5). The results show that the torsion angles at four comparable positions of 3p (43.16°, 24.53°, –164.91°, –42.00°) and 3q (37.18°, 27.62°, –163.20°, –41.69°) are quite close to each other, indicating similar molecular conformations (Fig. 7a). In addition, the packing modes of 3p and 3q are similar as well, giving two types of dimer modes (dimers I and II) (Fig. 7b). Further analysis demonstrates that they display slight differences in the fundamental structures of the two types of dimers (Fig. 7c). Specifically, in dimer I, the interplanar distances between the carbazole planes of 3p (3.386 Å) and 3q (3.413 Å) are quite similar. However, in dimer II, the four benzene rings between the two molecules constituting the dimer II in 3p exhibit intermolecular C‧‧‧H distances from 3.160 to 3.471 Å. The distances between the four benzene rings in 3q are quite long (5.283–7.577 Å), exceeding the normal intermolecular interactions, giving rise to the weaker solid-state emission of 3q69,70. The above described results suggest that in the case of very similar conformations subtle differences between dimers in the packing structures can nevertheless play a prominent role for the observed emission intensities.
In summary, a vanadium-catalyzed direct transformation of phenolic β-O-4 lignin segments, containing γ-OH with 3-alkenylated indoles has been developed for the construction of carbazole-based bioAIEgens without an external H/O resource. A selective tandem cleavage of two C-O bonds /Diels–Alder cycloaddition/dehydrogenative aromatization sequence is established as the reaction pathway, both through experimental studies and DFT calculations. As intended, the lignin-derived carbazoles with A-D-A configurations exhibit TICT-AIE performance, endowing the lignin-depolymerized derivatives with unique photophysical properties. The present protocol provides a concise and sustainable route to functionalized carbazoles with AIE properties from renewable substrates, creating a bridge between lignin and BioAIEgens.
Methods
Typical procedure for carbazole derivatives synthesis from lignin β-O-4 model compounds
Quantification of phenol derivatives: lignin model compound 1 (0.4 mmol), alkenylated indole 2 (0.2 mmol), catalyst (10 mol%) and toluene (4 mL) are added into a pressure tube (35 mL). The mixture was sealed and heated to 140 °C for 20 h. After reaction, the solution is cooled to room temperature, and diluted to 25 mL volumetric flask with DMF. The phenol derivatives are analyzed by HPLC using an external standard calibration curve method. Quantification of carbazole derivatives: lignin model compound 1 (0.4 mmol), alkenylated indole 2 (0.2 mmol), catalyst (10 mol%) and toluene (4 mL) are added into a pressure tube (35 mL). The mixture is sealed and heated to 140 °C for 20 h. After reaction, the solution is cooled to room temperature. The carbazole products are purified by column chromatography using petroleum ether/ethyl acetate (3:1) to give the isolated yield.
Computational details
All the calculations are performed at the (u)B3LYP-D3(BJ)71,72,73 level of theory using Gaussian 09 package74. The geometry optimizations are carried out with a mixed basis set of LANL2TZ(f) for V and 6–31 G(d,p) for other atoms. Frequencies are computed analytically at the same level of theory to confirm whether the structures are minima (no imaginary frequencies) or transition states (only one imaginary frequency). Selected transition-state structures are confirmed to connect the correct reactants and products by intrinsic reaction coordinate calculations75,76. To obtain better accuracy, energies for the optimized geometries were recalculated using the solution-phase single-point calculations with a larger basis set, which is LANL2TZ(f) for V and 6-311 + G(d,p) for all other atoms. Solvation effects (solvent = toluene, ε = 2.374) are taken into account by performing single-point calculations with the SMD model77. The final free energies reported in the article are the large basis set single-point energies corrected by gas-phase Gibbs free energy correction (at 298.15 K).
Data availability
All relevant data are available within the article as well as its Supplementary Information and from the corresponding authors on request. Crystallographic data for structures 3d, 3p and 3q reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2212869, 2212868 and 2212870, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc. cam.ac.uk/structures/.
References
Zhang, Z., Song, J. & Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 117, 6834–6880 (2017).
Sun, Z., Fridrich, B., de Santi, A., Elangovan, S. & Barta, K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 118, 614–678 (2018).
Zakzeski, J., Bruijnincx, P. C. A., Jongerius, A. L. & Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010).
Wong, S. S., Shu, R., Zhang, J., Liu, H. & Yan, N. Downstream processing of lignin derived feedstock into end products. Chem. Soc. Rev. 49, 5510–5560 (2020).
Li, C., Zhao, X., Wang, A., Huber, G. W. & Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 115, 11559–11624 (2015).
Espro, C. et al. Sustainable production of pharmaceutical, nutraceutical and bioactive compounds from biomass and waste. Chem. Soc. Rev. 50, 11191–11207 (2021).
Li, H., Bunrit, A., Li, N. & Wang, F. Heteroatom-participated lignin cleavage to functionalized aromatics. Chem. Soc. Rev. 49, 3748–3763 (2020).
Pelckmans, M., Renders, T., Van de Vyver, S. & Sels, B. F. Bio-based amines through sustainable heterogeneous catalysis. Green. Chem. 19, 5303–5331 (2017).
Chen, Z. et al. Formal direct cross-coupling of phenols with amines. Angew. Chem. Int. Ed. 54, 14487–14491 (2015).
Qiu, Z., Lv, L., Li, J., Li, C.-C. & Li, C.-J. Direct conversion of phenols into primary anilines with hydrazine catalyzed by palladium. Chem. Sci. 10, 4775–4781 (2019).
Chen, Z., Zeng, H., Gong, H., Wang, H. & Li, C.-J. Palladium-catalyzed reductive coupling of phenols with anilines and amines: efficient conversion of phenolic lignin model monomers and analogues to cyclohexylamines. Chem. Sci. 6, 4174–4178 (2015).
Elangovan, S. et al. From wood to tetrahydro-2-benzazepines in three waste-free steps: modular synthesis of biologically active lignin-derived scaffolds. ACS Cent. Sci. 5, 1707–1716 (2019).
Zhang, J., Liu, Y., Chiba, S. & Loh, T.-P. Chemical conversion of β-O-4 lignin linkage models through Cu-catalyzed aerobic amide bond formation. Chem. Commun. 49, 11439–11441 (2013).
Blondiaux, E. et al. Bio-based aromatic amines from lignin-derived monomers. ACS Sustainable Chem. Eng. 7, 6906–6916 (2019).
Li, H. et al. NH2OH–mediated lignin conversion to isoxazole and nitrile. ACS Sustainable Chem. Eng. 6, 3748–3753 (2018).
Liu, X. et al. Copper-catalyzed synthesis of benzanilides from lignin model substrates 2-phenoxyacetophenones under an air atmosphere. New J. Chem. 42, 1223–1227 (2018).
Guo, T., Liu, T., He, J. & Zhang, Y. One-pot transformation of lignin and lignin model compounds into benzimidazoles. Eur. J. Org. Chem. 2022, e202101152 (2022).
Cuypers, T. et al. Ni-Catalyzed reductive amination of phenols with ammonia or amines into cyclohexylamines. Green. Chem. 22, 1884–1893 (2020).
Zheng, B. et al. Production of alkoxyl-functionalized cyclohexylamines from lignin-derived guaiacols. Green. Chem. 23, 8441–8447 (2021).
Zhang, B. et al. Sustainable production of benzylamines from lignin. Angew. Chem. Int. Ed. 60, 20666–20671 (2021).
Zhang, B. et al. Transition-metal-free synthesis of pyrimidines from lignin β-O-4 segments via a one-pot multi-component reaction. Nat. Commun. 13, 3365 (2022).
Ding, Y. et al. Transition-metal-free synthesis of functionalized quinolines by direct conversion of β-O-4 model compounds. Angew. Chem. Int. Ed. 61, e202206284 (2022).
Liu, J. et al. From alkylarenes to anilines via site-directed carbon-carbon amination. Nat. Chem. 11, 71–77 (2019).
Liu, Y. et al. Successive cleavage and reconstruction of lignin β-O-4 models and polymer to access quinoxalines. ChemSusChem 15, e202201401 (2022).
Rinesch, T. & Bolm, C. Cobalt-catalyzed oxidation of the β-O-4 bond in lignin and lignin model compounds. ACS Omega 3, 8386–8392 (2018).
Zhang, C. et al. Cleavage of the lignin β-O-4 ether bond via a dehydroxylation–hydrogenation strategy over a NiMo sulfide catalyst. Green. Chem. 18, 6545–6555 (2016).
Cai, X.-M. et al. BioAIEgens derived from rosin: how does molecular motion affect their photophysical processes in solid state? Nat. Commun. 12, 1773 (2021).
Huang, S., Zhong, W., Zhang, X., Li, S. & Cai, X.-M. Research progress of photochemical-based aggregation-induced luminescent materials. J. For. Eng. 7, 34–45 (2022).
Zhao, Z., Zhang, H., Lam, J. W. Y. & Tang, B. Z. Aggregation-Induced emission: new vistas at the aggregate level. Angew. Chem. Int. Ed. 59, 9888–9907 (2020).
Li, X. et al. “Irregular” aggregation-induced emission luminogens. Coord. Chem. Rev. 418, 213358 (2020).
Lee, M. M. S. et al. Leveraging bacterial survival mechanism for targeting and photodynamic inactivation of bacterial biofilms with red natural AIEgen. Cell Rep. Phys. Sci. 3, 100803 (2022).
Ling, X. et al. Photoactivatable dihydroalkaloids for cancer cell imaging and chemotherapy with high spatiotemporal resolution. Mater. Horiz. 7, 2696–2701 (2020).
Li, W., Chen, Z., Yu, H., Li, J. & Liu, S. Wood-derived carbon materials and light-emitting materials. Adv. Mater. 33, 2000596 (2021).
Sun, L., Wang, X., Shi, J., Yang, S. & Xu, L. Kaempferol as an AIE-active natural product probe for selective Al3+ detection in Arabidopsis thaliana. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 249, 119303 (2021).
Long, R. et al. Novel natural myricetin with AIE and ESIPT characteristics for selective detection and imaging of superoxide anions in vitro and in vivo. Chem. Commun. 55, 10912–10915 (2019).
Yan, L., Li, R., Shen, W. & Qi, Z. Multiple–color AIE coumarin–based schiff bases and potential application in yellow OLEDs. J. Lumin. 194, 151–155 (2018).
Duan, L. et al. Slight substituent modification in coumarin molecular structures for strong solid emission and application in light-emitting devices. Dyes Pigment 174, 108117 (2020).
Ma, Z. et al. Seeking brightness from nature: J-aggregation-induced emission in cellulolytic enzyme lignin nanoparticles. ACS Sustainable Chem. Eng. 6, 3169–3175 (2018).
Shi, N. et al. Lignosulfonate/diblock copolymer polyion complexes with aggregation-enhanced and pH-switchable fluorescence for information storage and encryption. Int. J. Biol. Macromol. 187, 722–731 (2021).
Xue, Y. et al. Aggregation-induced emission: the origin of lignin fluorescence. Polym. Chem. 7, 3502–3508 (2016).
Mei, J. et al. Aggregation-induced emission: the whole is more brilliant than the parts. Adv. Mater. 26, 5429–5479 (2014).
Liu, C. et al. Functionalization of silk by AIEgens through facile bioconjugation: full-color fluorescence and long-term bioimaging. Angew. Chem. Int. Ed. 60, 12424–12430 (2021).
Chen, Y. et al. D–A type NIR-II organic molecules: strategies for the enhancement fluorescence brightness and applications in NIR-II fluorescence imaging-navigated photothermal therapy. Adv. Healthc. Mater. 11, 2201158 (2022).
Etherington, M. K. et al. Regio- and conformational isomerization critical to design of efficient thermally-activated delayed fluorescence emitters. Nat. Commun. 8, 14987 (2017).
Lei, Z. & Zhang, F. Molecular engineering of NIR-II fluorophores for improved biomedical detection. Angew. Chem. Int. Ed. 60, 16294–16308 (2021).
Wu, T.-L. et al. Diboron compound-based organic light-emitting diodes with high efficiency and reduced efficiency roll-off. Nat. Photonics 12, 235–240 (2018).
Gu, Y. et al. Chlorination-mediated π–π stacking enhances the photodynamic properties of a NIR-II emitting photosensitizer with extended conjugation. Angew. Chem. Int. Ed. 63, e202303476 (2023).
Li, D. et al. Add the finishing touch: molecular engineering of conjugated small molecule for high-performance AIE luminogen in multimodal phototheranostics. Small 17, 2102044 (2021).
Li, X. et al. Biodegradable π-conjugated oligomer nanoparticles with high photothermal conversion efficiency for cancer theranostics. ACS Nano 13, 12901–12911 (2019).
Wan, X., Li, C., Zhang, M. & Chen, Y. Acceptor–donor–acceptor type molecules for high performance organic photovoltaics – chemistry and mechanism. Chem. Soc. Rev. 49, 2828–2842 (2020).
Guo, T. et al. Copper-catalyzed three-component formal [3+1+2] benzannulation for carbazole and indole synthesis. J. Org. Chem. 85, 9117–9128 (2020).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Zhang, D. et al. Efficient and stable deep-blue fluorescent organic light-emitting diodes employing a sensitizer with fast triplet upconversion. Adv. Mater. 32, 1908355 (2020).
Liu, Z. et al. AIE-active luminogens as highly efficient free-radical ROS photogenerator for image-guided photodynamic therapy. Chem. Sci. 13, 3599–3608 (2022).
Zhang, Q. et al. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 134, 14706–14709 (2012).
Roy, J., Jana, A. K. & Mal, D. Recent trends in the synthesis of carbazoles: an update. Tetrahedron 68, 6099–6121 (2012).
Schmidt, A. W., Reddy, K. R. & Knölker, H.-J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 112, 3193–3328 (2012).
Banerjee, A., Kundu, S., Bhattacharyya, A., Sahu, S. & Maji, M. S. Benzannulation strategies for the synthesis of carbazoles, indolocarbazoles, benzocarbazoles, and carbolines. Org. Chem. Front. 8, 2710–2771 (2021).
Banerjee, A., Guin, A., Saha, S., Mondal, A. & Maji, M. S. Formal [4 + 2] benzannulation of 2-alkenyl indoles with aldehydes: a route to structurally diverse carbazoles and bis-carbazoles. Org. Biomol. Chem. 17, 1822–1826 (2019).
Saha, S., Banerjee, A. & Maji, M. S. Brønsted acid catalyzed one-pot benzannulation of 2-alkenylindoles under aerial oxidation: a route to carbazoles and indolo[2,3-a]carbazole alkaloids. Org. Lett. 20, 6920–6924 (2018).
Banerjee, A. & Maji, M. S. A Brønsted acid catalyzed cascade reaction for the conversion of indoles to α-(3-Indolyl) ketones by using 2-benzyloxy aldehydes. Chem. Eur. J. 25, 11521–11527 (2019).
Son, S. & Toste, F. D. Non-oxidative vanadium-catalyzed C-O bond cleavage: application to degradation of lignin model compounds. Angew. Chem. Int. Ed. 49, 3791–3794 (2010).
Pinheiro, P. F. et al. Semisynthetic phenol derivatives obtained from natural phenols: antimicrobial activity and molecular properties. J. Agric. Food Chem. 66, 323–330 (2018).
Shang, Y. et al. Pd-Catalyzed C–H olefination of (Hetero)arenes by using saturated ketones as an olefin source. Angew. Chem. Int. Ed. 52, 1299–1303 (2013).
Yu, Y. et al. How do molecular interactions affect fluorescence behavior of AIEgens in solution and aggregate states? Sci. China Chem. 65, 135–144 (2022).
Yuan, W. Z. et al. Efficient solid emitters with aggregation-induced emission and intramolecular charge transfer characteristics: molecular design, synthesis, photophysical behaviors, and OLED application. Chem. Mater. 24, 1518–1528 (2012).
Pashazadeh, R. et al. An iminodibenzyl–quinoxaline–iminodibenzyl scaffold as a mechanochromic and dual emitter: donor and bridge effects on optical properties. Chem. Commun. 54, 13857–13860 (2018).
Zuo, K. et al. Theoretical insight into the photodeactivation pathway of the tetradentate Pt(II) complex: the π-conjugation effect. Appl. Organomet. Chem. 32, e4220 (2018).
Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. +Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Fang, M. M., Yang, J. & Li, Z. Light emission of organic luminogens: generation, mechanism and application. Prog. Mater. Sci. 125, 100914–100982 (2022).
Lee, C., Yang, W. & Parr, R. G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 37, 785–789 (1988).
Becke, A. D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate Ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Frisch, M. J. et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, 2013.
Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 74, 4161–4163 (1970).
Fukui, K. The path of chemical reactions - The IRC Approach. Acc. Chem. Res. 14, 363–368 (1981).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B. 113, 6378–6396 (2009).
Acknowledgements
Support from the National Natural Science Foundation of China (22078317, 22108272, 21601087, 22073066), Natural Science Foundation of Jiangsu Province (BK20231296), the science and technology bureau of Dalian city (No. 2021RT04) and Technical University of Munich.
Author information
Authors and Affiliations
Contributions
T.Z., B.Z. and X.-M.C. conceived the study and directed the project; T.G. and B.Z. designed and performed the experiments; W.Z. and D.X. performed the control experiments; Y.L., X.Z. and X.-M.C. performed photophysical investigation for AIEgens; D.P. and G.H. performed the DFT calculations. H.W. performed a part of HPLC measurements. B.Z., X.-M.C. and G.H. wrote the manuscript. F.E.K. and T.Z. improved the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, T., Lin, Y., Pan, D. et al. Towards bioresource-based aggregation-induced emission luminogens from lignin β-O-4 motifs as renewable resources. Nat Commun 14, 6076 (2023). https://doi.org/10.1038/s41467-023-41681-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41681-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.