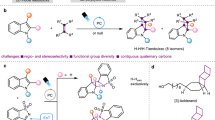
Abstract
Indole alkaloids are one of the largest alkaloid classes, proving valuable structural moiety in pharmaceuticals. Although methods for the synthesis of indole alkaloids are constantly explored, the direct single-step synthesis of these chemical entities with broad structural diversity remains a formidable challenge. Herein, we report a modular assembly of tetrahydrocarboline type of indole alkaloids from simple building blocks in a single step while showing broad compatibility with medicinally relevant functionality. In this protocol, the 2-alkylated or 3-alkylated indoles, formaldehyde, and amine hydrochlorides could undergo a one-pot reaction to deliver γ-tetrahydrocarbolines or β-tetrahydrocarbolines directly. A wide scope of these readily available starting materials is applicable in this process, and numerous structural divergent tetrahydrocarbolines could be achieved rapidly. The control reaction and deuterium-labelling reaction are conducted to probe the mechanism. And mechanistically, this multicomponent reaction relies on a multiple alkylamination cascade wherein an unusual C(sp3)–C(sp3) connection was involved in this process. This method could render rapid access to pharmaceutically interesting compounds, greatly enlarge the indole alkaloid library and accelerate the lead compound optimization thus facilitating drug discovery.
Similar content being viewed by others
Introduction
Indole alkaloids, one of the largest classes of naturally occurring alkaloids, have proven to be an important and rich source of pharmaceuticals1,2,3,4,5,6,7,8. Particularly, tetrahydrocarbolines are the common structural motif in most of the indole alkaloids and represent a key scaffold in a wide range of market drugs and clinical candidates9,10,11,12,13, such as hypotensive drug reserpine, antiphosphodiesterase-5 agent Tadalafil, and antiallergic therapeutics mebhydrolin and dimebolin (Fig. 1a)14,15,16,17,18. Therefore, the study of tetrahydrocarboline synthesis and drug discovery has inspired practitioners from a wide spectrum of the scientific community. Typically, the β-tetrahydrocarbolines biogenetically originate from tryptamine and secologanin via an enzymatic Pictet-Spengler reaction and sequential diversification19,20,21,22,23,24,25,26,27, while most of the chemical synthesis of these molecules uses the same Pictet-Spengler strategy, and these protocols dictate the functionality on C-1 position (Fig. 1b). Respecting the γ-tetrahydrocarbolines, which are less abundant in natural isolates, the most facile synthetic approach is the Fischer-indole reaction (Fig. 1b)28. Although the power of the aforementioned methods is undisrupted, these protocols still have limitations, including normally tedious synthetic procedures, and limited diversity in piperidine fragments. And more to be considered is that in the course of structure-activity relationship studies in medicinal chemistry, these alkaloids-relevant molecular optimization has to rely on long-step modifications wherein some designed molecules are synthetically unreachable. Given the prevalence of indole alkaloids and the limitations of present synthetic methods, the development of modular synthesis of tetrahydrocarboline type of indole alkaloids with broad diversity in a rapid and efficient manner would greatly enrich the toolbox of organic chemists, enlarge the compound library available for drug discovery and also accelerate the lead compound optimization process.
The rapid assembly of complex scaffolds in a single step from simple precursors identifies as an ideal reaction in terms of efficiency and sustainability. Typically, multicomponent reactions (MCRs) fall into this ideal reaction and have become powerful synthetic tools for the construction of complex and diverse chemical entities in a one-pot fashion from three or more starting materials29,30,31,32. Compared to conventional stepwise synthesis, the MCRs could rapidly assemble complex molecules with simple manipulation, high atom- and step-economy; therefore, they have found widespread applications in the facile synthesis of natural products, market drugs, biologically active compounds, and materials. The prominent MCRs, such as Ugi, Mannich, Strecker, Biginelli reaction, or Hantzsch dihydropyridine synthesis, have been robust preparative drug discovery methods33,34,35,36,37,38,39,40,41,42,43,44. However, the MCRs-promoted modular and straightforward synthesis of tetrahydrocarboline type of indole alkaloids still remains explored. On the other hand, most of the reported MCRs mechanistically relied on those activated carbon centers to render the multiple chemical bond formations and target molecules construction, whereas the invoking of unactivated carbon centers poses a substantial challenge.
Bearing these challenges in mind and inspired by the Mannich reaction for the biomimetic synthesis of tropinone alkaloids, as well as our previous studies on MCRs45,46,47, we speculated that if a functional group was incorporated into the N-atom of indole, the electron density distribution of indole moiety would be changed and this might have the opportunity to invoke the tethered unactivated carbon centers to involve in an MCR process toward the construction of indole alkaloids. Herein, we would like to report our successful implementation of this concept for the modular assembly of tetrahydrocarboline type of indole alkaloids through multicomponent reaction (Fig. 1c). This practical reaction could tolerate a broad range of functional groups so that a wide set of market drugs and drug-like alkaloids could be achieved in a single-step from simple precursors, and this could also greatly enlarge the alkaloid library and much simplified the optimization process in drug discovery.
Results
Reaction optimization
We commenced our study by choosing the model substrates of indole derivatives 1, formaldehyde 2, and methyl glycine ester hydrochloride 3a. Each reaction parameter was examined including solvent, temperature, and the N-substitution of indole (Table 1, for details, see Supplementary information), and we identified that an intriguing multicomponent reaction of N-benzyl-2-methyl indole 1a, 2, and 3a in the solvent of N,N’-dimethylformamide (DMF) under air could well occur to furnish γ-tetrahydrocarboline 4a in excellent yield (92%). This meant it has overcome the intrinsic challenge of modular assembly of tetrahydrocarboline type of indole alkaloids in a single step, wherein multiple chemical bond formations were observed including an unactivated carbon center involved C(sp3)–C(sp3) connection. Besides, the N-substitution was important in this process wherein the alkyl groups were optimal while the hydrogen atom and electron-withdrawing groups (t-butoxycarbonyl, Boc; p-toluenesulfonyl, Ts) would lead to diminished reaction efficiency (Supplementary Table 2). This is probably because the N-substitution of indole would render drastic changes to the electron density distribution of indole moiety which is essential to this MCR process and the alkaloid formation.
Reaction scope study and tetrahydrocarboline synthesis
We next focused on the generality of this multicomponent one-pot synthesis of indole alkaloids using this operationally simple protocol. Firstly, a wide set of amine-building blocks were explored (Fig. 2). For instance, diverse amino acid derivatives could well engage in this process with 2-methyl indoles 1 and formaldehyde 2 to deliver the corresponding products 4a–4l, and the chirality and functionality from amino acid could be directly incorporated into the indole alkaloids which could not be accessed by conventional methods. Besides, numerous flexible aliphatic amines were compatible with this protocol to furnish the indole alkaloids in good to excellent yields (4m–4ak), with a diversity of substituents attached, including chloride, bromide, sulfone, trifluoromethyl, cyano, pathalimide, acetoxy, alkene, alkyne, three- to six-membered cyclic and heterocyclic rings, phenol. Notably, these functionalities were widely used in drug discovery and they could be intuitively mapped onto the indole alkaloid frameworks from simple materials in this protocol. The structures (4f, 4af) were unambiguously confirmed by single-crystal x-ray crystallography. We next examined the compatibility of this protocol with indole building blocks. A variety of substitutions in the indole scaffold were well tolerated in this multicomponent synthesis of tetrahydrocarboline alkaloids, including fluoro, chloro, bromo, ester, methyl, phenyl, and pinacolboranyl (4al-4av). Notably, the 7-azaindole, a bioisostere of indole, was also applicable in this protocol (4aw). Meanwhile, various substitutions in the N-position of indole were varied, such as methyl, para-methoxybenzyl (PMB), 2-phenylethyl, allyl, cinnamyl, and tert-butyl carbonate (Boc) (4ax-4aac). Besides, we were pleased to find that the multi-substituted pyrroles were also applicable in this protocol for leading to pyrrole-piperidine fused products (4aad and 4aae). These functionalities pervade the structure-activity relationship studies in medicinal chemistry and serve as a productive springboard for further skeletal modification strategies.
Encouraged by the above results, we subsequently wondered whether β-tetrahydrocarboline could be assembled in the same manner using this MCR protocol (Fig. 3). Thus, the 3-methyl indole was employed as a building block for investigation, and the reaction parameters were examined including the substituents of indole, solvent, and reaction temperature. Gratifyingly, (Boc)2O, a widely used protecting group, was placed on the N-position of 3-methyl-5,6-dimethoxy indole (5) to render the occurrence of MCR with formaldehyde 2 and amine-building block 3. The generality was also examined, and a wide range of functionalities in the amines were well applicable in this process, including amino acid derivatives, alkene, alkyne, chloro, sulfone, cyano, phthalimide, and cyclobutane (6a–6n). Moreover, the prolonged aliphatic chain in the indole moiety with the functionality of ester and protected alcohol were also compatible to deliver the alkaloid products smoothly (6o–6p). Besides, a single group substitution in the indole skeleton such as methoxy, benzyloxy, or hydroxyl, would lead to diminished yields, probably due to the changed electron density distribution; however, when p-toluenesulfonic acid (p-TSA) was added as an additive, the corresponding β-tetrahydrocarbolines could be achieved in moderate to excellent yields (6q–6af; for details, see Supplementary information). In particular, We found that the reaction was dramatically affected by the N-substitutions, wherein the electron-withdrawing groups like benzyloxycarbonyl (Cbz) and N,N- dimethylformyl were optimal (6ag, 6ah, for details, see Supplementary information) and electron-donating groups were inferior. The structure (6v) was unambiguously confirmed by single-crystal x-ray crystallography. Considering the remarkable value of tetrahydrocarboline type of indole alkaloid in natural products and drug discovery, this protocol provides a rapid and distinct approach to new structured indole alkaloids available for drug discovery, and would definitely simplify the optimization process of lead molecule thus improving the accuracy and efficiency of candidate identification.
To highlight the value of this protocol, a collective and concise synthesis of market drugs and clinical candidates was exemplified (Fig. 4). For instance, Mebhydrolin (8) and γ-tetrahydrocarboline block (9), with the key structure of γ-tetrahydrocarboline, could be rapidly achieved from the feedstock 2-methyl indole (7a) in short step-synthetic routes under this protocol; while AVN-101 (10) and Dimebolin (11) could also be accessed from feedstock 7 g in only two-step synthesis under this protocol; Setipiprant (13) could also be rapidly achieved under this strategy. A 5 mmol scale reaction was established to demonstrate the practicability. Meanwhile, various new structured β-tetrahydrocarboline types of drug-like compounds (14–17) could be rapidly assembled and further merged with peptide and click chemistry, which could occupy new chemical space and biological space for drug discovery. Moreover, once a structure optimization of indole alkaloids is needed, this protocol provides a distinctive shortcut rather than a long-step modification.
Mechanistic investigations
Since the modular assembly of indole alkaloids and the unusual C(sp3)–C(sp3) connection occurrence in this process is impressive, the reaction mechanism was probed (Fig. 5). Control reaction, deuterium-labeling reaction, and cross-over reaction were set up to elucidate a multiple alkylamination cascade in this process48,49,50. For instance, the indole starting material 1a, the mono-alkylaminated intermediate 18, the di-alkylaminated intermediate 19, were individually subject to the reaction with deuterated formaldehyde (DCDO, [D2]-2) and glycine methyl ester hydrochloride 3a, and the equivalent amount of [D2]-2 and 3a was varied, and the results showed that the deuterated γ-tetrahydrocarboline 4a ([D4]-4a) could be formed with different deuteration ratio (Fig. 5a). Besides, when 18 was subjected to react with formaldehyde in the absence of 3a, there was not any product formation (Fig. 5b). Interestingly, either 18 or 19 was subjected to the reaction with formaldehyde and benzylamine hydrochloride (3af), 4af was formed as the major product rather than 4a. Meanwhile, a tertiary amine substituted indole 20 was prepared and subjected to reaction with 2 and 3a, the reaction did not deliver the γ-tetrahydrocarboline product. Additionally, kinetic isotope effect (KIE) studies were carried out and a KIE value of 3.25 was obtained, indicating that the C(sp3)−H bond cleavage might be involved in the rate-limiting step (Fig. 5c, for details, see Supplementary information). Taking together these results, a plausible reaction mechanism was proposed (Fig. 5d). At beginning, the indole ring triggered the iterative alkylaminations to deliver Int-2 and Int-3. Then, either 1a or Int-2 would undergo another alkylamination to deliver Int-4. At this stage, a further alkylamination via transition state TS-1 would induce the unactivated carbon center to undergo dehydrogenation and convert the unactivated C(sp3) to activated C(sp2) along with the formation of Int-5. The following iminium formation of Int-6, cyclization, and retro-Mannich reaction would achieve the construction of alkaloids and enable the cascade cycle51.
Similarly, control reaction and deuterium-labeling reaction were also conducted to investigate the mechanism for β-tetrahydrocarboline formation. For instance, the indole starting material 5a, the mono-alkylaminated intermediate 22, and the di-alkylaminated intermediate 23, were individually subject to the reaction with deuterated formaldehyde (DCDO, [D2]-2) and glycine methyl ester hydrochloride 3a, and the results showed that the deuterated γ-tetrahydrocarboline [D4]-6a could be formed with almost completed deuteration ratio (Fig. 6a). Besides, the one-pot reaction of 22, formaldehyde and benzylamine hydrochloride (3af) was set up, wherein 6f was formed as the major product rather than 6a (Fig. 6b). Additionally, kinetic isotope effect (KIE) studies were carried out and a KIE value of 2.64 was obtained, indicating that the C(sp3)−H bond cleavage might be involved in the rate-limiting step (Fig. 6c, for details, see Supplementary Information). These results indicated that the synthetic mechanism of β-tetrahydrocarboline is probably similar with γ-tetrahydrocarboline (Fig. 6a–c) and a plausible mechanism was then proposed (Fig. 6d). Initially, the indole ring triggered the iterative alkylaminations to deliver Int-7 and Int-8. Then, either 5a or Int-7 would undergo another alkylamination to deliver Int-9. At this stage, a further alkylamination via transition state TS-2 would induce the unactivated carbon center to undergo dehydrogenation and convert the unactivated C(sp3) to activated C(sp2) along with the formation of Int-10. The following iminium formation of Int-11, cyclization, and retro-Mannich reaction would achieve the construction of β-tetrahydrocarboline products 6 and enable the cascade cycle51.
Discussion
In conclusion, we have achieved a modular assembly of tetrahydrocarbolines through multicomponent reaction of 2-substituted or 3-substituted indoles, formaldehyde, and amino hydrochlorides, and this protocol provides expedient access to these indole alkaloids. The chirality and a wide scope of functional groups were compatible in this process thus significantly enlarging the chemical space and biological space of tetrahydrocarbolines. Because tetrahydrocarbolines are widely encountered as pharmaceutically relevant substrates, we believe that this protocol would be a widely applicable strategy in indole-related drug discovery. Besides, this work also provides a new vision for the reaction of unactivated C(sp3) center and C(sp3)–C(sp3) bond formation.
Methods
General procedure for the synthesis of γ-tetrahydrocarboline 4a-4aac
A mixture of 1 (0.2 mmol), formaldehyde 2 (37% in water, 0.08 mL, 5 equiv.), and corresponding primary amine hydrochloride 3 (0.4 mmol, 2 equiv.) in DMF (1.5 mL) was stirred at 60 °C until the reaction was completed. The reaction was quenched by saturated aqueous NaHCO3. The aqueous layer was extracted with ethyl acetate (three times), and the combined organic layer was dried over Na2SO4 and concentrated. Purification by silica gel column chromatography to give corresponding γ-tetrahydrocarboline products 4a–4aac.
General procedure for the synthesis of 4aad and 4aae
A mixture of 1t (or 1u) (0.2 mmol), formaldehyde 2 (37% in water, 0.08 mL, 5 equiv.), TsOH (0.1 mmol, 0.5 equiv.) and methyl glycinate hydrochloride 3a (0.4 mmol, 2 equiv.) in MeCN (1.5 mL) were stirred at 80 °C until the reaction was completed. The reaction was quenched by saturated aqueous NaHCO3. The aqueous layer was extracted with ethyl acetate (three times), and the combined organic layer was dried over Na2SO4 and concentrated. Purification by silica gel column chromatography to give products 4aad and 4aae.
General procedure for the synthesis of β-tetrahydrocarboline 6a–6p
A mixture of 5 (0.2 mmol), formaldehyde 2 (37% in water, 0.08 mL, 5 equiv.), and corresponding primary amine hydrochloride 3 (0.4 mmol, 2 equiv.) in MeCN (1.5 mL) was stirred at 80 °C until the reaction was completed. The reaction was quenched by saturated aqueous NaHCO3. The aqueous layer was extracted with ethyl acetate (three times), and the combined organic layer was dried over Na2SO4 and concentrated. Purification by silica gel column chromatography to give corresponding β-tetrahydrocarboline products 6a–6p.
General procedure for the synthesis of 6q–6ah
A mixture of 5 (0.2 mmol), formaldehyde 2 (37% in water, 0.08 mL, 5 equiv.), TsOH (0.1 mmol, 0.5 equiv.) and corresponding primary amine hydrochloride 3 (0.4 mmol, 2 equiv.) in MeCN (1.5 mL) was stirred at 60 °C until the reaction was completed. The reaction was quenched by saturated aqueous NaHCO3. The aqueous layer was extracted with ethyl acetate (three times), and the combined organic layer was dried over Na2SO4 and concentrated. Purification by silica gel column chromatography to give corresponding products 6q–6ah.
Data availability
The data that support the findings of this study are available in the main text or the supplementary materials. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2211850, 2211851 and 2235528. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Stempel, E. & Gaich, T. Cyclohepta[b]indoles: a privileged structure motif in natural products and drug design. Acc. Chem. Res. 49, 2390–2402 (2016).
Wan, Y., Li, Y., Yan, C., Yan, M. & Tang, Z. Indole: a privileged scaffold for the design of anti-cancer agents. Eur. J. Med. Chem. 183, 111691–111708 (2019).
Ishikura, M., Abe, T., Choshi, T. & Hibino, S. Simple indole alkaloids and those with a non-rearranged monoterpenoid unit. Nat. Prod. Rep. 27, 1630–1680 (2010).
Wibowo, J. T. et al. Marine-drived indole alkaloids and their biological and pharmacological activities. Mar. Drugs 20, 3 (2022).
Chauhan, M., Saxena, A. & Saha, B. An insight in anti-malarial potential of indole scaffold: a review. Eur. J. Med. Chem. 218, 113400–113412 (2021).
Taylor, W. I. The source of indole alkaloids. Science 153, 954–956 (1966).
Woo, J. et al. Scaffold hopping by net photochemical carbon deletion of azaarenes. Science 376, 527–532 (2022).
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 (2022).
Tan, J. et al. Synthesis and pharmacological evaluation of tetrahydro-γ-carboline derivatives as potent anti-inflammatory agents targeting cyclic GMP-AMP synthase. J. Med. Chem. 64, 7667–7690 (2021).
Herraiz, T. & Galisteo, J. Tetrahydro-β-carboline alkaloids occur in fruits and fruit juices. activity as antioxidants and radical scavengers. J. Agric. Food. Chem. 51, 7156–7161 (2003).
Beato, A., Gori, A., Boucherle, B., Peuchmaur, M. & Haudecoeur, R. β-carboline as a privileged scaffold for multitarget strategies in Alzheimer’s disease therapy. J. Med. Chem. 64, 1392–1422 (2021).
Cao, R., Peng, W., Wang, Z. & Xu, A. β-carboline alkaloids: biochemical and pharmacological functions. Curr. Med. Chem. 14, 479–500 (2007).
Dai, J., Dan, W. & Wan, J. Natural and synthetic β-carboline as a privileged antifungal scaffolds. Eur. J. Med. Chem. 229, 114057–114074 (2022).
Stitzel, R. E. The biological fate of reserpine. Pharmacol. Rev. 28, 179–208 (1976).
Galiè, N. et al. Al initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N. Engl. J. Med. 373, 834–844 (2015).
Zhou, R. et al. Repurposing of the antihistamine mebhydrolin napadisylate for the treatment of Zika virus infection. Bioorg. Chem. 128, 106024–106033 (2022).
Doody, R. S. et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled study. Lancet 372, 207–215 (2008).
Ustyugov, A. et al. New therapeutic property of demibon as a neuroprotective agent. Curr. Med. Chem. 25, 5315–5326 (2018).
Duan, Q. et al. Fungal indole alkaloid biogenesis through evolution of a bifunctional reductase/diels-alderase. Nat. Chem. 11, 972–980 (2019).
Mizoguchi, H., Oikawa, H. & Oguri, H. Biogenetically inspired synthesis and skeletal diversification of indole alkaloids. Nat. Chem. 6, 57–64 (2017).
Festa, A. A., Voskressensky, L. G. & Van der Eycken, E. V. Visible light-mediated chemistry of indoles and related heterocycles. Chem. Soc. Rev. 48, 4401–4423 (2019).
Schatz, D. J., Kuenstner, E. J., George, D. T. & Pronin, S. V. Synthesis of rearranged indole diterpenes of the paxilline type. Nat. Prod. Rep. 39, 946–968 (2022).
Lancianesi, S., Palmieri, A. & Petrini, M. Synthetic approaches to 3-(2-nitroalkyl) indoles and their use to access tryptamines and related bioactive compounds. Chem. Rev. 114, 7108–9149 (2014).
Zi, W., Zuo, Z. & Ma, D. Intramolecular dearomative oxidative coupling of indoles: a unified strategy for the total synthesis of indoline alkaloids. Acc. Chem. Res. 48, 702–711 (2015).
Zheng, C. & You, S.-L. Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products. Nat. Prod. Rep. 36, 1589–1605 (2019).
Liu, X.-Y. & Qin, Y. Indole alkaloid synthesis facilitated by photoredox catalytic cascade reactions. Acc. Chem. Res. 52, 1877–1891 (2019).
Rao, R. N., Maiti, B. & Chanda, K. Application of pictet-spengler reaction to indole-based alkaloids containing tetrahydro-β-carboline scaffold in combinatorial chemistry. ACS Comb. Sci. 19, 199–228 (2017).
Dai, J., Dan, W., Zhang, Y. & Wang, J. Recent developments on synthesis and biological activities of γ-carbolines. Europ. J. Med. Chem. 157, 447–461 (2018).
Reguera, L. & Rivera, D. Multicomponent reaction toolbox for peptide macrocyclization and stapling. Chem. Rev. 119, 9836–9860 (2019).
Dömling, A., Wang, W. & Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 112, 3083–3135 (2012).
Shiri, M. Indoles in multicomponent processes (MCPS). Chem. Rev. 112, 3508–3549 (2012).
Nia, R. H.; Taati, Z. & Mamaghani, M. Multi-component synthesis of indole-substituted heterocycles- a review. Polycycl. Aromat. Compd. https://doi.org/10.1080/10406638.2023.2173622 (2023).
Zhang, J. et al. Asymmetric phosphoric acid-catalyzed four-component Ugi reaction. Science 361, eaas8707 (2018).
Ugi, I., Meyr, R., Fetzer, U. & Steinbrückner, C. Versuche mit isonitrilen. Angew. Chem. Int. Ed. 71, 386 (1959).
Pan, S. C. & List, B. Catalytic three-component Ugi reaction. Angew. Chem. Int. Ed. 47, 3622–3625 (2008).
Shi, Y., Wang, Q. & Gao, S. Recent advances in the intramolecular Mannich reaction in natural products total synthesis. Org. Chem. Front. 5, 1049–1066 (2018).
Arrayás, R. G. & Carretero, J. C. Catalytic asymmetric direct Mannich reaction: a powerful tool for the synthesis of α,β-diamino acids. Chem. Soc. Rev. 38, 1940–1948 (2009).
Noble, A. & Anderson, J. C. Nitro-Mannich reaction. Chem. Rev. 113, 2887–2939 (2013).
Zuend, S. J., Coughlin, M. P., Lalonde, M. P. & Jacobsen, E. N. Scalable catalytic asymmetric strecker syntheses of unnatural α-amino acids. Nature 461, 968–970 (2009).
Wang, J., Liu, X. & Feng, X. Asymmetric srecker reactions. Chem. Rev. 111, 6947–6983 (2011).
Kappe, C. O. 100 years of the biginelli dihydropyrimidine synthesis. Tetrahedron 49, 6937–6963 (1993).
Wu, H. B., Wang, Z. M. & Tao, L. The Hantzsch reaction in polymer chemistry: synthesis and tentative application. Polym. Chem. 8, 7290–7296 (2017).
Hu, X. et al. Enantioselective catalytic hantzsch dihydropyridine synthesis. ACS Catal. 13, 6675–6682 (2023).
Ruijter, E., Scheffelaar, R. & Orru, R. V. A. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 50, 6234–6247 (2011).
Lai, Z. et al. Multicomponent double Mannich alkylamination involving C(sp2)-H and benzylic C(sp3)-H bonds. Nat. Commun. 13, 435–442 (2022).
Wang, C., Lai, Z., Xie, H. & Cui, S. Triazenyl alkynes as versatile building blocks in multicomponent reactions: diastereoselective synthesis of β-amino amides. Angew. Chem. Int. Ed. 60, 5147–5151 (2020).
Huang, B., Zeng, L., Shen, Y. & Cui, S. One-pot multicomponent synthesis of β-amino amides. Angew. Chem. Int. Ed. 56, 4565–4568 (2017).
Kumar, R., Flodén, N. J., Whitehurst, W. G. & Gaunt, M. J. A general carbonyl alkylative amination for tertiary amine synthesis. Nature 581, 415–421 (2020).
Trobridge, A., Reich, D. & Gaunt, M. J. Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation. Nature 561, 522–527 (2018).
Klose, I., Mauro, G. D., Kaldre, D. & Maulide, N. Inverse hydride shuttle catalysis enables the stereoselective one-step synthesis of complex frameworks. Nat. Chem. 14, 1306–1310 (2022).
Bandarage, U. K., Kuehne, M. E. & Glick, S. D. Total syntheses of racemic albifloranine and its anti-addictive congeners, including 18-methoxycoronaridine. Tetrahedron 55, 9405–9424 (1999).
Acknowledgements
We thank Zhenjun Mao (Department of Chemistry, Zhejiang University) and Jianyang Pan (Research and Service Center, College of Pharmaceutical Sciences, Zhejiang University) for performing NMR and HRMS spectrometry. We thank Jiyong Liu (Department of Chemistry, Zhejiang University) for performing the x-ray analysis. We are grateful for financial support from the NSFC (21971222, 22277106), the National Program for Support of Top-notch Young Professionals (grant 2022), the Natural Science Foundation of Zhejiang Province (Distinguished Young Scholar Program, LR23H300001), the Zhejiang Provincial Key R&D Program (grant 2023C03118).
Author information
Authors and Affiliations
Contributions
S.C. conceived and directed the project. Experiments and data analysis were conducted by J.L., W.Z., Z.L., and L.Z., S.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Gianfranco Favi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Lai, Z., Zhang, W. et al. Modular assembly of indole alkaloids enabled by multicomponent reaction. Nat Commun 14, 4806 (2023). https://doi.org/10.1038/s41467-023-40598-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40598-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.