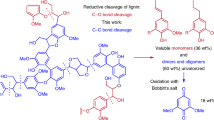
Abstract
Efficient valorization of lignin, a sustainable source of functionalized aromatic products, would reduce dependence on fossil-derived feedstocks. Oxidative depolymerization is frequently applied to lignin to generate phenolic monomers. However, due to the instability of phenolic intermediates, repolymerization and dearylation reactions lead to low selectivity and product yields. Here, a highly efficient strategy to extract the aromatic monomers from lignin affording functionalized diaryl ethers using oxidative cross-coupling reactions is described, which overcomes the limitations of oxidative methods and affords high-value specialty chemicals. Reaction of phenylboronic acids with lignin converts the reactive phenolic intermediates into stable diaryl ether products in near-theoretical maximum yields (92% for beech lignin and 95% for poplar lignin based on the content of β−O−4 linkages). This strategy suppresses side reactions typically encountered in oxidative depolymerization reactions of lignin and provides a new approach for the direct transformation of lignin into valuable functionalized diaryl ethers, including key intermediates in pharmaceutical and natural product synthesis.
Similar content being viewed by others
Introduction
Among the plentiful biomass, lignin is the only sustainable source of aromatic compounds1,2,3,4,5,6 and is available in abundant quantities as a waste product from the pulp and paper and bioethanol industries6. Nonetheless, lignin is under-exploited as a renewable chemical feedstock due to the limited number of efficient and selective downstream processing strategies available.
Various methods have been extensively studied for the conversion of lignin into aromatic products that can broadly be classified as catalytic oxidative degradation, catalytic reductive degradation and acid/base-catalyzed degradation2,3,4,6. Catalytic oxidative degradation has a number of advantages compared to other catalytic fractionation methods including hydrogenolysis (a reductive process), and acid/base-catalyzed degradation4,7,8. Catalytic oxidative degradation advantageously take place under mild and environmentally benign conditions, which contrasts with hydrogenolysis that uses noble metal catalysts, high reaction temperatures and pressures, or the use of corrosive acid/base-catalyzed degradation reagents. In addition, catalytic oxidative degradation has the potential to retain key functionality in the products that could be relevant in subsequent synthetic steps9,10,11. Many homogeneous and heterogeneous catalytic oxidative methods that cleave the C–C bonds of the alkyl side chains to depolymerize lignin have been reported, but typically they are limited by poor selectivity and consequently low product yields (Fig. 1)12,13,14,15,16,17,18,19,20,21,22,23. The catalysts reported for the oxidative fragmentation of lignin are summarized in Supplementary Table S1.The critical issue that must be solved to overcome the aforementioned limitations is that the phenolic hydroxy group is unstable under oxidative conditions24, leading to side reactions including repolymerization and ring opening reactions, which generates complex polymers, oligomers, and non-aromatic side products25,26,27,28. Hence, aromatic products are isolated in low yields in certain direct oxidative degradation reactions.
Diaryl ethers are typically prepared from cross-coupling reactions between petrochemical-derived substrates, specifically, phenols with excess electrophiles, i.e. aryl halides, or nucleophiles, i.e. boronic acids29. Inspired by Cu-catalyzed oxidative cross-coupling reactions between of nucleophiles (phenols, anilines, etc.) with organoboronic acids to afford poly-functionalized diaryl ethers and diaryl amines30,31,32,33,34, we decided to explore the utility of organoboronic acids to extract the reactive phenolic intermediates generated during the oxidative degradation of lignin (Fig. 1). We discovered that this protocol prevents common side reactions initialed by phenolic intermediates allowing functionalized diaryl ethers to be obtained in near-theoretical maximum yields. Using lignin as a starting material to synthesize functionalized diaryl ether is advantageous as lignin is an abundant, inexpensive and renewable material1,2,3,4,5,6. The direct conversion of lignin to diaryl ethers in a single step process requires fewer reagents and solvents than a two-step process in which phenols are initially generated from lignin and then further transformed5,10,23.
Results and discussion
Initially, organosolv beech lignin and 4-chlorophenylboronic acid (1a) were reacted with O2 in the presence of various Cu salts in a weakly alkaline solution that mimic the conditions typical of coupling30,31,32,33,34 and C–C bond cleavage reactions12,13,15,18 (Fig. 2a). After the oxidative degradation/coupling step, a methylation step was performed to transforms any carboxylic acid groups into methyl ester groups, to facilitate gas chromatography (GC) analysis. From the Cu salts screened, copper(II) triflate (Cu(OTf)2) was found to be the most effective catalyst together with bathophenanthroline (L1 in Fig. 2b) co-catalyst, affording the diaryl ether products (1-4b) in 92% yield (Fig. 2a, Entry 5). Note that other ligands were evaluated (Fig. 2a, b), but none were as effective as L1. Since the triflate anions in Cu(OTf)2 are weakly coordinating and readily displaced35,36. Cu(OTf)2 is expected to react with L1, a bidentate N-donor ligand, to form the active catalyst in situ. L1 is an electron-rich ligand that is expected to increase the electron density on the Cu center, which facilitates oxidation Cu(II) to Cu(III), a key step in the reaction (the more electron rich the Cu center the easier it is to oxidize)31,37. A CuL1 complex is formed in situ (evidenced by mass spectrometry, Supplementary Fig. 1), which serves as the actual catalyst, and is sufficiently stable to be isolated after reaction, recycled and reused with only a minor loss in activity (Supplementary Fig. 1). The GC spectrum showing the product distribution is given in Fig. 2c and Supplementary Fig. 2. As a representative hardwood2, the aromatic rings of beech wood consist of 79.5% syringyl (S), and 20.5% guaiacyl (G) units (Supplementary Fig. 3). The main diaryl ether products are the syringyl type methyl ester (1b, 68%) and the guaiacyl type methyl ester (3b, 15%), along with syringyl- and guaiacyl-type aldehyde products (2b, 8% and 4b, 1%, respectively), demonstrating that the aromatic rings of lignin may be extracted successfully as functionalized aromatic diaryl ether products using this method. Detail optimization of the reaction parameters is summarized in Supplementary Tables 2–11.
a Reaction and optimization of the catalyst and co-catalyst, further details of the optimization of the reaction parameters are provided in the SI. Reaction conditions: (1) beech lignin (40 mg), 4-chlorophenylboronic acid (1.5 equiv.), Cu salt (30 mol%), co-catalyst (30 mol%), K2CO3 (4 equiv.), biphenyl (0.02 mmol, internal standard), DMSO (2 mL), O2 (3 atm), 140 °C, 6 h. (2) K2CO3 (3 equiv.), MeI (10 equiv.), 25 °C, 12 h. The methylation step is required so the products can be analyzed by GC-MS. Yield of diaryl esters is based on the content of β–O–4 ether linkages in lignin. b Structures of co-catalysts. c GC spectrum of the reaction mixture (Entry 5, Fig. 2a) showing the presence of four products and their relative abundance. d Short-range HSQC spectra before and after reaction. Assignment of contours is provided by the numbering in the structures on the right. Overlying green contours in the HSQC spectra correspond to 1b.
The evolution of the reaction was monitored by short-range 13C-1H correlation (HSQC) NMR spectroscopy (Fig. 2d shows the spectra before and after reaction). The beech lignin structure consists of syringyl and guaiacyl aromatic rings, together with their main alkyl side chains (β–O–4 (A) and β–β (B) linkages)2, clearly identified in the HSQC spectrum before reaction. After reaction, signals corresponding to the alkyl side chains and electron-rich aromatic rings are no longer present, indicative of complete degradation of the lignin structure. The HSQC spectrum of the reaction mixture is consistent with that of the expected major product 1b (Fig. 2d, green contour) and other products, based on comparisons with full HSQC spectra of 1b–4b (Supplementary Figs. 7–10). The remaining HSQC signals may be attributed to unreacted linkages of lignin and side products derived from the excess boronic acid used (Supplementary Fig. 2).
In the absence of 1a, no aromatic monomer products were detected under the optimized reaction conditions, confirming that the boronic acid captures the reactive phenolic intermediates (Fig. 3). The composition and structure of lignin varies with the type of wood used (Supplementary Figs. 3–5), hence several types were evaluated (Fig. 3). Lignin from poplar, a hardwood, is primarily composed of S aromatic units. Poplar lignin was evaluated under the optimized reaction conditions, affording diaryl ethers in 95% yield, with high selectivity to the syringyl-type product 1b, which was obtained in 71% yield (Fig. 3). Pine lignin, containing G and coumaryl (H) aromatic units without S units, affords the G-type diaryl ether 3b as main product in 71% yield (Fig. 3). Raw beech wood sawdust was tested using the standard reaction conditions with diaryl ether products obtained in only 10% yield (Supplementary Fig. 11).
Other side products corresponds to polymers, oligomers, and non-aromatic side products25,26,27,28. Standard reaction conditions: (1) Lignin (40 mg), 4-chlorophenylboronic acid (1.5 equiv.), Cu(OTf)2 (30 mol%), L1 (30 mol%), K2CO3 (4 equiv.), biphenyl (0.02 mmol, internal standard), DMSO (2 mL), O2 (3 atm), 140 °C, 6 h. (2) K2CO3 (3 equiv.), MeI (10 equiv.), 25 °C, 12 h.
The scope of the boronic acid and related coupling reagents was also evaluated using beech wood lignin under the optimized reaction conditions (Fig. 4). Specifically, phenylboronic acid (2a) affords the S type diaryl ether (6b) in 66% yield. Phenylboronic acids functionalized with electron-withdrawing halogen (1a, 3-4a) or trifluoromethyl (5-6a) substituents are tolerated and afford the desired diaryl ether products in 64–71% yield. Nitro (7a), ester (8a), and methoxy (9a) substituents at the para-position result in slightly lower yields (49–56%). Biphenyl boronic acid (10a) was transformed in 61% yield. Several organic borate esters (11a–13a) were also tested and are less effective in the aromatic extraction reaction (yields ranging from 23 to 55%), and the phenyltrifluoroborate salt (14a) and alkyl boronic acid (15a, 16a) do not function as coupling reagents. In general, phenylboronic acids and borate esters could be employed as effective extraction reagents for the transformation of lignin into functionalized diaryl ether products. Diaryl ethers with ortho-methoxy and/or para-carboxy groups are commonly encountered as intermediates in the preparation of pharmaceutical and natural products, such as chrysophaentins38,39, himalain A40 and certain biological inhibitors41,42.
Mechanistic studies were conducted to probe the reaction pathway and based on the product analysis (Figs. 2d and 3), the transformation of lignin to diaryl ether products appears to involve well-ordered C–C and C–O bond cleavage to release reactive phenol intermediates, which subsequently undergo Cham-Lam coupling with the boronic acid. Control experiments employing diol (c, 1-phenyl-1,2-propandiol), benzyl alcohol (d), and acetophenone (e) as substrates were conducted to confirm that Cα–Cβ bond cleavage takes place under the reaction conditions (Fig. 5a). All three different kinds of Cα–Cβ bonds were successfully cleaved affording methyl benzoate in excellent yield (90–98%). These results are in agreement with previous reports concerning Cu catalyzed C–C activation initiated by the oxidation of hydroxy groups (Supplementary Figs. 12 and 13)13,15,18,43,44. In addition, the reaction of protected phenols in which the hydroxy group is modified with glycol (f), acetic acid (g) and formic acid (h) groups with phenyl boronic acid (2a) affords the expected diphenyl ether product in 97–99% yield (Fig. 5b). Hence, it would appear that under the oxidative conditions the protective groups are unstable15, and release reactive phenolic intermediates (Supplementary Fig. 14), that react with the boronic acid.
a Control experiments probing Cα–Cβ bond cleavage. Reaction conditions are the same as in Fig. 4, but without boronic acid. b Control experiments to investigate C(alkyl)−O bond cleavage. Reaction conditions are the same as Fig. 4. c Exploration of C–C and C–O bond cleavage in β–O–4 lignin model compounds. Reaction conditions were the same as Fig. 4. d Plausible reaction mechanism.
Aromatic dimers with alkyl β–O–4 linkages were employed as lignin model compounds and reacted with 2a under the standard conditions (Fig. 5c). Dimer i containing a stable methoxy group at the para position provides ester j and diaryl ether 16b in good yield, demonstrating the ability of the catalytic system to degrade the alkyl β–O–4 linkage via C–C and C–O bond cleavage. Alternatively, with a hydroxy group at the para position (dimer k) diaryl ethers 17b and 16b are obtained, which implies the boronic acid reacts directly with the phenolic hydroxy group.
The roles of Cu(OTf)2, L1 and K2CO3 were investigated through a series of control experiments (Supplementary Fig. 15). The Cu complex and base are indispensable for aerobic −OH group oxidation, C–C bond activation and Cham-Lam coupling. L1 coordinated to the Cu center to promote the Cham-Lam coupling of the boronic acid with the phenol intermediates. The kinetic study demonstrates that C–C bond activation to release reactive phenolic intermediates is slower Cham-Lam coupling between phenol and boronic acid, which ensures rapid capture of phenolic intermediates with boronic acid, preventing side reactions (Supplementary Fig. 16).
Based on the mechanistic studies a tentative reaction pathway is proposed (Fig. 5d). Under the basic reaction conditions lignin 1 is activated by the Cu catalyzed oxidation of the hydroxy groups to give intermediate 2 containing a carbonyl group (Supplementary Fig. 12)45,46. Cu catalyzed Cα–Cβ bonds cleavage of 2 results in the depolymerization of lignin to the oxalic acid protected phenol monomer 3 (Supplementary Fig. 13)13,18,43,44. Thermal decomposition of 3 would release the phenolic hydroxy group to generate reactive intermediate 4 (Supplementary Fig. 14)47,48,49, which is captured by Cham-Lam coupling with an appropriate boronic acid to afford the carboxylic acid containing product 550,51. Methylation of the carboxylic acid in 5 to ester 6 is performed to facilitate analysis.
We developed an in-situ method to extract the aromatic rings in lignin by adding aryl boronic acids or borate esters during oxidative decomposition. Diaryl ether products are generated in near-theoretical maximum yield as repolymerization and dearylation side reactions of the reactive phenol intermediates are suppressed. Studies indicate that the reaction is initiated by Cu catalyzed C–C and C–O bond cleavage of the alkyl side chains, degrading the polymeric structure of lignin and releasing reactive phenol intermediates, which are captured by cross-coupling with the aryl boronic acids or borate esters to afford stable diaryl ether products. This study paves the way to alternative approaches to transform lignin via reactive phenol intermediates into high-value specialty chemicals, including pharmaceutical and natural product intermediates and other chemicals.
Methods
Typical reaction to prepare ether monomers from lignin
Beech lignin (40 mg, content of β–O–4 linkages is 0.245 mmol/g lignin), 4-chlorophenylboronic acid (1.5 equiv.), Cu(OTf)2 (30 mol%), bathophenanthroline (L1, 30 mol%), K2CO3 (4 equiv.), biphenyl (internal standard, 2 equiv.), and DMSO (2 mL) were added into autoclave. The reactor was heated to 140 °C for 6 h. After cooling to room temperature, the crude product was methylated by addition of K2CO3 (3 equiv.), MeI (10 equiv.) and stirring for 12 h. After reaction, the mixture was added ethyl acetate (EA, 3 mL) and a saturated NH4Cl (5 mL) aqueous solution was added to extract the organic products. The aqueous solution was extracted into EA (3 × 3 mL). For qualitative and quantitative analysis of the products, the combined organic phase was filtered over silica gel to remove the inorganic salts and ligand. Further purification was achieved using column chromatography.
Data availability
All data supporting this study are available in the article and Supplementary Information files, and also are available from the corresponding author if request.
References
Ragauskas, A. J. et al. Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843 (2014).
Rinaldi, R. et al. Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 55, 8164–8215 (2016).
Sun, Z., Fridrich, B., de Santi, A., Elangovan, S. & Barta, K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 118, 614–678 (2018).
Schutyser, W. et al. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 47, 852–908 (2018).
Questell-Santiago, Y. M., Galkin, M. V., Barta, K. & Luterbacher, J. S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 4, 311–330 (2020).
Abu-Omar, M. M. et al. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 14, 262–292 (2021).
Behling, R., Valange, S. & Chatel, G. Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: what results? What limitations? What trends? Green. Chem. 18, 1839–1854 (2016).
Yang, C., Maldonado, S. & Stephenson, C. R. J. Electrocatalytic lignin oxidation. ACS Catal. 11, 10104–10114 (2021).
Cai, Z. et al. Selective production of diethyl maleate via oxidative cleavage of lignin aromatic unit. Chem 5, 2365–2377 (2019).
Luo, H. et al. Oxidative catalytic fractionation of lignocellulosic biomass under non-alkaline conditions. J. Am. Chem. Soc. 143, 15462–15470 (2021).
Subbotina, E. et al. Oxidative cleavage of C–C bonds in lignin. Nat. Chem. 13, 1118–1125 (2021).
Sedai, B. & Baker, R. T. Copper catalysts for selective C−C bond cleavage of β-O-4 lignin model compounds. Adv. Synth. Catal. 356, 3563–3574 (2014).
Wang, M. et al. Oxidative C(OH)−C bond cleavage of secondary alcohols to acids over a copper catalyst with molecular oxygen as the oxidant. J. Catal. 348, 160–167 (2017).
Schutyser, W. et al. Revisiting alkaline aerobic lignin oxidation. Green. Chem. 20, 3828–3844 (2018).
Liu, M. et al. Stepwise degradation of hydroxyl compounds to aldehydes via successive C–C bond cleavage. Chem. Commun. 55, 925–928 (2019).
Liu, M. et al. Nitrogen dioxide catalyzed aerobic oxidative cleavage of C(OH)–C bonds of secondary alcohols to produce acids. Angew. Chem. Int. Ed. 58, 17393–17398 (2019).
Liu, J. et al. From alkylarenes to anilines via site-directed carbon–carbon amination. Nat. Chem. 11, 71–77 (2019).
Liu, M. et al. Aerobic oxidative cleavage and esterification of C(OH)–C bonds. Chem 6, 3288–3296 (2020).
Luo, H. et al. Cobalt nanoparticles-catalyzed widely applicable successive C−C bond cleavage in alcohols to access esters. Angew. Chem. Int. Ed. 59, 19268–19274 (2020).
Zhou, H. et al. Selectively upgrading lignin derivatives to carboxylates through electrochemical oxidative C(OH)−C bond cleavage by a Mn-doped cobalt oxyhydroxide catalyst. Angew. Chem. Int. Ed. 60, 8976–8982 (2021).
Cui, T. et al. Atomically dispersed Pt–N3C1 sites enabling efficient and selective electrocatalytic C–C bond cleavage in lignin models under ambient conditions. J. Am. Chem. Soc. 143, 9429–9439 (2021).
Ma, L., Zhou, H., Kong, X., Li, Z. & Duan, H. An electrocatalytic strategy for C–C bond cleavage in lignin model compounds and lignin under ambient conditions. ACS Sustain. Chem. Eng. 9, 1932–1940 (2021).
Shi, S.-H., Liang, Y. & Jiao, N. Electrochemical oxidation induced selective C–C bond cleavage. Chem. Rev. 121, 485–505 (2021).
Rahimi, A., Ulbrich, A., Coon, J. J. & Stahl, S. S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014).
Esguerra, K. V. N., Fall, Y., Petitjean, L. & Lumb, J.-P. Controlling the catalytic aerobic oxidation of phenols. J. Am. Chem. Soc. 136, 7662–7668 (2014).
Niederer, K. A., Gilmartin, P. H. & Kozlowski, M. C. Oxidative photocatalytic homo- and cross-coupling of phenols: nonenzymatic, catalytic method for coupling tyrosine. ACS Catal. 10, 14615–14623 (2020).
Xu, W., Huang, Z., Ji, X. & Lumb, J.-P. Catalytic aerobic cross-dehydrogenative coupling of phenols and catechols. ACS Catal. 9, 3800–3810 (2019).
Neuhaus, W. C. & Kozlowski, M. C. Total synthesis of pyrolaside B: phenol trimerization through sequenced oxidative C−C and C−O coupling. Angew. Chem. Int. Ed. 59, 7842–7847 (2020).
Frlan, R. & Kikelj, D. Recent progress in diaryl ether synthesis. Synthesis 2006, 2271–2285 (2006).
Vantourout, J. C., Miras, H. N., Isidro-Llobet, A., Sproules, S. & Watson, A. J. B. Spectroscopic studies of the Chan–Lam amination: a mechanism-inspired solution to boronic ester reactivity. J. Am. Chem. Soc. 139, 4769–4779 (2017).
West, M. J., Fyfe, J. W. B., Vantourout, J. C. & Watson, A. J. B. Mechanistic development and recent applications of the Chan–Lam amination. Chem. Rev. 119, 12491–12523 (2019).
Bose, S., Dutta, S. & Koley, D. Entering chemical space with theoretical underpinning of the mechanistic pathways in the Chan–Lam amination. ACS Catal. 12, 1461–1474 (2022).
Doyle, M. G. J. & Lundgren, R. J. Oxidative cross-coupling processes inspired by the Chan–Lam reaction. Chem. Commun. 57, 2724–2731 (2021).
Walker, B. R., Manabe, S., Brusoe, A. T. & Sevov, C. S. Mediator-enabled electrocatalysis with ligandless copper for anaerobic Chan–Lam coupling reactions. J. Am. Chem. Soc. 143, 6257–6265 (2021).
Hardouin Duparc, V., Bano, G. L. & Schaper, F. Chan–Evans–Lam couplings with copper iminoarylsulfonate complexes: scope and mechanism. ACS Catal. 8, 7308–7325 (2018).
Duparc, V. H., Thouvenin, A. & Schaper, F. Anion influences on the structures of pyridyl-iminosulfonate copper(II) complexes and their reactivity in Chan–Lam couplings. Can. J. Chem. 98, 502–510 (2020).
Naka, S., Okada, H., Onnagawa, H. & Tsutsui, T. High electron mobility in bathophenanthroline. Appl. Phys. Lett. 76, 197–199 (2000).
Plaza, A., Keffer, J. L., Bifulco, G., Lloyd, J. R. & Bewley, C. A. Chrysophaentins A−H, antibacterial bisdiarylbutene macrocycles that inhibit the bacterial cell division protein FtsZ. J. Am. Chem. Soc. 132, 9069–9077 (2010).
Fullenkamp, C. R. et al. Synthesis of 9-dechlorochrysophaentin A enables studies revealing bacterial cell wall biosynthesis inhibition phenotype in B. subtilis. J. Am. Chem. Soc. 142, 16161–16166 (2020).
Zhou, S.-Y. et al. A new diphenyl ether derivative from Mirabilis himalaica. Nat. Prod. Res. 31, 1034–1041 (2017).
Chhibber, M. et al. Novel diphenyl ethers: design, docking studies, synthesis and inhibition of enoyl ACP reductase of Plasmodium falciparum and Escherichia coli. Bioorg. Med. Chem. 14, 8086–8098 (2006).
Gupta, S., Chhibber, M., Sinha, S. & Surolia, A. Design of mechanism-based inhibitors of transthyretin amyloidosis: studies with biphenyl ethers and new structural templates. J. Med. Chem. 50, 5589–5599 (2007).
Huang, X. et al. From ketones to esters by a Cu-catalyzed highly selective C(CO)–C(alkyl) bond cleavage: aerobic oxidation and oxygenation with air. J. Am. Chem. Soc. 136, 14858–14865 (2014).
Liang, Y.-F. & Jiao, N. Oxygenation via C–H/C–C bond activation with molecular oxygen. Acc. Chem. Res. 50, 1640–1653 (2017).
Ryland, B. L. & Stahl, S. S. Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. 53, 8824–8838 (2014).
Liu, M. & Li, C.-J. Catalytic Fehling’s reaction: an efficient aerobic oxidation of aldehyde catalyzed by copper in water. Angew. Chem. Int. Ed. 55, 10806–10810 (2016).
Chang, J.-G., Chen, H.-T., Xu, S. & Lin, M. C. Computational study on the kinetics and mechanisms for the unimolecular decomposition of formic and oxalic acids. J. Phys. Chem. A 111, 6789–6797 (2007).
Molt, R. W., Lecher, A. M., Clark, T., Bartlett, R. J. & Richards, N. G. J. Facile Csp2–Csp2 bond cleavage in oxalic acid-derived radicals. J. Am. Chem. Soc. 137, 3248–3252 (2015).
Moldoveanu, S. C. Chapter 12 - Pyrolysis of carboxylic acids. In Pyrolysis of organic molecules 2nd ed. (ed. Moldoveanu, S. C.) 483–553 (Elsevier, 2019).
Chan, D. M. T., Monaco, K. L., Wang, R.-P. & Winters, M. P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 39, 2933–2936 (1998).
Evans, D. A., Katz, J. L. & West, T. R. Synthesis of diaryl ethers through the copper-promoted arylation of phenols with arylboronic acids. An expedient synthesis of thyroxine. Tetrahedron Lett. 39, 2937–2940 (1998).
Acknowledgements
This publication was supported by EPFL and was created was created as part of NCCR Catalysis (Grant number 180544, Paul Dyson), a National Centre of Competence in Research funded by the Swiss National Science Foundation. We also thank Dr. Manli Hua for helpful discussions.
Author information
Authors and Affiliations
Contributions
M.L. and P.D. designed the research, analyzed data, and wrote the paper. M.L. performed the research.
Corresponding author
Ethics declarations
Competing interests
The catalytic method is described in a patent invented by M.L. and P.D. (EP Patent, No. 22178410.1).
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer reviewer report is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, M., Dyson, P.J. Direct conversion of lignin to functionalized diaryl ethers via oxidative cross-coupling. Nat Commun 14, 2830 (2023). https://doi.org/10.1038/s41467-023-38534-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-38534-1
This article is cited by
-
Tandem catalysis enables chlorine-containing waste as chlorination reagents
Nature Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.