Abstract
The performances of rechargeable batteries are strongly affected by the operating environmental temperature. In particular, low temperatures (e.g., ≤0 °C) are detrimental to efficient cell cycling. To circumvent this issue, we propose a few-layer Bi2Se3 (a topological insulator) as cathode material for Zn metal batteries. When the few-layer Bi2Se3 is used in combination with an anti-freeze hydrogel electrolyte, the capacity delivered by the cell at −20 °C and 1 A g−1 is 1.3 larger than the capacity at 25 °C for the same specific current. Also, at 0 °C the Zn | |few-layer Bi2Se3 cell shows capacity retention of 94.6% after 2000 cycles at 1 A g−1. This behaviour is related to the fact that the Zn-ion uptake in the few-layer Bi2Se3 is higher at low temperatures, e.g., almost four Zn2+ at 25 °C and six Zn2+ at −20 °C. We demonstrate that the unusual performance improvements at low temperatures are only achievable with the few-layer Bi2Se3 rather than bulk Bi2Se3. We also show that the favourable low-temperature conductivity and ion diffusion capability of few-layer Bi2Se3 are linked with the presence of topological surface states and weaker lattice vibrations, respectively.
Similar content being viewed by others
Introduction
Performance loss or failure occurs in nearly all types of cell systems (e.g. lithium/sodium/magnesium/zinc ion cell) in cold climates1,2,3. Advanced batteries imperatively need powerful energy density and excellent lifespan, even at subzero temperatures. Substantial effort has been devoted to investigating cell degradation at low temperature and proposing approaches for performance enhancement4,5. Several mechanisms are generally responsible for poor cell performance at low temperatures: (a) a reduced rate of ion transfer in the electrode materials; (b) lower-than-usual electronic conductivity of the electrodes, especially for the widely used metal oxide electrodes; (c) lower-than-usual ionic conductivity of the electrolyte; and (d) sluggish charge-transfer kinetics induced by a decreased rate of chemical reactions. Fading electrochemical performance at low temperatures can be mitigated by introducing electrolyte additives6, coating surfaces with some material that is highly electronically conductive7,8, and heteroatom doping9,10, but the attenuation is inevitable, and to date the highest retention of 86% at −25 °C for a sodium ion cell is achieved by Goodenough’s group using an organic electrolyte5. Although this progress is valuable, for a cell required to operate over a long period in a cold climate, performance degradation remains unavoidable5. In addition, previously proposed methods of low-temperature cell performance enhancement largely focus on electrolyte modification, which cannot solve the sluggish kinetics of electrode reactions.
Recently, a family of materials, namely topological insulators with unique thermal and electrical properties, gain widespread interests11,12. These particular insulators have exotic metallic states formed by topological protection; their interiors function as insulators, but their surfaces act as conductors because their electrons are subject to strong spin-orbit interaction existing on their electrons13. Moreover, topological effects give these materials an inherent advantage: their properties are constant under any deformations. Bi2Se3, a topological insulator with a 0.3-eV nontrivial bulk gap and superficial single Dirac cone, is formed by periodic layers composed of five atomic planes (Se-Bi-Se-Bi-Se; namely, a quintuple layer denoted QL) connected through van der Waals forces; numerous unoccupied tetrahedral and octahedral exist between the Se atomic planes and result in the material’s potential as an intercalating cathode in batteries14,15. Especially, for low-dimensional Bi2Se3, the reduced concentration of bulk carriers highlights the contribution of conductivity from surface topological states. Particularly, the conductivity of most of the Bi2Se3 nanoribbon devices increases as temperature goes down16, and this metallic behavior provides an opportunity to circumvent the issue of cell electrodes operating at low temperatures. As topological surface states of Bi2Se3 protected by time inversion symmetry are robust at near ambient temperatures with exposure to liquids17,18,19,20,21,22, topological quantum states of Bi2Se3 have potential applications in cell environments.
Herein, we report an aqueous Zn ion cell (AZIB) consisting of a few QLs of Bi2Se3 nanosheets (E-Bi2Se3) as the cathode, a Zn anode, and an antifreeze polyacrylamide (PAM) hydrogel electrolyte with highly concentrated salts incorporated along with ethylene glycol (denoted HC-EGPAM). Bulk Bi2Se3 powder (P-Bi2Se3) is readily exfoliated into E-Bi2Se3 through hydrothermal intercalation to enhance the coupling advantage in topological surface states. Surprisingly, the Zn||E-Bi2Se3 cell exhibits abnormally low-temperature performance (a capacity of up to 524 mAh g−1 at −20 °C and 0.3 A g−1) that is even better than its performance at 25 °C (a capacity of 327 mAh g−1 at 0.3 A g−1) and all previously reported measurements of low-temperature batteries. Even at −40 °C, the capacity retentions remain to 106 and 113% compared to the capacities at 25 °C. Specifically, per molecular E-Bi2Se3 holds up to four Zn2+ on discharge at 25 °C and six Zn2+ at −20 °C along with greatly increased unit cell parameter (c). The unusual performance is attributed to the topological nature of E-Bi2Se3 improving kinetics of the cell reactions at lower temperatures. This is confirmed in this study by the observed higher electronic conductivity and good ion diffusion of the topological E-Bi2Se3 cathode at lower temperatures. Interestingly, after intercalation of Zn2+ into the E-Bi2Se3 cathode, enhanced contribution of trivial metal states for ZnxBi2Se3 is revealed and proved theoretically and experimentally by conducting an electrical behavior test, density-functional theory (DFT) calculation, and ab initio molecular dynamics (MD) simulation. We believe that the developed topological insulator electrode and unusually better cell performance at lower temperatures will provide opportunities for producing batteries that will operate for long periods in cold climates.
Results
Characterization of P-Bi2Se3 and E-Bi2Se3
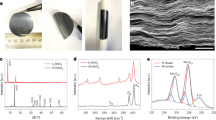
A few QLs of E-Bi2Se3 has been testified the existence of coupling-enhanced topological surface states in contrast to the bulk P-Bi2Se3. The single-phase rhombohedral P-Bi2Se3 structure and \({D}_{3d}^{5}\) (\(R\bar{3}m\)) space group of the prepared samples are confirmed using X-ray powder diffraction (XRD; Fig. 1a). Hydrothermal intercalation is used to exfoliate the P-Bi2Se3 into E-Bi2Se3 (Supplementary Fig. 1). Although LiOH is involved during the exfoliation, analyses of Bi and Se ions by inductively coupled plasma atomic emission spectrometry (ICP-AES) and Li ion by ICP-mass spectrometry (ICP-MS) evidence the Li/Bi/Se atomic ratio for the E-Bi2Se3 sample is 0.00:1.00:1.48, respectively, indicating the absence of Li in the final product (Supplementary Table 1). For the E-Bi2Se3, exfoliation causes the number of stacking layers in one Bi2Se3 particle reduced, and the intensity of related host peaks (such as the (006) peak) is considerably lower than that in the P-Bi2Se3 spectrum23. E-Bi2Se3 lattice parameters of a = 4.14(3) Å, b = 4.14(3) Å, and c = 28.67(4) Å are obtained through Rietveld refinement, and these parameters favorably match the experimental profile displayed in Fig. 1b. The refined atomic models are illustrated in Fig. 1c and Supplementary Fig. 2 where the framework consists of covalently bonded QLs coupled through van der Waals interactions in the [001] direction, accommodating the intercalant at high density and providing large tunnels for ion diffusion at interstitial sites or interlayer galleries in the van der Waals gap24. Rietveld refinement of the XRD pattern of P-Bi2Se3 are likewise shown in 3, and the refined structure parameters are listed in Supplementary Table 2. The transmission electron microscopy (TEM) analysis of P-Bi2Se3 presented in Supplementary Fig. 4a indicates irregular granules ranging in size from 200 nm to 1 μm. The corresponding selected area electron diffraction (SAED) spot pattern indicates that the P-Bi2Se3 is polycrystalline (Supplementary Fig. 4b). After exfoliation, ultrathin and stacked nanosheets with ripples of E-Bi2Se3 and sized within 1 μm are obtained (Fig. 1d), and a typical hexagonal SAED pattern of E-Bi2Se3 consistent with the (110) and (300) planes is distinctly observed, confirming the high level of single crystallinity. The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of E-Bi2Se3 recorded along the [010] zone axis (Fig. 1e) is a magnified view from the A region of Supplementary Fig. 5a, where 1 QL is well assigned to the layered structure. The corresponding electron energy loss spectroscopy (EELS) elemental maps confirm the uniform distribution of Se and Bi (Fig. 1e). Magnified view of a rectangular area in Fig. 1e shows consistency with the E-Bi2Se3 rhombohedral structure at the atomic resolution (Supplementary Fig. 5b–d and Fig. 1f)25,26. A structural model is shown on the left of the HAADF image. Figure 1g shows the intensity line scan along the orange dashed line in the HAADF image. Clear peaks are observed due to the Se-Bi-Se atoms column. We averaged several plots across Se-Bi-Se cluster along pink dashed lines (Supplementary Fig. 5e). Assume the atom is a Gaussian shape. The averaged plot (average of 8 line profiles) is fitted with Gaussians (Supplementary Fig. 5f). Two Se atoms do not distribute symmetrically around Bi site. The phenomena are due to that the atomic configuration at different areas may have certain distortion after HEMM and hydrothermal exfoliating processes as capturing the atomic-scaled HAADF image along [010] zone axis (see Supplementary Fig. 5 for details). Two peaks together in Fig. 1g corresponds to a QL of Se-Bi-Se-Bi-Se with a thickness approaching 1 nm, confirming the ordered arrangement of QLs originating from the layered structure. High-resolution HAADF-STEM image from the B region of Supplementary Fig. 5a shown in Supplementary Fig. 5g has a (003) crystal plane orientation confirmed by corresponding Fourier transform (FT) pattern, which highlights the lamellar structure of E-Bi2Se3 (Supplementary Fig. 5h). The electron energy loss spectrum of E-Bi2Se3 shows that E-Bi2Se3 does not contain Li as there is no peak at 55 eV in Supplementary Fig. 5i. X-ray photoelectron spectroscopy (XPS) also indicate the composition of P-Bi2Se3 and E-Bi2Se3 (Supplementary Fig. 6a). No liquid intercalation was observed (Supplementary Fig. 6b). The high-resolution spectra reveal the valence states of the Bi and Se (Fig. 1h). The Bi 4f spectrum of E-Bi2Se3 has two peaks at 163.37 and 158.02 eV, corresponding to the Bi 4f5/2 and 4f7/2 binding energies attributable to Bi2Se3, respectively, whereas deconvolution of the Bi 4f core-level peaks in the P-Bi2Se3 spectrum indicates that the Bi 4f5/2 and 4f7/2 peaks are shifted to higher energies by 0.9 eV, which is likely attributable to the formation of oxides (BiOx), as discovered in a previous study on Bi2Se3 nanowires signifying higher oxidation states than those of E-Bi2Te327. Moreover, one peak of Se 3d level for E-Bi2Se3 reveal the contributions of Se2- in Bi2Se3 from the Se 3d5/2 level at binding energy of 52.85 eV, and Se 3d3/2 level at 53.74 eV, and a similar phenomenon emerges in P-Bi2Se3. The blueshift of the Se 3d peak at 58.2 eV in P-Bi2Se3 is attributed to amorphous SeO2 introduced during HEMM process28,29 (Supplementary Fig. 7). After washing with tetramethylammonium hydroxide (TMAH)/NaOH/NaCl aqueous solution and DI water, SeO2 impurity can be fully removed. Figure 1i presents a typical atomic force microscopy (AFM) image showing the smooth surface of E-Bi2Se3, and Fig. 1j shows the corresponding height profile, revealing that the thickness of E-Bi2Se3 is approximately 6 nm and that the material thus consists of 6 QLs given than the thickness of a QL is approximately 0.96 nm30. The thickness of numerous flakes is calculated from the AFM image in Supplementary Fig. 8, the average thickness is statistically estimated to be 6 nm.
a XRD analyses of P-Bi2Se3 and E-Bi2Se3 (matched with powder diffraction file cards No. 00-033-0214). b Rietveld refinement of the XRD pattern of E-Bi2Se3 with reliability factors Rwp and Rp of 5.83% and 4.32%, respectively. c Layered crystal structure of E-Bi2Se3 with quintuple layers aligned perpendicular to the trigonal c-axis. The dashed red square indicates a quintuple layer in the \({{{{{{\rm{Se}}}}}}}^{1}\)-\({{{{{{\rm{Bi}}}}}}}^{1}\)-Se2-\({{{{{{\rm{Bi}}}}}}}^{1{\prime} }\)-\({{{{{{\rm{Se}}}}}}}^{1{\prime} }\) sequence, and the dimensions shown here are calculated from the lattice constants. d TEM image of E-Bi2Se3 and corresponding SAED pattern. e HAADF- STEM image of E-Bi2Se3 and its corresponding EELS elemental mappings along [010] zone axis. f A magnified view of the yellow rectangular area in e with the E-Bi2Se3 crystal structure. g An intensity profile from orange line in f. h XPS spectra of Bi 4f and Se 3d signals for P-Bi2Se3 and E-Bi2Se3. i Typical AFM image of individual E-Bi2Se3 and j corresponding height profile.
Electrochemical performance comparison in Zn||P-Bi2Se3 and Zn||E-Bi2Se3 cells
The temperature-dependent electrochemical performance of the rechargeable Zn||P-Bi2Se3 and Zn||E-Bi2Se3 cells is characterized using HC-EGPAM as the electrolyte. The preparation and characterization of HC-EGPAM hydrogel electrolyte are detailed in Supplementary Fig. 9. The HC-EGPAM hydrogel has high adhesiveness, favorable freezing tolerance, and flexibility even at −35 °C (Supplementary Fig. 9h–j)6. The ionic conductivity of HC-EGPAM hydrogel is much higher than that of PAM containing highly concentrated salts (HC-PAM) at low temperatures (Supplementary Fig. 9f). However, when the temperature is decreased from 25 to −20 °C, 50% of the ionic conductivity of HC-EGPAM is still lost (from 7.85 to 3.90 ms cm−1).
We first test the electrochemical performance of the quasi-solid Zn||P-Bi2Se3 cell, in which the P-Bi2Se3 exhibits limited topological insulating states. Figure 2a shows a typical cyclic voltammogram (CV) with weakened intensity at decreased temperature. The temperature-dependent rate performance is illustrated in Fig. 2b. At 25 °C, capacities values of 249, 199, 152, 129, and 88 mAh g−1 are obtained at current rates of 0.3, 0.5, 1, 3, and 5 A g−1, respectively. At lower temperature, the reversible capacity of the Zn||P-Bi2Se3 cell is severely decreased. At 0.3 A g−1, the Zn||P-Bi2Se3 cell maintains reversible capacities values of 231, 205, 130, and 87 mAh g−1 at 10, 0, −10, and −20 °C, respectively, which are 92%, 82%, 52%, and 35% of the capacities delivered at 25 °C, respectively. At higher temperature or lower specific current, characteristic low coulombic efficiency (CE) is noted on account of the irreversible side reaction (Fig. 2c)31. Figure 2d displays the corresponding galvanostatic charge/discharge (GCD) curves obtained at various temperatures from −20 to 25 °C at 1 A g−1. Discharge capacities of 42, 57, 122, 156, and 156.3 mAh g−1 are obtained at temperatures of −20, −10, 0, 10, and 25 °C, respectively. The GCD profile obtained at −20 °C is almost a straight line, which is attributable to the sluggish Zn2+-transport kinetics32. The Zn||P-Bi2Se3 cell exhibits poor cycling performance at a low temperature of 0 °C in Fig. 2e, f: the capacity retention is less than 16% after 1200 cycles at 1 A g−1, in which P-Bi2Se3 electrode delamination leads to the cell failure33. Our observation clearly indicates that even when an anti-freeze electrolyte is employed, performance degradation of Zn||P-Bi2Se3 at low temperature is unavoidable, which is similar to other reported cells. The working mechanism of P-Bi2Se3 is studied in Supplementary Fig. 10, and the capacitive behavior with poor bulk diffusion is observed.
We further evaluate the electrochemical performance of the as-prepared E-Bi2Se3 cathodes in an AZIB at different temperatures with the E-Bi2Se3 exhibiting enhanced topological states16,34. Unlike what we have observed for the Zn||P-Bi2Se3 cell, as the temperature is decreasing, the cathodic peaks shift to higher potential and anodic peaks shift to lower potential, which can be ascribed to reduced polarization (Fig. 3a)35. Exceptionally, the area of redox peaks is remarkably enlarged at lower temperature, indicating higher reaction kinetics of E-Bi2Se3 electrodes at low temperature than at 25 °C36. Figure 3b, c depict the good rate capability of Zn||E-Bi2Se3 cells at −20 to 25 °C. Unlike all previously reported cells, including the Zn||P-Bi2Se3 cells for which attenuation inevitably occurs at low temperatures, the Zn||E-Bi2Se3 cells anomaly deliver better performances at lower temperatures. At −20 °C, discharge specific capacities of 526.3, 400.9, 301.3, and 206.1 mAh g−1 are obtained at specific currents of 0.3, 0.5, 1.0, and 3.0 A g−1, respectively. Compared with the capacities of 326.7, 278, 231, and 159 mAh g−1 at 25 °C, the capacity retention values at −20 °C are 161%, 144%, 130%, and 129.6%, respectively. When the current rate returns to 0.3 A g−1 from 3 A g−1, discharge capacities of 499 (the rate capacity retention of 95%), 468, 405, 335, and 317 mAh g−1 are recovered at −20, −10, 0, 10, and 25 °C, respectively. The corresponding GCD profiles at different temperatures at 1 A g−1 are displayed in Fig. 3d, all containing a discharge plateau at approximate 1.6 V and a subsequent slope at around 1.0 V. In sharp contrast to the Zn||P-Bi2Se3 cells, the Zn||E-Bi2Se3 cells exhibit surprisingly enhanced discharge capacity (269 mAh g−1) and cycling capacity retention (94.6% after 2000 cycles at 0 °C) at 1 A g−1 with a high CE nearly 100% (Fig. 3e, f). The capacity in Fig. 3e sharply increases in the first tens cycles due to the activation of the electrode. In addition, the polarization of the cell is negligible, being as low as 0.08 V at −20 °C. We further extend the temperature range (Supplementary Fig. 11). At the temperature ranging from −20 °C to −50 °C (or from 50 °C to 30 °C), electrolyte has a greater influence on the cell performance (Supplementary Fig. 11a–d) as the ionic conductivity may remarkably decrease with the declined temperature37. For the region from 30 °C to −20 °C, the contribution of E-Bi2Se3 electrode to the cell performance enhancement is greater than that of the electrolyte (Supplementary Fig. 11e). Supplementary Figure 11f summarizes capacity retention of Zn||E-Bi2Se3 cells at various temperatures and current rates compared to the corresponding discharge capacities at 25 °C. Remarkably, even at −40 °C the capacity retentions at 1 and 3 A g−1 remain to be over 100% (106 and 113 %, respectively). The Zn||E-Bi2Se3 cells still deliver discharge capacities of 280, 245, 206, 140, and 113 mAh g−1 at specific currents from 0.3 to 5 A g−1 at −50 °C with record-high capacity retentions of 86, 88, 88, 90, and 81%, respectively (Supplementary Fig. 11f).
Figure 4a and Supplementary Fig. 12a summarize comparisons of temperature-dependent discharge gravimetric and volumetric capacities of Zn||E-Bi2Se3 and Zn||P-Bi2Se3 cells at various current rates, respectively. Clearly, unlike the Zn||P-Bi2Se3 with a degraded performance at lower temperatures, the specific capacity of Zn||E-Bi2Se3 increases as the temperature decreases. To rule out the contribution from the electrolyte, we compare Zn||MnO2 cells using HC-EGPAM with other reported antifreeze hydrogel (Supplementary Fig. 12b), and also compare our topological insulator E-Bi2Se3 electrode with PBAs and MnO2 electrodes in other antifreeze hydrogel electrolytes (Supplementary Fig. 12c, details of discussion are given in the Supporting Information). This anomalous low-temperature cell performance has also been observed in a Na3VCr(PO4)3 (NVCP) cathode38. However, it should be noted that this is because NVCP is not stable at a relatively high temperature (30 °C), leading to a poorer performance output at higher temperature. At low temperature, the high temperature induced irreversible electrochemical reaction can be suppressed in NVCP38. With a totally different mechanism, here exfoliation endows Bi2Se3 with large electrode/electrolyte interfacial contact areas (reduced the ion diffusion length) and fast ion diffusion, which improves the cells performance. More importantly, exfoliation enhances the topological protection performance of the electrodes, significantly increasing electronic conductivity and boosting the electron transfer kinetics (Supplementary Fig. 13)13,16. What really gives the cell remarkably improved performance at low temperature is the synergistic effect of exfoliation and exfoliation-enhanced topological surface state. Compared with all other low-temperature cells including lithium/sodium/zinc ion cells in aqueous6,39 or organic3,5,7,9,32,40,41 electrolytes, as shown in Fig. 4b, the temperature-dependent capacity of Zn||E-Bi2Se3 is among the best. Moreover, the variation trend of capacity versus temperature is clearly reversed. Figure 4c discloses the comparison profile of the dependence of specific capacity on current rate for the prepared E-Bi2Se3 cathode and representative Mn42,43, V44,45,46,47,48, and Ni-based cathodes49,50, PBAs51,52, organic cathodes35,53,54, and others55,56 in AZIBs. The Zn||E-Bi2Se3 possesses the highest specific capacity of 526.3 mAh g−1 at 0.3 A g−1 at −20 °C, which is even prominently outstrip those of the most room-temperature reports. The E-Bi2Se3 cathode is also amenable to high mass loadings of 1–4 mg cm−2. They are tested at specific currents of 0.1 A g−1 to 5 A g−1 at different temperatures. All samples exhibit a higher capacity at lower temperatures, confirming our previous observation (see Supplementary Fig. 14a–d for details). A high areal capacity of 2.1 mAh cm−2 (0.1 A g−1) at a mass loading of 4 mg cm−2 and −20 °C is achieved. Besides, we also assemble flexible quasi-solid Zn||E-Bi2Se3 cells with electrodeposited Zn on carbon cloth as the anode (Supplementary Fig. 15), which can successfully power a wristwatch at low temperature. Considering high-voltage applications, we boost the Zn||E-Bi2Se3 cell voltage to 3.3 V through a DC-DC boost converter with a transition efficiency of 90% (Supplementary Fig. 16a). We first charge Zn||E-Bi2Se3 to 2.3 V, then connect it to the converter following by discharging at −20 °C. Even at 3.3 V, considerable capacity of 438 mAh cm−3 (0.5 Ag−1), and 386 mAh cm−3 (1 Ag−1) are obtained, verifying the low-temperature application potential. Zn||E-Bi2Se3 exhibits a maximum specific energy of 441 Wh kg−1 at a specific power of 683 W kg−1, which is remarkable better than that of other reported aqueous AZIBs, especially at low temperature (Supplementary Fig. 16b).
a Comparison of discharge specific capacity versus temperature of Zn||P-Bi2Se3 and Zn||E-Bi2Se3 cells at various current rates and temperatures. b Specific capacity versus temperature of various low-temperature cells including lithium/sodium/zinc ion cells, among which LNCM-37, F-LTO, LTO-LNCM, PB-CNT, NVPF-NTP, and LFP stand for LiNi0.6Co0.2Mn0.2O2, fluorinated Li4Ti5O129, Li4Ti5O12/Li[Ni0.45Co0.1Mn1.45]O43, Prussian blue-carbon nanotubes5, Na3V2(PO4)2O2F nanotetraprisms32, and LiFePO439, respectively. c Comparison of specific capacity between the present E-Bi2Se3 cathode at −20 °C and representative cathodes in AZIBs at room temperature and various current rates. All the current rates herein are in the range 0.3–5.0 A g−1 except for Prussian blue analogs (PBAs), for which they are 0.05–3.00 A g−1. The exfoliated and nanoscale electrodes are circled with dotted lines.
The Zn-ion storage mechanistic study of E-Bi2Se3
Our findings clearly indicate that topological insulating states play a crucial role in the unique low-temperature performance of the Zn||E-Bi2Se3 system. To reveal the mechanism underlying this performance, we first employed ex situ XRD to investigate the E-Bi2Se3 cathode during charge and discharge. Figure 5a presents the XRD patterns of the E-Bi2Se3 cathode at selected charge/discharge states corresponding to the GCD profile at 0.3 A g−1 in the liquid electrolyte (see Supplementary Fig. 17 for electrochemical performance of the rechargeable Zn||E-Bi2Se3 cells in 1 m Zn(TFSI)2 and 21 m LiTFSI/H2O aqueous electrolyte), and magnified XRD patterns of characteristic peaks are shown in Fig. 5b. During the discharge process (samples 1–8 in the red region), three sets of peaks at 17.7°, 24.7°, and 43.4° [indexed to the (006), (101), and (110) planes of Bi2Se3] shift to lower angles, indicating that the intercalation of Zn2+ triggers expansion of the interlayer spacing. During subsequent charging of the cell to 2.3 V (samples 9–16 in the blue region), these peaks gradually migrate back to their initial positions, reflecting the highly reversible crystal structure evolution of E-Bi2Se3. The peaks at approximately 29°, 40°, and 53.2°— assigned to the (015), (1 0 10), and (205) planes — remains almost unchanged during the charge-discharge process (Supplementary Fig. 18). Ex situ Raman spectra are obtained to analyze the cathode’s structural stability during the electrochemical process (Supplementary Fig. 19). The representative Raman-active modes of Bi2Se3 (1A1g, 2Eg, and 2A1g located at 72, 132, and 174 cm−1, respectively) are enlarged in Fig. 5c. The corresponding measurements of intensity and full width at half maximum (FWHM) of Raman-active modes (1A1g, 2Eg, and 2A1g) in Supplementary Fig. 19c reveals that upon discharge to 0.1 V, the three modes become broader and weaker, which corresponds to the embedding of Zn2+ into the interlayers of E-Bi2Se3. When charging to 2.3 V, these characteristic peaks gradually become stronger and finally return to their initial states, which can be attributed to the deintercalation of Zn2+ from the electrode framework. The Raman results further rule out solvent insertion, confirming that Zn2+ is exclusively responsible for the enlarged spacing. TEM is conducted to probe the structural and morphological evolution of E-Bi2Se3 after it has been fully discharged, presenting the interlaced ultra-thin nanosheets (Fig. 5d). Under higher magnification in Fig. 5e with SAED patterns in Supplementary Fig. 20a, b, the high crystallinity of E-Bi2Se3 is accompanied by an increase in the interplanar spacing from 0.206 to 0.215 nm, indexed to the (110) plane. Also, a characteristic superlattice pattern is observed in which a hexagon of six superlattice spots surrounds each of the host lattice spots as shown in Fig. 5f and Supplementary Fig. 20c. The experimental intensity of the spots and the interplanar spacing are analyzed to be 0.274 and 0.160 nm which corresponds to the (107) and (0 2 10) planes of ZnxBi2Se3, respectively. These planes are in good agreement with those of the simulated electron diffraction pattern of Zn4Bi2Se3 crystal taken along the same zone axis of [001] demonstrating the intercalated Zn2+ in the interlayer (Supplementary Fig. 20d). While there are no significant changes in the spacing of (018), (015), and (107) planes (Fig. 5g and Supplementary Fig. 21), which is in agreement with the XRD results. The HAADF-STEM image taken from a red dotted rectangular box in Supplementary Fig. 22a is shown in Fig. 5h. The layered structure of ZnxBi2Se3 follows the layered ZnxBi2Se3 lattice (Supplementary Fig. 22b). Inset is an FFT pattern showing the (003) crystallographic plane. Figure 5i is a magnified view from a rectangular area from Fig. 5h. Bi atom is located in the middle position between two Se atoms (Supplementary Fig. 22c–f). Figure 5j shows an intensity profile from the magnified part of Fig. 5i. The Zn2+ occupies the position between the Se layers (quantification of the HAADF image is discussed in detail in Supplementary Fig. 22e, f. Comparison of the vertical line scan profiles of the HAADF images of E-Bi2Se3 and ZnxBi2Se3 is shown in Supplementary Fig. 22g. TEM-EDS elemental mappings (Fig. 5k) and corresponding TEM-EDS spectrum (Supplementary Fig. 23) reveal that Zn uniformly intercalates in the E-Bi2Se3 nanosheets. The electrode remains in the original pattern of ultrathin interconnected nanosheets anchored stably on the base (see Supplementary Fig. 24 for different magnifications), and Supplementary Fig. 25 depicts the Zn 2p core-level spectra of the E-Bi2Se3 cathode in three stages. In its original state, the spectrum doesn’t manifest the Zn 2p1/2-2p3/2 spin-orbit doublet, whereas the spectrum of the cathode discharged to 0.1 V confirms successful intercalation of Zn2+ (insertion state), and the weak signal in the spectrum obtained for the cathode on charge clearly reveals the retention of a few intercalated Zn2+ (extraction state), which offers clear evidence of reversible Zn2+ intercalation/deintercalation into the E-Bi2Se3 cathode.
a Evolution of ex situ XRD patterns during the charge/discharge process for E-Bi2Se3 for the third cycle in the liquid electrolyte at 0.3 A g−1 (the solid dots numbered sequentially from 1 to 16 refer to the positions at which the XRD patterns are obtained, and the red region is corresponding to the discharge process while the blue corresponding to the charge process). b Magnified XRD patterns from a for 17°–19°, 24°–26°, and 43°–44°. c Magnified part (50–200 cm−1) of ex situ Raman spectra of the E-Bi2Se3 at the selected states corresponding to the charge/discharge process illustrated in a. d TEM image and e high-resolution TEM (HRTEM) image of E-Bi2Se3 at fully discharged state after three cycles. f SAED pattern of E-Bi2Se3 at fully discharged state. g HRTEM image of fully discharged E-Bi2Se3 revealing (018) and (015) planes with insets displaying fast Fourier transform patterns. h HAADF-STEM image of ZnxBi2Se3, and its corresponding FT pattern. i Magnified part from the red box in h with the ZnxBi2Se3 crystal structure and j corresponding intensity profile. The red arrows in j indicate the presence and specific location of Zn2+ sites. k TEM-EDS elemental maps of Bi, Se and Zn are given in colors of purple, green and yellow dots, respectively. l Schematic of Zn2+ intercalation and deintercalation in the E-Bi2Se3 cathode upon electrochemical charge and discharge process at −20 or 25 °C. Gold sequins represent topological surface states.
To gain additional insights into the potential mechanism underlying the unique low-temperature electrochemical properties, we investigate the Zn2+ insertion model, electronic structures, and Zn2+ ion diffusion kinetics for E-Bi2Se3 by using DFT calculations and ab initio MD simulation. A reversible crystal structure transformation between E-Bi2Se3 and ZnxBi2Se3 during the charge-discharge process is illustrated in Fig. 5l. During the discharge process, the discharge capacity of 327 mAh g−1 under a specific current of 0.3 A g−1 at 25 °C corresponds to the Zn insertion phase, with the stoichiometry of Zn4Bi2Se3 amounting to almost 99.88% of the calculated capacity (327.4 mAh g−1), and strikingly, the low-temperature (−20 °C) discharge capacity of 524 mAh g−1 at 0.3 A g−1 corresponds to Zn6Bi2Se3. After intercalation at 25 °C, the unit cell parameter (c, perpendicular to QLs) increases from 29.16 to 32.92 Å (Zn4Bi2Se3). Upon the subsequent charge process, Zn2+ is reversibly deintercalated from Zn4Bi2Se3 and, together with two electrons, is transformed into the original Zn. Overall, Supplementary Fig. 26 depicts a schematic of the full rechargeable Zn | |E-Bi2Se3 cell.
Low-temperature electronic conductivity and ion diffusion kinetics study of E-Bi2Se3
Because the performance of the electrolyte is worse at low temperatures than that at room temperature, it is believed that the enhanced electrochemical performances of Zn||E-Bi2Se3 cells is mainly contributed by improved electrode properties. We then investigate the electronic conductivity and ion diffusion kinetics of E-Bi2Se3 electrodes at different temperatures. The electrochemical impedance spectra (EIS) of Zn||E-Bi2Se3 (Fig. 6a) and Zn||P-Bi2Se3 cells (Fig. 6b) are conducted, and the fitting results with corresponding equivalent circuits are summarized in Supplementary Fig. 27. The Ri of Zn||E-Bi2Se3 is much smaller relative to that of Zn||P-Bi2Se3, especially at −20 °C (252 vs. 2320 Ω), indicating the far better wettability of the E-Bi2Se3 electrode for the HC-EGPAM electrolyte, caused by the enlarged interlayer spacing. The slightly increased Rs of Zn||E-Bi2Se3 from 6.2 Ω at 20 °C to 8.55 Ω at −20 °C—which involves the resistance of the HC-EGPAM, separator, and E-Bi2Se3 electrode—reflects the good electric conductivity of the cells. The faradic impedance (namely the combination of Rct and ZW) reflects the kinetics of the cell reactions57. Herein, the considerably lower Rct at −20 °C (288 Ω) of Zn||E-Bi2Se3 than the Rct of Zn||P-Bi2Se3 (500 Ω) favorably escorts fast Zn2+ diffusion kinetics. Additionally, Zw is assigned to the Zn2+ diffusion in the cell58. As the slope is proportional to Zw, the Zn||E-Bi2Se3 cell with smaller slopes incidates a faster Zn2+ diffusion59. These results reveal that the stable ionic transport and higher electric conductivity of the Zn||E-Bi2Se3 cell in cold environments far surpass those of the Zn||P-Bi2Se3 cell.
EIS of the a Zn||E-Bi2Se3 and b Zn||P-Bi2Se3 cells, obtained at −20, 0, and 20 °C. c Diffusivity coefficient (D) of Zn2+, calculated using the GITT, of Zn||E-Bi2Se3 and Zn||P-Bi2Se3 cells in the third cycle at 25 °C. d MSD of the Zn between 4 × 4 × 1 supercell Bi2Se3 bilayers, as discovered using ab initio MD simulation at 275 and 300 K, respectively. The inset displays electron density plot for the ZnxBi2Se3 skeleton. e I–V curves of ZnxBi2Se3 with inset showing schematic of the ZnxBi2Se3 nanosheet device fabricated for the electrical transport experiment and f the corresponding temperature-dependent current analysis on the ZnxBi2Se3 device.
In addition, the average Zn2+ diffusion coefficient of the Zn||E-Bi2Se3 cell is calculated using the galvanostatic intermittence titration technique (GITT) to be 10−10–10−11 cm2 s−1, higher than that of the Zn | |P-Bi2Se3 cell (Fig. 6c and Supplementary Fig. 28). To unravel the low-temperature Zn2+ diffusion of the E-Bi2Se3 cathode, we calculate the mean square displacement (MSD) by selecting last 4000-steps in the simulation. Figure 6d graphs the calculated MSD of the Zn ions between the bilayer E-Bi2Se3 at 275 and 300 K. The MSD of Zn in E-Bi2Se3 at 275 K is clearly higher than that at 300 K. On the basis of the calculated MSD, we compute the diffusion coefficient of Zn ions within the bilayer Bi2Se3 at 275 and 300 K, 5.993 × 10−5 m2 s−1 and 4.603 × 10−5 m2 s−1, respectively, indicating that the Zn ions diffuse more quickly at the lower temperature (275 K) than the higher temperature (300 K) in the topological E-Bi2Se3. This phenomenon can be attributed to the weaker lattice vibration of the E-Bi2Se3 bilayer at 275 K than at 300 K, which facilitates the movement of Zn2+. Additionally, the inset of Fig. 6d shows an electron density plot for the ZnxBi2Se3 skeleton, and Bader analysis reveals that some electrons from Zn are transferred to E-Bi2Se3 after Zn2+ intercalation into the E-Bi2Se3 cathode. Consequently, the intercalated Zn2+ bears an effective charge of 1.89 (smaller than the nominal charge of 2), contributing to the ions’ high mobility in E-Bi2Se360.
To elaborate the temperature-dependent conductance characteristics of the topological electrode, several E-Bi2Se3 and ZnxBi2Se3 nanosheets, obtained by discharging the E-Bi2Se3 cathode to 0.1 V at 0.3 A g−1, are separately configured for direct-current transport measurements by using standard electron beam lithography and thermal evaporation of Cr/Au contacts as displayed in the inset of I-V curves of ZnxBi2Se3 (Fig. 6e). The corresponding current versus 1000/T plots over the temperature range −195 to 25 °C for the E-Bi2Se3 and ZnxBi2Se3 devices are presented in Supplementary Fig. 29a and Fig. 6f, respectively. The linear I–V relationship proves that the ohmic-like contacts between the ZnxBi2Se3 nanosheets and Cr/Au electrodes61. Notably, the resistance of the as-prepared E-Bi2Se3 nanosheets and discharging-product ZnxBi2Se3 nanosheets decrease as the temperature declines from 25 to −195 °C, in favorable agreement with the expected metallic behavior. Here, the effect of heavily doped properties of bulk defects is not ruled out. When the temperature drops, the current of the ZnxBi2Se3 nanosheet device steadily rises, indicating a continued reduction in resistance; the decrease in resistance of E-Bi2Se3 tends to saturation, and the resistance of ZnxBi2Se3 is much lower than that of E-Bi2Se3, demonstrating the metallic temperature dependence of both materials and prominently improved conductivity (~1.2–1.5 times) after Zn2+ embedding in ZnxBi2Se3 nanosheets.
Spin-orbit coupling band structure calculation of ZnXBi2Se3
The information from the electronic structures of these materials is vital to multivalent chemistries62. Figure 7a shows the calculated spin-orbit coupling (SOC) band structure of Zn2+ intercalated in Bi2Se3 interlayers (Zn2+Bi2Se3), which gives a band gap of 0.17 eV, illustrating the semiconducting nature in the bulk. The 6-QL slab models with different type of elements terminated at the top and bottom surface are employed to investigate the surface electronic structure of the nanosheet (Fig. 7b–d). For all the Se-Se, Zn-Zn, and Se-Zn terminated slabs, the band gap close and make the system metallic, and the band structures do not show any noticeable difference. Compared with the bulk band structure, the conduction bands shift downward and mix with the valence bands, indicating the existence of metallic surface states. In addition, because Bi2Se3 has the intrinsic topological surface states (Supplementary Figs. 29b and 30a), even if the topological surface states still exist after Zn2+ incorporation, the number of bands will remain unchanged. The metallic surface states play a leading role in the material’s conductivity. Due to the overlapping of the conduction and valence bands, it is difficult to distinguish the topological states even if the topological surface states of Zn2+Bi2Se3 are still retained. The neutral system with intercalated charge-neutral zinc atoms is also calculated for comparison (see Supplementary Fig. 30b–e for details), which implies the existence of topological surface states. It can be concluded that after Zn2+ intercalated into the E-Bi2Se3 cathode, Zn2+ tends to be located around Se atoms on one side, elongating the Bi-Se bonds and resulting in an increased lattice parameter along the c axis, as illustrated in Supplementary Fig. 31. A bandgap exists in the bulk, and the results obtained through the calculation for the 6-QL slab model display surface metallicity; the conductivity of ZnxBi2Se3 benefits from metallic surface states, as proposed in the schematic plot of Supplementary Fig. 32.
The dominant mechanisms responsible for the abnormal low-temperature Zn storage performance of Zn||E-Bi2Se3 can be briefly summarized as follows: the surface metallic feature and topological protection result in enhanced electrical conductivity of E-Bi2Se3 at low temperatures. In addition, weak lattice vibration of the E-Bi2Se3 bilayer at low temperature results in rapid Zn2+ diffusion. The highlights of the previous discussion are that electronic and ionic conductivities are improved at low temperatures, accelerating Zn2+ (de)intercalation reactions. Additionally, the decent ionic conductivity of the anti-freeze HC-EGPAM electrolyte also contributes to the enhanced performance. It should be noted that while HC-EGPAM provide a good platform to study the unique features of the topological insulating E-Bi2Se3 electrode, the high concentration of LiTFSI (21 m) in the electrolytes will inevitably increase the cost of the cell. Alternatives can be developed in future to address this problem63,64. The surface metallic feature and topological protection result in enhanced electrical conductivity open a venue for low temperature cells to achieve enhanced performances. Other materials such as topological semimetals with high electrical conductivity and suitable carrier density near Fermi level are enticing candidate materials for low-temperature electrochemical energy storage meriting from their topologically protected surface states.
Discussion
All cell systems suffer from performance loss or failure at low temperatures, which is a long-standing problem. Herein, we demonstrate a low-temperature cell based on a few-layer Bi2Se3 topological insulator cathode, Zn anode, and HC-EGPAM electrolyte. The dependence of cell capacity on temperature is unusual—the lower the temperature is, the better the cell performance is. This is different from all previously reported low-temperature cells. The Zn||E-Bi2Se3 cell delivers remarkable capacities of 327 mAh g−1 (25 °C) and 524 mAh g−1 (−20 °C) at 0.3 A g−1, and decent cycling performance, with 94.6% of its capacity retained over 2000 cycles at 0 °C and a CE approaching 100%. The mechanism underlying the unusual temperature-dependent cell performance is interpreted by cooperatively utilizing experimental and theoretical strategies. It is revealed that, at a lower temperature, E-Bi2Se3 realizes higher electrical conductivity, which is ascribed to a coupling advantage in the topological surface states. In addition, more rapid Zn ion diffusion in the E-Bi2Se3 is observed and is attributed to weaker lattice vibration of the E-Bi2Se3 bilayer at lower temperature. In particular, the discharging-product ZnxBi2Se3 exhibits higher conductivity than E-Bi2Se3, taking advantage of reinforced trivial metallic surface states because of much-enlarged interlayer spacing and structure distortion after Zn2+ intercalation. These aspects, together with the prominent anti-freeze capability of the developed HC-EGPAM electrolyte, result in better cell performance at lower temperatures. Our study indicates that use of a topological insulator as an electrochemical electrode may result in substantially enhanced low-temperature cell performances, even better than that at room temperature. The developed cells can be an excellent choice for powering systems that operate in cold areas for long periods. Undoubtedly, this research will inspire research on low-temperature cells from the perspective of using a topological insulator as an electrode.
Methods
Preparation of anti-freeze hydrogel electrolyte with high concentration of salts and ethylene glycol (HC-EGPAM)
Zn(TFSI)2 (99% purity) and LiTFSI (99% purity) are purchased from Macklin reagent Co., LTD. The salts are used as received without further purification. A high concentration salt electrolyte is prepared by adding 1 m Zn(TFSI)2 and 21 m LiTFSI [where m is molality (mol kg−1) and TFSI denotes bis(trifluoromethanesulfonyl)imide] to 2 mL of deionized water under vigorous stirring at room temperature for 30 min. After the salts are fully dissolved in the water, 2 mL of ethylene glycol (EG) antifreeze is mixed in. Acrylamide, used as the monomer, 0.05 g of ammonium persulfate, used as the initiator, and 0.005 × g of bisacrylamide, used as the crosslinker, are added together and stirred until a transparent dispersion is obtained. EG (99.5% purity), acrylamide (99% purity), ammonium persulfate (99% purity), and bisacrylamide (99% purity) are purchased from Macklin reagent Co., LTD and used as received. Subsequently, free-radical polymerization proceeds in an oil bath at 70 °C for 3 h. For comparison, the polyacrylamide-based hydrogel electrolyte (HC-PAM) is prepared by replacing the EG additive with an equal amount of deionized water in the aforementioned process. 70% EG/water has the lowest freezing point of −55 °C. In our highly concentrated salts system, EG content can be up to 50%.
Synthesis of few-layer Bi2Se3 nanosheets (E-Bi2Se3)
E-Bi2Se3 nanosheets are readily fabricated using a facile two-step high-energy mechanical milling (HEMM)/lithium intercalation approach, and Bi2Se3 powder (P-Bi2Se3) obtained through HEMM can be easily exfoliated into a stable suspension by using the hydration force (Supplementary Fig. 1). High-purity bismuth shot (Bi, 99.999%; Alfa Aesar) and selenium shot (Se, 99.999%; Alfa Aesar), weighed according to the stoichiometric ratio of Bi2Se3 are loaded into a stainless-steel ball-milling jar in a glove box at room temperature under argon atmosphere (<1 ppm of H2O and O2). The jar is subjected to ball milling for 20 h at 1200 rpm. Afterward, 150 mg of ball-milled powder is added to a 70-mL EG solution of lithium hydroxide (8 g L−1) under continuous stirring for 30 min, after which the mixture is placed into a 100-mL Teflon-lined autoclave. The autoclave is heated at 200 °C for 28 h to obtain the fully exfoliated Bi2Se3 by plating/stripping Li+ dispersed in the solution. The dispersion is centrifuged and then washed thoroughly using acetone (the amount of volume used is 250 mL) and deionized water (300 mL) six times. Lithium hydroxide (99.7% purity) and acetone (>99.5% purity) are purchased from Macklin reagent Co., LTD and used as received. A small amount of SeO2 impurity introduced during the HEMM process can be removed after washing with the tetramethylammonium hydroxide (TMAH, 0.023 M)/NaOH (0.006 M)/NaCl (0.016 M) aqueous solution and deionized water (Supplementary Fig. 7)30. TMAH (25 wt.% in H2O), NaOH (68 g/L in H2O) and NaCl (>99.5% purity) are purchased from Macklin reagent Co., LTD and used as received. After vacuum filtering through porous polyvinylidene fluoride (PVDF) membranes of 0.45-mm nominal pore size, the resulting samples are dried in vacuum at 40 °C overnight.
Fabrication and electrochemical characterization of the quasi-solid Zn | |E-Bi2Se3 cell
To prepare the cathode, E-Bi2Se3, acetylene black (>99.99% purity, Macklin reagent), and PVDF adhesive (Solvay 5130 with a purity > 99.5%, Solvay Specialty Polymers) are mixed uniformly in a 7:2:1 weight ratio with N-methyl-2pyrrolidone (NMP, AR, Aladdin Reagent); then, the slurry is cast on a carbon cloth with a thickness of 1 mm (WOS 1009, Cetech Co., LTD), which is then vacuum dried. The areal loading of E-Bi2Se3 is approximately 1.6 mg cm−2. Electrodeposited Zn on carbon cloth (in flexible cells for demonstration) or Zn foil with purity of 99.99% and thickness of 0.15 mm purchased from Shanghai Ailiai Metallic Material Co., LTD (in CR2032 coin-type cells to evaluate the electrochemical properties) is used as an anode. The coin cell is crimped with a pressure of 5 MPa. The quasi-solid Zn||E-Bi2Se3 cell is assembled by sandwiching HC-EGPAM between the Zn anode and E-Bi2Se3 cathode. CVs and EIS are obtained using a CHI 760E workstation. EIS is conducted at a quasi-stationary potential with two-electrode system in the frequency range from 1 MHz to 0.1 Hz at open-circuit voltage, 5 mV amplitude. The recording number of data points is 12 per decade. GCD profiles, rate capability, and cycling performance are determined using the LAND testing system. In order to avoid the influence of cell activation, the order of measurement of temperature-dependent rate ability is inititated from a small specific current of 0.3 A g−1 to a large specific current of 5 A g−1. The test begins after the completion of activation at 0.3 A g−1 at 25 °C (around three cycles). The ionic conductivity (σ) of the hydrogels can be calculated from alternating-current impedance spectra obtained using two stainless-steel planar electrodes sandwiching the hydrogels. Then, σ is calculated as a function of the ohmic resistance (R; namely the X-axis intercept of the profile), thickness (l), and test area of the hydrogel (A) through the following equation:
Material characterization
XRD data are collected using a Bruker D2 Phaser diffractometer and Cu-Kα radiation (λ = 0.154 nm). XRD refinement is performed using the GSAS-EXPGUI program. The morphology of the electrodes is examined using TEM (JEOL-2001F) and field-emission SEM (FEI/Philips XL30). Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) is performed using the PerkinElmer Optima 8300. Raman spectroscopy is conducted using a multichannel modular triple Raman system (WITec alpha300 access). Chemical state and composition are analyzed using XPS (ESCALAB 250 photoelectron spectroscopy). For ex situ measurements of the cathode, batteries are firstly charged/discharged up to the specific potential using a LAND workstation. At the end of charged/discharge, these batteries are opened in the air to collect cathodes. Afterward, these cathodes are washed with deionized water three times. Finally, cathodes are dried in an oven at 60 °C for 20 min for further ex situ measurements. For electrical transport experiments on the as-fabricated E-Bi2Se3 and discharging-product ZnxBi2Se3, shadow masking is used to define the drain and source regions. Cr/Au film electrodes of thickness 5/80 nm are deposited using electron beam evaporation. The channel lengths are 10 μm for all devices. The devices are characterized using a standard electrical probe station and Agilent 4155C semiconductor analyzer in an ambient atmosphere. The low temperature test is done in ESPEC high and low temperature test chamber (GPS-3).
Calculation
The DFT calculations are performed using the Vienna ab initio simulation package (VASP)61 with the Perdew-Burke-Ernzerhof-type generalized gradient approximation (GGA-PBE)65. The electron-ion interaction is described by projected augmented wave (PAW) method66. The cutoff energy for the plane-wave basis sets is set to be 350 eV. The spin-orbit coupling (SOC) is taken into account due to the strong relativistic effect in Bi element. A slab model contained 6-QLs with a vacuum region of more than 15 Å is used to model the E-Bi2Se3 nanosheets. The Brillouin zone is sampled by a 12 × 12 × 2 and 12 × 12 × 1 Γ-centered k-mesh for bulk and slab model, respectively. All structures are completely relaxed until the residual force on each atom is <0.01 eV/Å. The Grimme’s DFT-D3 method is used to describe the van der Waals interactions67. In order to simulate the Zn2+ diffusion process in the E-Bi2Se3 interlayers, we perform the ab initio MD (AIMD) simulations using the Nosé algorithm68, with a time step of 2 fs and a simulation period of 10 ps. A 4 × 4 × 1 supercell containing 161 atoms and a k-point sampling at Γ-point is used in the AIMD.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available within the text including the Methods, and Supplemental information. Raw datasets related to the current work are available from the corresponding author on reasonable request.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Goodenough, J. B. Rechargeable batteries: challenges old and new. J. Solid State Electrochem. 16, 2019–2029 (2012).
Jung, H. G. et al. A high-rate long-life Li4Ti5O12/Li[Ni0.45Co0.1Mn1.45]O4 lithium-ion battery. Nat. Commun. 2, 516 (2011).
Jaguemont, J. et al. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 164, 99–114 (2016).
You, Y. et al. Subzero-temperature cathode for a sodium-ion battery. Adv. Mater. 28, 7243–7248 (2016).
Mo, F. et al. A flexible rechargeable aqueous zinc manganese-dioxide battery working at −20 °C. Energy Environ. Sci. 12, 706–715 (2019).
Sun, Z. et al. Grafting benzenediazonium tetrafluoroborate onto LiNixCoyMnzO2 materials achieves subzero-temperature high-capacity lithium-ion storage via a diazonium soft-chemistry method. Adv. Energy Mater. 9, 1802946 (2018).
Zhao, Y. et al. Inhibiting grain pulverization and sulfur dissolution of bismuth sulfide by ionic liquid enhanced poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) for high-performance zinc-ion batteries. ACS Nano 13, 7270–7280 (2019).
Zhang, Y. et al. Enhanced rate capability and low-temperature performance of Li4Ti5O12 anode material by facile surface fluorination. ACS Appl. Mater. Interfaces 9, 17145–17154 (2017).
Luo, D. et al. Low-concentration donor-doped LiCoO2 as a high performance cathode material for Li-ion batteries to operate between −10.4 and 45.4 °C. J. Mater. Chem. 22, 22233–22241 (2012).
Moore, J. E. The birth of topological insulators. Nature 464, 194–198 (2010).
Hsieh, D. et al. A topological Dirac insulator in a quantum spin Hall phase. Nature 452, 970–974 (2008).
Peng, H. et al. Aharonov-Bohm interference in topological insulator nanoribbons. Nat. Mater. 9, 225–229 (2010).
Nia, J. et al. Bismuth chalcogenide compounds Bi2X3 (X = O, S, Se): Applications in electrochemical energy storage. Nano Energy 34, 356–366 (2017).
Mishra, S. K. et al. Electronic structure and thermoelectric properties of bismuth telluride and bismuth selenide. J. Phys. Condens. Matter 9, 461–470 (1997).
Hong, S. S. et al. Ultrathin topological insulator Bi2Se3 nanoribbons exfoliated by atomic force microscopy. Nano Lett. 10, 3118–3122 (2010).
Moore, J. E. The next generation. Nat. Phys. 5, 378–380 (2009).
Xia, Y. et al. Observation of a large-gap topological-insulator class with a single Dirac cone on the surface. Nat. Phys. 5, 398–402 (2009).
Zhang, H. et al. Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat. Phys. 5, 438–442 (2009).
Hsieh, D. et al. A tunable topological insulator in the spin helical Dirac transport regime. Nature 460, 1101–1105 (2009).
Yashina, L. V. et al. Negligible surface reactivity of topological insulators Bi2Se3 and Bi2Te3 towards oxygen and water. ACS Nano 7, 5181–5191 (2013).
Chen, C. et al. Robustness of topological order and formation of quantum well states in topological insulators exposed to ambient environment. Proc. Natl Acad. Sci. USA 109, 3694–3698 (2012).
Koski, K. J. et al. High-density chemical intercalation of zero-valent copper into Bi2Se3 nanoribbons. J. Am. Chem. Soc. 134, 7584–7587 (2012).
Chen, K. P. et al. Dual element intercalation into 2D layered Bi2Se3 nanoribbons. J. Am. Chem. Soc. 137, 5431–5437 (2015).
de la Mata, M. et al. Polarity assignment in ZnTe, GaAs, ZnO, and GaN-AlN nanowires from direct dumbbell analysis. Nano Lett. 12, 2579–2586 (2012).
Zhou, W. et al. Identification of active Zr-WOx clusters on a ZrO2 support for solid acid catalysts. Nat. Chem. 1, 722–728 (2009).
Kong, D. et al. Rapid surface oxidation as a source of surface degradation factor for Bi2Se3. ACS Nano 5, 4698–4703 (2011).
Hobbs, R. G. et al. Resist-substrate interface tailoring for generating high-density arrays of Ge and Bi2Se3 nanowires by electron beam lithography. J. Vac. Sci. Technol. B 30, 041602 (2012).
Hou, Y. B. et al. Bi2Se3 nanosheets: advanced nanofillers for reinforcing and flame retarding polyethylene nanocomposites. Compos. Part A Appl. Sci. Manuf. 100, 371–380 (2017).
Sobolev, V. V., Shutov, S. D., Popov, Yu. V. & Shestatskii, S. N. et al. Reflectivity spectra of the rhombohedral crystals Bi2Te3, Bi2Se3, and Sb2Te3 over the Range from 0.7 to 12.5 eV. Phys. Status Solidi 30, 349–355 (1968).
Aurbach, D. et al. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000).
Guo, J. Z. et al. High-energy/power and low-temperature cathode for sodium-ion batteries: in situ XRD study and superior full-cell performance. Adv. Mater. 29, 1701968 (2017).
Xu, H. et al. Hierarchical Bi2Se3 microrods: microwave-assisted synthesis, growth mechanism and their related properties. CrystEngComm 15, 1618–1625 (2013).
Hong, M. et al. Enhanced thermoelectric performance of ultrathin Bi2Se3 nanosheets through thickness control. Adv. Electron. Mater. 1, 1500025 (2015).
Kundu, D. et al. Organic cathode for aqueous zn-ion batteries: taming a unique phase evolution toward stable electrochemical cycling. Chem. Mater. 30, 3874–3881 (2018).
Liu, C. et al. Expanded hydrated vanadate for high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 12, 2273–2285 (2019).
Ding, M. S. et al. Conductivity, viscosity, and their correlation of a super-concentrated aqueous electrolyte. J. Phys. Chem. C 121, 2149–2153 (2017).
Rui, L. et al. Counter-intuitive structural instability aroused by transition metal migration in polyanionic sodium ion host. Adv. Energy Mater. 11, 2003256 (2021).
Liang, Y. et al. Universal quinone electrodes for long cycle life aqueous rechargeable batteries. Nat. Mater. 16, 841–848 (2017).
Wu, X. et al. Carbon-nanotube-decorated nano-LiFePO4@C cathode material with superior high-rate and low-temperature performances for lithium-ion batteries. Adv. Energy Mater. 3, 1155–1160 (2013).
Elia, G. A. et al. Nanostructured tin-carbon/LiNi0.5Mn1.5O4 lithium-ion battery operating at low temperature. J. Power Sources 275, 227–233 (2015).
Zhang, N. et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 138, 12894–12901 (2016).
Pan, H. et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 1, 16039 (2016).
Xia, C. et al. Rechargeable aqueous zinc-ion battery based on porous framework zinc pyrovanadate intercalation cathode. Adv. Mater. 30, 1705580 (2018).
He, P. et al. Layered VS2 Nanosheet-based aqueous Zn ion battery cathode. Adv. Energy Mater. 7, 201601920 (2017).
Kundu, D. et al. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 1, 119 (2016).
Yang, Y. et al. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 11, 3157–3162 (2018).
Pang, Q. et al. H2V3O8 Nanowire/graphene electrodes for aqueous rechargeable Zinc ion batteries with high rate capability and large capacity. Adv. Energy Mater. 8, 1800144 (2018).
Liu, J. et al. A flexible quasi-solid-state nickel-zinc battery with high energy and power densities based on 3D electrode design. Adv. Mater. 28, 8732–8739 (2016).
Zeng, Y. et al. Oxygen-vacancy and surface modulation of ultrathin nickel cobaltite nanosheets as a high-energy cathode for advanced Zn-ion batteries. Adv. Mater. 30, 1802396 (2018).
Zhang, L. et al. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: the zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 5, 1400930 (2015).
Yang, Q. et al. Activating C-coordinated iron of iron hexacyanoferrate for Zn hybrid-ion batteries with 10 000-cycle lifespan and superior rate capability. Adv. Mater. 31, 1901521 (2019).
Huang, J. et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 9, 2906 (2018).
Ji, X. et al. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Li, H. et al. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-ion batteries. Energy Storage Mater. 19, 94–101 (2018).
Ma, L. et al. Initiating a mild aqueous electrolyte Co3O4/Zn battery with 2.2 V-high voltage and 5000-cycle lifespan by a Co(iii) rich-electrode. Energy Environ. Sci. 11, 2521–2530 (2018).
Zhang, S. S. et al. Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochim. Acta 49, 1057–1061 (2004).
Mohamedi, M. et al. Electrochemical investigation of LiNi0.5Mn1.5O4 thin film intercalation electrodes. Electrochim. Acta 48, 79–84 (2002).
Macdonald, D. D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 51, 1376–1388 (2006).
Wang, F. et al. A rechargeable aqueous Zn2+-battery with high power density and a long cycle-life. Energy Environ. Sci. 11, 3168–3175 (2018).
Meng, Y. et al. Direct vapor-liquid-solid synthesis of all-inorganic perovskite nanowires for high-performance electronics and optoelectronics. ACS Nano 13, 6060–6070 (2019).
Kresse, G. et al. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Xie, J. et al. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 19, 1006–1011 (2020).
Bi, H. B. et al. A universal approach to aqueous energy storage via ultralow-cost electrolyte with super-concentrated sugar as hydrogen-bond-regulated solute. Adv. Mater. 32, 2000074 (2020).
Perdew, J. P. et al. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B Condens Matter 50, 17953–17979 (1994).
Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511 (1984).
Acknowledgements
This research was supported by the National Key R&D Program of China (no. 2019YFA0705104 (C.Z.)). The work was also partially sponsored by GRFs under Project CityU 11305218 (C.Z.), CityU 11212920 (C.Z.), and the Guangdong Innovative and Entrepreneurial Research Team Program (no. 2016ZT06G587 (W.L.)). The authors would like to thank Mr T. F. Hung for HRTEM analysis.
Author information
Authors and Affiliations
Contributions
Yw.Z.: conceptualization, methodology, and writing. Y.L.: methodology. H.L.: methodology. Yb.Z.: methodology. Y.M.: methodology. N.L.: methodology. D.W.: methodology. F.J.: methodology. F.M.: methodology. C.L.: methodology. Y.G.: methodology. X.L.: methodology. Z.H.: methodology. Q.L.: methodology. J.C.H.: methodology. J.F.: methodology. M.S.: methodology. F.C.: methodology, review, and editing. W.Z.: methodology, review & editing. W.L.: methodology, review, and editing. C.Z.: conceptualization, funding acquisition, writing – review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Lu, Y., Li, H. et al. Few-layer bismuth selenide cathode for low-temperature quasi-solid-state aqueous zinc metal batteries. Nat Commun 13, 752 (2022). https://doi.org/10.1038/s41467-022-28380-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-28380-y
This article is cited by
-
Halogen-powered static conversion chemistry
Nature Reviews Chemistry (2024)
-
An Electrochemical Perspective of Aqueous Zinc Metal Anode
Nano-Micro Letters (2024)
-
Recent Advances in Structural Optimization and Surface Modification on Current Collectors for High-Performance Zinc Anode: Principles, Strategies, and Challenges
Nano-Micro Letters (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.