Abstract
Antibiotic exposure at the beginning of life can lead to increased antimicrobial resistance and perturbations of the developing microbiome. Early-life microbiome disruption increases the risks of developing chronic diseases later in life. Fear of missing evolving neonatal sepsis is the key driver for antibiotic overtreatment early in life. Bias (a systemic deviation towards overtreatment) and noise (a random scatter) affect the decision-making process. In this perspective, we advocate for a factual approach quantifying the burden of treatment in relation to the burden of disease balancing antimicrobial stewardship and effective sepsis management.
Similar content being viewed by others
Introduction
Physicians caring for neonates presenting with respiratory distress or other nonspecific symptoms often prescribe empiric antibiotics because early-onset sepsis (EOS) cannot be ruled out. The rationale behind this practice is the possible risk of infection and the potential lifesaving effect of early initiation of antibiotics. Serious bacterial infections lead to the death of around 400,000 newborns worldwide each year and puts survivors at risk of lifelong disability1,2. Antibiotic therapy is one of the key achievements in medicine in the last century, reducing infection-related morbidity and mortality3. For neonates with culture-proven sepsis, prompt initiation of antibiotic treatment is undoubtedly lifesaving4. However, the potential involvement of bacterial infections in many clinical syndromes, together with the limited accuracy of current sepsis diagnostic tests resulted in a massive use of antibiotics5. After implementation of Group B streptococcal sepsis prevention strategies in the 1990s, the incidence of culture-proven EOS has substantially decreased in high-income countries, and is continuing to go down in the new millennium6,7,8. Mortality of neonatal sepsis has similarly decreased in high-income countries9. Despite these positive changes, antibiotics are still the most commonly prescribed medication during the first days of life6,9. Up to 14% of late-preterm and term neonates and up to 90% of extremely preterm neonates receive intravenous antibiotics10. This exposure is disproportionate to the burden of disease, as only 0.5 to 2% of neonates treated with antibiotics have a culture-proven bacterial infection11.
During the last decades, the impact of misuse and overuse of antibiotic therapy on growing antimicrobial resistance (AMR) has been increasingly acknowledged as a global public health threat. The World Health Organization (WHO) has called for urgent action to improve antibiotic treatment by increased awareness and evidence through education, surveillance and research to avoid AMR crisis12. Whereas the impact of antibiotic use on AMR is well known, evidence as to the effect of antibiotic therapy on the developing microbiome is relatively new and dynamic13. This is particularly important at the beginning of life, where the commonly prescribed course of 48 h of empiric antibiotic therapy for suspected sepsis in term infants may exert major effects on the microbiome and AMR gene selection, which remain detectable at one year14,15. In preterm infants, unwarranted and prolonged antibiotic exposure within the first few weeks of life may increase the subsequent risk of necrotizing enterocolitis, bronchopulmonary dysplasia, late-onset sepsis and death. Some studies, with an inherent bias due to the retrospective design, reported conflicting results; reinforcing the need for well-powered, prospective studies to clarify the causal relationship and the effect-size16,17,18,19,20. Further, early life antibiotic exposure is associated with future health problems like obesity, allergic predisposition, asthma, diabetes, juvenile idiopathic arthritis, celiac and inflammatory bowel disease14,21,22. Whereas causal associations between early exposure to antibiotics, changes in the microbiome and diseases presenting later in life are not fully understood or analysed, growing evidence from the current literature underlines the interplay between the microbiome and genomics, proteomics and metabolomics. Various mechanisms within the gut-brain, gut-lung, and gut-skin axis, and immune modulation are reported to contribute to the pathogenesis for diseases presenting later in life23,24,25,26,27,28,29,30. The example of microbe-induced obesity may serve as proof of concept: In a mouse-model, Cox et al. were able to proof the causal role of penicillin-altered microbiome for long-term metabolic changes and obesity31. Therefore, lowering exposure to antibiotics at the beginning of life may reduce the risk for future chronic health conditions. That is why less antibiotics at the beginning of life is urgently needed.
In this perspective, we review the current state of antibiotic exposure at the beginning of life and the incidence and outcome of culture-proven EOS. We hypothesize that a factual approach quantifying the burden of treatment in relation to the burden of disease may help balancing antibiotic stewardship and efficient sepsis management.
The current state
There is an inappropriate antibiotic exposure in relation to the number of culture-proven infections at the beginning of life with a potentially high societal cost of treatment due to AMR-development and perturbations of the developing microbiome13,32,33,34,35. In 2019, an estimated 5 million people worldwide died from illnesses in which bacterial AMR played a part, including 1.27 million deaths directly caused by drug resistant infections13. The number of people living with chronic conditions potentially triggered by early-life microbiome disruption is increasing, leading to substantial mortality and morbidity36. Obesity and diabetes mellitus show an exponential rise within the last decades, and are associated with subsequent mortality due to cardiovascular disease37. Globally, the prevalence of asthma has increased to 11.5% in the population aged between 5 and 69 years38,39. Inflammatory bowel disease (IBD) has a high impact on individual health and on healthcare systems. In the 21st century, IBD has become a global disease with a prevalence of 0.3% in Europe and North America and its incidence is rising in newly industrialized countries from Asia, South America and Africa40. As preventive interventions have a limited success rate, there is an urgent call for new strategies41. Preservation of a healthy microbiome through a more rational use of antibiotics is an important point of action to prevent numerous diseases and improve global health42,43,44,45.
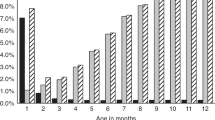
The burden of EOS in high-income countries is decreasing within the new millennium, as the incidence of EOS and sepsis-related mortality are declining in term and late-preterm neonates7. Important to note, the situation in low-income countries may differ due to limitations in access to health care and much higher sepsis-related mortality46. The recently published AENEAS study (Antibiotic Exposure for Suspected Neonatal Early-onset Sepsis) shows the current situation in 13 different networks from Europe, North America, and Australia11. In a cohort of 757,979 late-preterm and term infants, 21,703 (2.9%) infants received intravenous antibiotics during the first postnatal week. This proportion is in the lower range compared to the literature with reports up to 14% all infants treated32,47,48,49. However, wide practice variation from 1.2% to 12.5% of all infants started on antibiotics was observed among different networks11. Infants with culture-proven EOS were treated for a median of 9 (IQR 7–14) days and those who were started on antibiotics but did not have a positive blood culture were treated for a median of 4 (IQR 3–6) days, in line with the recent literature, but in contrast to international guidelines requesting to discontinue antibiotics after 36 to 48 h50,51,52. Within the AENEAS cohort, the incidence of culture proven EOS was 0.49/1000 live births (range 0.18–1.45) and EOS-associated case fatality was 3.2%11. In the recent literature, EOS rates between 0.13 and 0.95/1000 term and late-preterm live births were reported in Europe, the United States and Australia32,53,54,55,56,57. Reports from these areas show a low mortality due to EOS and are in line with the AENEAS study results32,50,53,58. Between 50 to more than 100 term and late-preterm neonates are therefore started on antibiotics for each case of EOS (Fig. 1). Thus, antibiotic exposure at the start of life is very high, and the risks and cost of treatment are inappropriate compared to the burden of disease. Therefore, a new balance between effective sepsis care and antimicrobial stewardship (AMS) is urgently needed.
The rate of antibiotic exposure at the beginning of life of life varies between 1.2 and 14%11,32,47,48,49. The incidence of culture-proven early-onset sepsis (EOS) varies between 0.13 and 1.45/1000 term and late-preterm neonates11,32,53,54,55,56,57. Therefore, between 50 to more than 100 term and late-preterm neonates are started on antibiotics for each case of culture-proven EOS.
A number of today’s pediatricians and neonatologists were trained in an era with a prevailing mind-set that it is better to treat every neonate with clinical signs possibly related to bacterial infection, even if the risk of sepsis is low. Shifting this paradigm requires a culture change59. Initiating and implementing change successfully first requires an understanding of why change is necessary. The 8-steps-framework for leading change by John Kotter requests to create a sense of urgency as a mandatory first step, which was successfully applied in different healthcare setting60,61. The current overexposure of antibiotics at the beginning of life and its unintended consequences are why we must change.
The needed change: A factual approach
Given that more than 98% of neonates started on antibiotics do not turn out to have a microbiologically documented infection, there is enormous potential for improving antibiotic prescription practices11,32. Currently, guidelines to decide when to initiate antibiotics in the first days of life show wide variations among countries62. There is no obvious single best strategy to safely reduce neonatal antibiotic exposure at the beginning of life and improve the balance of efficient sepsis care and AMS11.
AMS is a key topic in current neonatal care and many clinicians are focused on improving antibiotic prescription practices. Nevertheless, safety concerns, fear of medical malpractice litigation, peer pressure and the tendency not to change antibiotic treatments started by colleagues commonly overrule AMS intentions63. Fear to miss a sepsis case is a key driver for antibiotic prescriptions in medicine64. The perceived vulnerability of children and seeking safety in the face of uncertainty are two important aspects guiding the decision to start antibiotics in paediatric care (Fig. 2)63,65. A qualitative study in paediatric intensive care regarding decision making for antibiotic therapy observed an overruling of the clinical reasoning process by disease severity and safety concerns63. Importantly, possible adverse effects of antibiotics play a minor role in the prescribing process66. Fear however is a poor advisor for decision making and we have to calculate the risk to control the fear instinct67. A factual approach using data regarding the absolute risk of EOS and sepsis-related adverse outcomes may help to overcome the fear instinct and to improve the balance between efficient sepsis care and AMS.
Fear to miss a true sepsis case and the impact of bias and noise on decision-making are important factors contributing to antibiotic overtreatment. In addition, there is a time preference for now with a discount over time for potential late benefits: the perceived immediate safety of antibiotic treatment for suspected sepsis is of much higher value than the possible decrease in risk for chronic diseases years or decades later71. These challenges of decision-making result in overtreatment with antibiotics at the beginning of life.
Whereas fear can induce a bias with systematic deviation towards increased antibiotics use, many other factors impact neonatal antibiotic prescription. In addition to objective differences between guidelines, patient populations and implemented AMS programs, it is difficult to assess the impact of habits, collaboration across hierarchy and previous experiences68,69,70. The theory of time preference of behaviour and economic scholarship offers another explanation on how physicians trade off time and outcomes: there is a time preference for now with a discount over time for potential late benefits71. In this context, the perceived immediate safety of antibiotic treatment for suspected sepsis is of much higher value than the possible decrease in risk for chronic diseases years or decades later (Fig. 2). Nevertheless, with a factual approach illustrating the true risk, the bias from time preference may be reduced72. In addition, physicians make different decisions in similar situation at different points in time73. This phenomenon is described as noise, resulting in a random scatter of decision making (Fig. 2)74.
Physicians caring for neonates with nonspecific clinical signs such as respiratory distress often prescribe antibiotics due to their concern that the patient may have bacterial sepsis. This judgment is only verifiable if the blood cultures become positive. Otherwise, the quality of the decision remains blurred and only the quality of the decision-making process can be assessed74. However, this seldom happens and an inadequate circle based on causal thinking, confirmation and desirability bias may lead to overconfidence of physicians (Box 1). Learning from error is difficult. Failure can trigger a threatening feeling and these emotions may hinder learning75. An important step for every critical review and to enter a circle of learning (Box 1) is to use measures of decision hygiene74. The most important measure of decision hygiene is to take an outside view and to assess the baseline-rate of events. As for the fear instinct, a factual approach may help to improve the decision-making process by taking the outside perspective analysing baseline-rates and outcomes. Different baseline-rates and outcomes in different settings may further highlight the importance of using a factual approach.
The first steps towards a factual approach
In discussions with neonatologists, antibiotic prescriptions are often not acknowledged as a problem. This statement is rarely supported by data. To think we are doing well, is not good enough—we must know. The first step towards a factual approach is to measure one’s own performance (Fig. 3). Measuring performance is a mandatory step to get better76. Auditing routinely available outcome data is becoming more common and may help to overcome this gap. Importantly, weaknesses and shortcomings in routine data research is a cause of concern and must be addressed77. A recently published WHO review regarding the use of digital health technologies reported an increasing problem of global inequity78. Transparent and open-access data presentation is key to minimize inequity. Therefore, we must measure our own performance and make these data available to all physicians involved in care in every institution.
The first step towards a factual approach is to measure one’s own performance and to make data available within the institution. International, open-access data presentation may help for collaboration and benchmarking improving learning and decision-making. In future, algorithms and artificial intelligence will play an increasing role for individualized decision-making, taking into account routine data and new variables. In the end, a factual approach may help to balance effective sepsis care and antimicrobial stewardship.
The COVID-19 pandemic showed us the potential benefit of international collaboration and transparent data sharing. Timely and accurate presentation of data with dashboards was the base for benchmarking and collaborative learning. The team of the Johns Hopkins University in Baltimore reported several challenges with their collected data, such as ambiguous and inconsistent parameter definition, and lack of reporting standardization including support for machine-readable data and unstable reporting practices in terms of metrics and frequency79. Lessons learnt from scientific data presentation include freely accessible results as standardised graphs and figures with a high grade of simplicity and clarity, attention to concise titles and subtitles, regular feedback of users to facilitate the education of people and ensuring trust by full transparency80. In addition, the authors conclude that the positive experience of the COVID-19-dashboard may be transferred to other topics or other medical diseases80.
The conduct of the AENEAS study allowed us to learn important lessons to take the next step in our endeavour to reduce antibiotic use at start of life without affecting sepsis-related outcomes and to improve the balance between efficient sepsis care and AMS11. Feasibility, high data quality and insightful data presentation were key factors. Therefore, most important for feasibility and high data quality is the definition of a standardized, minimal data set with concise definitions of parameters. The AENEAS study may serve as a pilot trial. With only 6 key parameters we were able to analyse the burden of disease (1. incidence of EOS; 2. sepsis-related mortality), the burden of antibiotic treatment (3. proportion of live births started on antibiotics; 4. duration of antibiotic treatment) and a baseline description of the cohort (5. gestational age; 6. all-cause neonatal mortality)11. The timely, transparent display of data within a dashboard is the main difference from more conventional registries. Data of registries are often only accessible after being published and transparency is limited to participating centres81. In conclusion, international collaboration and benchmarking of open-accessible data may leverage learning and decision-making to improve care at the beginning of life.
Due to the low incidence of culture-proven EOS and sepsis-related mortality, randomized clinical trials (RCT) require a very large study population to prove the safety of an approach51. In addition, RCTs in neonatal sepsis are challenging and not always feasible due to high ethical requirements, difficulties to obtain parental consent during an emotional time period, and high complexities and costs. Nevertheless, the recently published REASON trial demonstrates that a RCT evaluating the effect of antibiotics on the microbiome, metabolome and immune markers at the beginning of life is feasible82,83. On the other hand, new clinical trial designs using routine data from the real world may help to overcome these problems84. The aim is to develop a tight link between clinical care and research. Therefore, we need a change in the mind-set of many care providers: On one hand, as physicians, we are responsible to deliver the best possible care. On the other hand, clinical research is mandatory to define best possible care. The new stream of clinical research, using routine data from the real world, necessitates that physicians and other health care workers act as care providers and as clinical researchers: To deliver the best possible care and to strive to get better84. Therefore, the development of new algorithms including artificial intelligence analysing routine data may help to reach the next level of a factual approach. In the future, data-based individualization including results from multiomics analyses may further improve the accuracy of prediction85,86,87.
Limitations and next steps
The strengths of the AENEAS six-key point dashboard to consolidate a factual approach lies in the feasibility and standardized data including two dimensions; the burden of disease and the burden of treatment11. The presentation of both dimensions is mandatory to balance efficient sepsis care and AMS. However, such a dashboard also has limitations. First, AENEAS was limited to late-preterm and term neonates with suspected EOS. Antibiotic exposure of more immature preterm infants with suspected EOS and late-onset sepsis (LOS) needs to be evaluated as well and is achievable, with sufficient resources. Whereas very preterm infants account for less than 2% of life births, the rate of EOS (13.5/1000 very preterm infants) and LOS (93/1000 very preterm infants) are significantly higher88,89,90. Secondly, due to the minimal data set, more in-depth information regarding morbidity of EOS and management strategies are lacking. With a more detailed data sampling in a subset of patient cohorts and centres, we may mitigate these limitations. Analyses of more detailed and richer data sets may reveal currently unappreciated risk profiles affecting antibiotic treatment thresholds and management strategies. In addition, there is a need for regular quality improvement cycles after start of the initiative and the initial data set may be changed accordingly.
Most importantly, a dashboard showing the burden both of confirmed EOS cases as well as antibiotic exposure is not the final aim. Registry data are rarely used to evaluate the impact of therapeutic interventions on patient outcomes81. To allow collaborative learning and to promote change, longitudinal presentation of standardized key indicators and transparency are mandatory91,92. Longitudinal (inter)national benchmarking allows clinicians to assess their own performance and to guide improvements93. The development of adjustable benchmarks may facilitate global use in diverse settings. Insightful data presentation showing the balance between the burden of EOS and the burden of antibiotic treatment may help to guide clinicians regarding future steps to improve their performance and to foster collaborative learning. Among many challenges ahead, the most urgent is to develop better diagnostic tools for neonatal sepsis. Non-bacterial infections can also cause sepsis, and the lack of a clear definition impedes our studies and efforts to improve the management of neonatal sepsis94. A recent review demonstrated the highly variable sepsis definitions used even in clinical trials95. Whereas adult and pediatric sepsis definitions mandate evidence of organ dysfunctions, neonatal sepsis definitions are generally restricted to clinical signs, microbiology, and laboratory results, and intention to antibiotic treatment.
Culture-negative sepsis represents another common, poorly defined and challenging condition. The AENEAS study reported only on culture-positive EOS. There is no consensus definition of culture-negative sepsis and in literature, the rate of culture-negative sepsis is reported to be 6–16 times higher than culture-positive sepsis70. Neonatal, culture-negative sepsis exists, but non-infectious conditions that mimic sepsis are far more common and include prematurity, transient tachypnea of the neonate, surfactant deficiency, persistent pulmonary hypertension and many more. Fear of true culture-negative sepsis is a driver for prolonged antibiotic therapy. Optimal blood culture technique and intensified diagnostic search for non-bacterial sepsis-like causes are key in every neonate with suspected EOS. In their recently published framework, Cantey et al. used a factual approach to tackle the overdiagnosis of culture-negative sepsis: They calculated the risk of culture-negative sepsis based on the incidence of sepsis, the probability of bacterial detection in a culture, and the risk of bacterial ultra-low concentration below the detection threshold of cultures with a need of antibiotic treatment. They concluded that even with conservative assumption, the rate of culture-negative sepsis should be 8 to 10 times lower than the rate of culture-positive sepsis35. This is around 100 times lower than observed in the literature11,70. New and improved blood culture techniques, viral and bacterial detection with PCR panels, and omics techniques that can detect host response to microbial invasion are potentially beneficial techniques when considering the differential diagnosis of culture-negative sepsis. Thus, rule-out sepsis is not a diagnosis, and culture-negative sepsis is most likely often a wrong diagnosis.
In the recent literature, the EOS-calculator has been the most discussed approach to reduce antibiotic therapy for suspected EOS in late preterm and term infants48. The EOS-calculator is a fact-based model including the local incidence of EOS, maternal risk factors and neonatal clinical signs resulting in management advice. In settings of high exposure, studies using the EOS-calculator report a substantial reduction in the use of antibiotics in the first week of life48. The impact of the EOS-calculator in settings with a strong culture of AMS is not studied and therefore debatable. The risk threshold of 3 EOS-cases out of 1000 as an indication to start antibiotics may be a possible reason for the questionable benefit of the EOS-calculator in settings with relatively low antibiotic use48. Moreover, the EOS-calculator does not identify all cases of EOS, but this is also the case with other approaches96. Interestingly, accepting an algorithm only until an error or limitation is found is a general known barrier in the implementation of algorithms for decision making, even if reports prove the overall superiority of the algorithm74. The AENEAS study reported networks with a substantially lower antibiotic use as reported with the use of the EOS-calculator. A strategy of serial physical examination has been associated with markedly lower antibiotic exposure than in networks using the EOS-calculator, but more prospective studies are needed97.
As we know from the management literature, there is no lack of good ideas in the world, but a lack of implemented good ideas98. The AENEAS study was a start to show the feasibility and possible analyses for a factual approach. An open-access data dashboard including international data from high-, middle- and low-income countries may help to leverage collaborative learning and new thinking within an initiative aiming to conciliate efficient sepsis care and AMS.
Conclusions
Antibiotic exposure at the start of life is high and the risks and costs of treatment are disproportionate compared to the burden of EOS. Fear to miss a true sepsis case, the impact of bias, noise, and time preference on decision-making are important factors contributing to antibiotic overtreatment. We hypothesize that the decision-making process regarding antibiotic therapy at the beginning of life may be improved with a factual approach. First, we must measure one’s own performance and make these data available. This feedback may produce a sense of urgency regarding the needed change of behaviour. Secondly, collaboration and benchmarking using an open-access dashboard quantifying the burden of disease versus the burden of treatment may be an important step to initiate a global initiative to conciliate effective sepsis care and antimicrobial stewardship. In particular, the focus should be made on educational interventions to improve the decision-making process regarding the start and the stop of antibiotics. And thirdly, the development of new algorithms using artificial intelligence analysing routine data may leverage decision-making to the next level of a factual approach. The aim of a preserved microbiome and reduced antimicrobial resistance may improve the health of future generations.
References
Perin, J. et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc. Health 6, 106–115 (2022).
Fleischmann, C. et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch. Dis. Child. 106, 745–752 (2021).
Armstrong, G. L., Conn, L. A. & Pinner, R. W. Trends in infectious disease mortality in the United States during the 20th century. JAMA 281, 61–66 (1999).
Sankar, J. et al. Delayed administration of antibiotics beyond the first hour of recognition is associated with increased mortality rates in children with sepsis/severe sepsis and septic shock. J. Pediatr. 233, 183–190.e3 (2021).
Magalhães, C., Lima, M., Trieu-Cuot, P. & Ferreira, P. To give or not to give antibiotics is not the only question. Lancet Infect. Dis. 21, e191–e201 (2021).
Stark, A. et al. Medication Use in the Neonatal Intensive Care Unit and Changes from 2010 to 2018. J. Pediatr. 240, 66–71.e4 (2022).
Benitz, W. E. & Achten, N. B. Finding a role for the neonatal early-onset sepsis risk calculator. EClin.Med. 19, 100255 (2020).
Verani, J. R., McGee, L. & Schrag, S. J., Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease-revised guidelines from CDC, 2010. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 59, 1–36 (2010).
Agyeman, P. K. A. et al. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study. Lancet Child Adolesc. Health 1, 124–133 (2017).
Flannery, D. D. et al. Temporal trends and center variation in early antibiotic use among premature infants. JAMA Netw. Open 1, e180164 (2018).
Giannoni, E. et al. Analysis of antibiotic exposure and early-onset neonatal sepsis in Europe, North America, and Australia. JAMA Netw. Open 5, e2243691 (2022).
WHO | Global action plan on AMR. WHO http://www.who.int/antimicrobial-resistance/global-action-plan/en/.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet Lond. Engl. 399, 629–655 (2022).
Reyman, M. et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: a randomized trial. Nat. Commun. 13, 893 (2022).
Stiemsma, L. T. & Michels, K. B. The Role of the Microbiome in the Developmental Origins of Health and Disease. Pediatrics 141, (2018).
Dierikx, T. H. et al. Association between duration of early empiric antibiotics and necrotizing enterocolitis and late-onset sepsis in preterm infants: a multicenter cohort study. Eur. J. Pediatr. 181, 3715–3724 (2022).
Vatne, A. et al. Early empirical antibiotics and adverse clinical outcomes in infants born very preterm: a population-based cohort. J. Pediatr. S0022-3476(22), 00851–00854 (2022).
Ting, J. Y. et al. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics 143, e20182286 (2019).
Krediet, T. G. et al. Microbiological factors associated with neonatal necrotizing enterocolitis: protective effect of early antibiotic treatment. Acta Paediatr. Acta Paediatr. Oslo Nor. 1992 92, 1180–1182 (2003).
Berkhout, D. J. C. et al. Risk factors for necrotizing enterocolitis: a prospective multicenter case-control study. Neonatology 114, 277–284 (2018).
Dydensborg Sander, S. et al. Association between antibiotics in the first year of life and celiac disease. Gastroenterology 156, 2217–2229 (2019).
Clarke, S. L. N. et al. Moving from nature to nurture: a systematic review and meta-analysis of environmental factors associated with juvenile idiopathic arthritis. Rheumatol. Oxf. Engl. 61, 514–530 (2022).
VanEvery, H., Franzosa, E. A., Nguyen, L. H. & Huttenhower, C. Microbiome epidemiology and association studies in human health. Nat. Rev. Genet. 24, 109–124 (2023).
Brodin, P. Immune-microbe interactions early in life: a determinant of health and disease long term. Science 376, 945–950 (2022).
Dhariwala, M. O. & Scharschmidt, T. C. Baby’s skin bacteria: first impressions are long-lasting. Trends Immunol. 42, 1088–1099 (2021).
Stevens, J. et al. The balance between protective and pathogenic immune responses to pneumonia in the neonatal lung is enforced by gut microbiota. Sci. Transl. Med. 14, eabl3981, (2022).
Constantinides, M. G. & Belkaid, Y. Early-life imprinting of unconventional T cells and tissue homeostasis. Science 374, eabf0095 (2021).
Deshmukh, H. S. et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 20, 524–530 (2014).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Radjabzadeh, D. et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 13, 7128 (2022).
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Schulman, J. et al. Newborn antibiotic exposures and association with proven bloodstream infection. Pediatrics 144, (2019).
Kimpton, J. A. et al. Comparison of NICE guideline CG149 and the sepsis risk calculator for the management of early-onset sepsis on the postnatal ward. Neonatology 118, 562–568 (2021).
Schulman, J. et al. Variations in neonatal antibiotic use. Pediatrics 142, e20180115 (2018).
Cantey, J. B. & Prusakov, P. A proposed framework for the clinical management of neonatal ‘Culture-Negative’ sepsis. J. Pediatr. 244, 203–211 (2022).
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond. Engl. 386, 743–800 (2015).
Glovaci, D., Fan, W. & Wong, N. D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep. 21, 21 (2019).
Song, P. et al. Global, regional, and national prevalence of asthma in 2019: a systematic analysis and modelling study. J. Glob. Health 12, 04052 (2022).
Serebrisky, D. & Wiznia, A. Pediatric asthma: a global epidemic. Ann. Glob. Health 85, 6 (2019).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet Lond. Engl. 390, 2769–2778 (2017).
Beasley, R., Semprini, A. & Mitchell, E. A. Risk factors for asthma: is prevention possible? Lancet Lond. Engl. 386, 1075–1085 (2015).
Jie, Z. et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8, 845 (2017).
Zhang, Y. et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 11, 5015 (2020).
Zhu, F. et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 11, 1612 (2020).
Pathak, J. L., Yan, Y., Zhang, Q., Wang, L. & Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 185, 106475 (2021).
Kissoon, N. & Uyeki, T. M. Sepsis and the global burden of disease in children. JAMA Pediatr. 170, 107–108 (2016).
Goel, N. et al. Implementation of an adapted Sepsis Risk Calculator algorithm to reduce antibiotic usage in the management of early onset neonatal sepsis: a multicentre initiative in Wales, UK. Arch. Dis. Child. Fetal Neonatal Ed. 107, 303–310 (2022).
Achten, N. B. et al. Association of use of the neonatal early-onset sepsis calculator with reduction in antibiotic therapy and safety: a systematic review and meta-analysis. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2019.2825 (2019).
Kuzniewicz, M. W. et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 171, 365–371 (2017).
Mundal, H. S., Rønnestad, A., Klingenberg, C., Stensvold, H. J. & Størdal, K. Antibiotic use in term and near-term newborns. Pediatrics 148, e2021051339 (2021).
Stocker, M. et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet Lond. Engl. 390, 871–881 (2017).
Fjalstad, J. W. et al. Early-onset sepsis and antibiotic exposure in term infants: a nationwide population-based study in Norway. Pediatr. Infect. Dis. J. 35, 1–6 (2016).
Stoll, B. J. et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 174, e200593 (2020).
Braye, K. et al. Epidemiology of neonatal early-onset sepsis in a geographically diverse Australian health district 2006-2016. PLoS ONE 14, e0214298 (2019).
Cailes, B. et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch. Dis. Child. Fetal Neonatal Ed. 103, F547–F553 (2018).
Schrag, S. J. et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics 138, e20162013 (2016).
Giannoni, E. et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J. Pediatr. 201, 106–114.e4 (2018).
Escobar, G. J. et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics 133, 30–36 (2014).
Cantey, J. B. & Baird, S. D. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics 140, (2017).
Campbell, R. J. Change management in health care. Health Care Manag 39, 50–65 (2020).
Ravi, S., Patel, S. R., Laurence, S. K., Sebok-Syer, S. S. & Gharahbaghian, L. Kotter’s 8 stages of change: implementation of clinical screening protocols for assessing patients for COVID-19 - a review of an academic medical centre’s preparedness. BMJ Lead. 6, 319–322 (2022).
van Herk, W. et al. Variation in current management of term and late-preterm neonates at risk for early-onset sepsis: an international survey and review of guidelines. Pediatr. Infect. Dis. J. 35, 494–500 (2016).
Fontela, P. S. et al. Clinical reasoning behind antibiotic use in PICUs: a qualitative study. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 23, e126–e135 (2022).
Teixeira Rodrigues, A., Roque, F., Falcão, A., Figueiras, A. & Herdeiro, M. T. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int. J. Antimicrob. Agents 41, 203–212 (2013).
Cabral, C., Lucas, P. J., Ingram, J., Hay, A. D. & Horwood, J. ‘It’s safer to …’ parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: an analysis across four qualitative studies. Soc. Sci. Med. 1982 136–137, 156–164 (2015).
Livorsi, D., Comer, A., Matthias, M. S., Perencevich, E. N. & Bair, M. J. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect. Control Hosp. Epidemiol. 36, 1065–1072 (2015).
Rosling, H., Rosling, O. & Rönnlund, A. R. Factfulness: Ten Reasons We’re Wrong About The World - And Why Things Are Better Than You Think. (Sceptre, 2018).
Steinmann, K. E. et al. Impact of empowering leadership on antimicrobial stewardship: a single center study in a neonatal and pediatric intensive care unit and a literature review. Front. Pediatr. 6, 294 (2018).
Mukhopadhyay, S. et al. Variation in sepsis evaluation across a national network of nurseries. Pediatrics 139, e20162845 (2017).
Klingenberg, C., Kornelisse, R. F., Buonocore, G., Maier, R. F. & Stocker, M. Culture-negative early-onset neonatal sepsis - at the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr. 6, 285 (2018).
Mahboub-Ahari, A., Pourreza, A., Akbari Sari, A., Rahimi Foroushani, A. & Heydari, H. Stated time preferences for health: a systematic review and meta analysis of private and social discount rates. J. Res. Health Sci. 14, 181–186 (2014).
Andreoni, J. & Sprenger, C. Risk preferences are not time preferences. Am. Econ. Rev. 102, 3357–3376 (2012).
Al-Azzawi, R., Halvorsen, P. A. & Risør, T. Context and general practitioner decision-making - a scoping review of contextual influence on antibiotic prescribing. BMC Fam. Pract. 22, 225 (2021).
Kahneman D., Sibony O., Sunstein CR. Noise: A Flaw in Human Judgment. (Little Brown Spark, 2021).
Eskreis-Winkler, L. & Fishbach, A. You think failure is hard? So is learning from it. Perspect. Psychol. Sci. J. Assoc. Psychol. Sci. 17, 1511–1524 (2022).
Gawande, A. Better: A Surgeon’s Notes on Performance. (Macmillan USA, 2008).
Dron, L. et al. Data capture and sharing in the COVID-19 pandemic: a cause for concern. Lancet Digit. Health 4, e748–e756 (2022).
Equity within digital health technology within the WHO European Region: a scoping review. https://www.who.int/europe/publications/i/item/WHO-EURO-2022-6810-46576-67595.
Dong, E. et al. The Johns Hopkins University Center for Systems Science and Engineering COVID-19 Dashboard: data collection process, challenges faced, and lessons learned. Lancet Infect. Dis. S1473–3099, 00434–0 (2022).
Peeples, L. Lessons from the COVID data wizards. Nature 603, 564–567 (2022).
Hoque, D. M. E. et al. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS ONE 12, e0183667 (2017).
Ruoss, J. L. et al. Routine early antibiotic use in symptomatic preterm neonates: a pilot randomized controlled trial. J. Pediatr. 229, 294–298.e3 (2021).
Russell, J. T. et al. Antibiotics and the developing intestinal microbiome, metabolome and inflammatory environment in a randomized trial of preterm infants. Sci. Rep. 11, 1943 (2021).
Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 29, 49–58 (2023).
Mahajan, P. et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA 316, 846–857 (2016).
Serna, E., Parra-Llorca, A., Panadero, J., Vento, M. & Cernada, M. miRNomic signature in very low birth-weight neonates discriminates late-onset gram-positive sepsis from controls. Diagn. Basel Switz. 11, 1389 (2021).
Cernada, M. et al. Genome-wide expression profiles in very low birth weight infants with neonatal sepsis. Pediatrics 133, e1203–e1211 (2014).
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2019).
Flannery, D. D., Edwards, E. M., Puopolo, K. M. & Horbar, J. D. Early-onset sepsis among very preterm infants. Pediatrics 148, e2021052456 (2021).
Huncikova, Z. et al. Late-onset sepsis in very preterm infants in Norway in 2009–2018: a population-based study. Arch. Dis. Child. Fetal Neonatal Ed. fetalneonatal-2022-324977 https://doi.org/10.1136/archdischild-2022-324977 (2023).
Flannery, D. D. et al. Neonatal infections: insights from a multicenter longitudinal research collaborative. Semin. Perinatol. 46, 151637 (2022).
Larsen, G. Y. et al. Development of a quality improvement learning collaborative to improve pediatric sepsis outcomes. Pediatrics 147, e20201434 (2021).
Madden, K. Risk and resistance: examining our antibiotic use. Pediatr. Crit. Care 23, 227–228 (2022).
Wynn, J. L. Defining neonatal sepsis. Curr. Opin. Pediatr. 28, 135–140 (2016).
Hayes, R. et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr. Res. https://doi.org/10.1038/s41390-021-01749-3 (2021).
Achten, N. B. et al. Stratification of culture-proven early-onset sepsis cases by the neonatal early-onset sepsis calculator: an individual patient data meta-analysis. J. Pediatr. https://doi.org/10.1016/j.jpeds.2021.01.065 (2021).
Vatne, A. et al. Reduced antibiotic exposure by serial physical examinations in term neonates at risk of early-onset sepsis. Pediatr. Infect. Dis. J. 39, 438–443 (2020).
Malik, F. Führen Leisten Leben: Wirksames Management für eine neue Welt, plus E-Book inside. (Campus Verlag, 2019).
Hollnagel, E. Safety-I and Safety-II: The Past and Future of Safety Management. (Routledge, 2014).
Bartman, T., Merandi, J., Maa, T., Kuehn, S. & Brilli, R. J. Developing tools to enhance the adaptive capacity (Safety II) of Health Care Providers at a Children’s Hospital. Jt. Comm. J. Qual. Patient Saf. 47, 526–532 (2021).
Author information
Authors and Affiliations
Contributions
M.S. and E.G. conceptualised the manuscript. M.S. wrote the first draft. C.K., L.N., V.N., A.B., S.H., G.F., J.B., D.L., V.D., N.G., J.S., P.M., D.L., J.M., J.J., P.A., R.P., G.L., G.M., N.L., E.M., K.S., T.S., E.G. contributed substantially by revising the manuscript. All authors approved the submitted version and are fully accountable for every aspect of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stocker, M., Klingenberg, C., Navér, L. et al. Less is more: Antibiotics at the beginning of life. Nat Commun 14, 2423 (2023). https://doi.org/10.1038/s41467-023-38156-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-38156-7
This article is cited by
-
Antibiotic use in infants at risk of early-onset sepsis: results from a unicentric retrospective cohort study
BMC Pediatrics (2024)
-
A decade of neonatal sepsis in Stockholm, Sweden: Gram-positive pathogens were four times as common as Gram-negatives
European Journal of Clinical Microbiology & Infectious Diseases (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.