Abstract
How retinol as a clinical indicator of vitamin A status is related to long-term mortality is unknown. Here we report the results of a prospective analysis examining associations between serum retinol and risk of overall and cause-specific mortality. During a 30-year cohort follow-up, 23,797 deaths were identified among 29,104 men. Participants with higher serum retinol experienced significantly lower overall, CVD, heart disease, and respiratory disease mortality compared to men with the lowest retinol concentrations, reflecting 17–32% lower mortality risk (Ptrend < 0.0001). The retinol-overall mortality association is similar across subgroups of smoking intensity, alcohol consumption, body mass index, trial supplementation, serum alpha-tocopherol and beta-carotene concentrations, and follow-up time. Mediation analysis indicated that <3% of the effects of smoking duration and diabetes mellitus on mortality were mediated through retinol concentration. These findings indicate higher serum retinol is associated with lower overall mortality, including death from cardiovascular, heart, and respiratory diseases.
Similar content being viewed by others
Introduction
Vitamin A is a class of essential, fat-soluble compounds of which retinol is the major form in circulation and considered the biochemical indicator of human vitamin A status1. Animal products are the primary food sources of retinol, including liver, butter, milk, cheese, and egg. Retinol and related retinoids act as ligands for cellular retinoic acid and retinoid X receptors (RARs and RXRs) through which they modulate transcription of genes regulating a wide-range of physiological processes including embryonic development, post-natal growth, vision, epithelial differentiation and maintenance, and immunity1,2,3. The deficiency of vitamin A can lead to xerophthalmia and blindness, infections and inflammation, chronic diseases, and even death. Despite the well-established clinical importance of vitamin A sufficiency for growth and development, physiological homeostasis, and overall human health, a comprehensive assessment of the long-term relationship between vitamin A status and overall and cause-specific mortality is lacking.
Population-based studies have demonstrated inconsistent associations between circulating retinol and risk of cancer, cardiovascular disease (CVD), and death4,5,6,7,8,9,10. A meta-analysis of four studies revealed no material association between circulating vitamin A (retinol) and all-cause mortality11. However, most of the previous studies were of limited sample size and the number of events (i.e., ranging from 62 to 720 deaths)4,5,6,9,10, controlled for confounding factors inconsistently (e.g., only controlling for age and sex6), and had relatively low power to examine cause-specific mortality, effect modification by other factors4,5,6,7,8,9, and dose-response associations4,5,6,9.
In order to more robustly evaluate the vitamin A-mortality relationship, we conducted a prospective analysis of a large cohort of men with serum retinol measured at study entry and over three decades of subsequent observation with nearly 24,000 deaths from heart disease, stroke, other CVD, cancer, respiratory disease, diabetes mellitus, and other causes. Dose-response and population subgroups of lifestyle and other selected factors were also examined. Here, we show statistically significant inverse associations between higher serum retinol and lower risk of overall and cause-specific mortality, including death from cardiovascular, heart, and respiratory diseases. The inverse retinol-overall mortality association is similar across several cohort subgroups, including smoking intensity, alcohol consumption, body mass index, trial supplementation, serum alpha-tocopherol, and beta-carotene concentrations, and duration of follow-up.
Results
Baseline characteristics
The median baseline serum retinol concentration in the cohort was 576 µg/L (interquartile range = 500 µg/L to 662 µg/L), and the mean retinol value in the fifth quintile was 84% higher than that in the first quintile. According to the WHO criteria, the present study had only two participants identified with severe vitamin A deficiency (≤98 µg/L), and 21 participants identified with subclinical vitamin A deficiency (98 to ≤196 µg/L). When compared with men in the lowest quintile category, men in higher quintiles of retinol were slightly younger, had higher blood pressure, BMI, and educational level, and were more likely to have a history of CVD, use vitamin supplements and be more physically active (Table 1). Serum retinol was also positively associated with serum total and HDL cholesterol and alcohol consumption (as expected)12,13,14, and inversely associated with serum beta-carotene. With regard to the magnitude of the correlation, serum retinol was negligibly or weakly correlated with these factors (all Pearson correlation coefficients, −0.12 < r ≤ 0.22)15.
Serum retinol and overall and cause-specific mortality
During 31 years of follow-up (median 18 years), there were 514,289 person-years of accumulated observation, with 23,797 deaths, including 9,869 deaths from CVD (8,064 heart disease and 1,764 stroke), 7,695 deaths from cancer, 2,161 deaths from respiratory disease, 119 deaths from diabetes, 1,255 deaths from injuries and accidents, and 2,698 deaths from other causes. The age-adjusted analysis showed that, compared with those in the lowest quintile, men in the higher quintiles of serum retinol experienced lower mortality from all-causes combined, cancer, and respiratory disease, reflecting 10−38% reductions (fifth versus first quintile, HRs [95% CIs]: 0.90 [0.87, 0.94], 0.90 [0.83, 0.96], 0.62 [0.54, 0.71], respectively; all Ptrend ≤ 0.0013; Table 2). The multivariate-adjusted models for mortality from all-causes combined, CVD, heart disease, respiratory disease and other causes, indicated 17−32% lower mortality among men in the highest quintile (fifth versus first quintile, HRs [95% CIs]: 0.83 [0.80, 0.87], 0.81 [0.76, 0.86], 0.79 [0.74, 0.85], 0.68 [0.59, 0.78], 0.83 [0.74, 0.94], respectively; all Ptrend < 0.001; overall mortality per one SD HR, 0.95 [0.94, 0.96]). The findings of absolute risk difference for the associations remained qualitatively similar when truncating the follow-up observation periods at 10- or 20-years. The adjusted 30-year one SD ARDs (95% CIs) were −1.19% (−1.54%, −0.87%), −0.80% (−1.17%, −0.43%), −0.83% (−1.20%, −0.44%), −0.87% (−1.25%, −0.53%), and −0.75% (−1.18%, −0.30%), for all-causes combined, CVD, heart disease, respiratory disease and other causes, respectively (Table 3).
The restricted 4-knot cubic spline linear regression of the serum retinol-mortality association substantiated a non-linear dose-response, with all-cause and cause-specific mortality (mortality from CVD and heart disease) increasing among men with serum retinol concentrations below 483 µg/L (cutoff reference value of the lowest quintile) (Fig. 1). Increasing serum retinol was related to decreasing overall, CVD and heart disease mortality, with greatest risk reduction apparent for concentrations near 660 µg/L. The multivariable fractional polynomials analysis, after adjustment for confounding factors, showed similar patterns of the dose-response associations between serum retinol and overall mortality as well as CVD and heart disease mortality (P values < 0.0001 for multivariable fractional polynomials compared with linear, Supplemental Fig. 1). By contrast, serum retinol appeared to be linearly associated with respiratory disease mortality (on the log scale of the hazard ratio) (restricted cubic splines model, P nonlinear = 0.04, Fig. 1; multivariable fractional polynomials model, P nonlinear > 0.05, Supplemental Fig. 1). Kaplan−Meier survival plots show that compared with those in the higher quintiles, men in the lowest category of serum retinol experienced excess cumulative overall mortality and mortality from CVD, heart disease and respiratory disease (all log-rank P values < 0.0001; Fig. 2).
The reference value (483 µg/L; hazard ratio = 1) corresponds to the cutoff value of the first quintile category of serum retinol concentration. A Overall mortality. B Cardiovascular disease (CVD) mortality. C Heart disease mortality. D Respiratory disease mortality. The solid line represents the hazard ratio (HR) for mortality and serum retinol with a 4-knot spline (knots were selected at the 5th, 25th, 75th, and 95th percentiles of the serum retinol); dashed lines suggested the 95% confidence intervals. The total number of participants: 29,104. Event number of overall-, CVD-, heart disease-, respiratory disease death is 23,797, 9,869, 8,064, and 2,161, respectively. Multivariate-adjusted models adjusted for age, BMI, serum total and serum HDL cholesterol, cigarettes smoked per day, years of smoking, alcohol intake, intervention assignment, systolic and diastolic blood pressure, history of CVD, and history of diabetes. ATBC Alpha-Tocopherol, Beta-Carotene Cancer Prevention; BMI body mass index; CVD cardiovascular disease; HDL high-density lipoprotein.
Stratified analyses
Stratified analyses revealed no significant differences in the retinol-overall mortality association across subgroups of smoking intensity, alcohol consumption, BMI, trial supplementation, serum alpha-tocopherol, and beta-carotene concentrations, and duration of follow-up (Fig. 3). There was, however, a significant interaction with both age and serum total cholesterol where the inverse association appeared stronger among men who were older than 54 years and those with low total cholesterol concentrations (Pinteraction < 0.005, respectively). Findings were similar across subgroups for CVD, heart disease, and respiratory disease mortality with no significant differences observed (Supplemental Tables 1–3).
Hazard ratios of overall mortality were for one SD increment of serum retinol concentration. We used Cox proportional hazard regression models to estimate HRs and 95% CIs. Models were adjusted for age, BMI, serum total and serum HDL cholesterol, cigarettes smoked per day, years of smoking, alcohol intake, intervention assignment, systolic and diastolic blood pressure, history of CVD, and history of diabetes. P value for interaction: according to the likelihood test to assess the statistical significance of the cross-product term entered into the Cox proportional hazard regression model. Abbreviations: BMI body mass index; CI confidence interval; CVD cardiovascular disease; HDL high-density lipoprotein; HR hazard ratios. *P value for interaction achieved the Bonferroni corrected threshold: 0.05/10 = 0.005.
Sensitivity analyses
The inverse serum retinol associations with mortality from CVD, heart disease, and respiratory disease were accentuated after excluding the 12,488 men with a baseline history of CVD or diabetes mellitus (mortality from CVD, heart disease, and respiratory disease in the multivariable-adjusted model, fifth versus first quintile: HRs = 0.72, 0.68 and 0.60, respectively; all Ptrend < 0.0001). By contrast, the retinol-overall mortality association remaining unchanged (HR = 0.81, Ptrend < 0.0001; Supplemental Table 4). The findings were similar after excluding the first 5 years of follow-up (Supplemental Table 5) and when used serum retinol concentration data from the third follow-up year (fifth versus first quintile, HR [95% CIs] for overall mortality: 0.85 (0.81, 0.89), Ptrend < 0.0001; Supplemental Table 6).
Mediation analyses
As reported, traditional risk factors including obesity, intensity of smoking, duration of smoking, diabetes mellitus, and alcohol drinking were statistically significantly associated with increased risk of overall mortality, whereas light or moderate physical activity was inversely associated risk of mortality in the cohort (Table 4). Our results from the mediation analysis suggest that 2.2% (1.5−3.2) of the smoking duration association with mortality, as well as 1.2% (0.5−3.1) of the diabetes mellitus association, were mediated through serum retinol concentration.
Discussion
In this large prospective serological analysis involving 29,104 cohort participants and 23,797 deaths over three decades, we found significant inverse associations between serum vitamin A (retinol, subsequently referred as “vitamin A”) and mortality from overall, CVD, heart disease and respiratory disease after adjusting for several important risk factors, with subjects in the highest serum quintile experiencing 17–32% lower mortality when compared with those in the lowest quintile category (5–12% per one SD increment). The beneficial overall mortality association was evident across all population subgroups with the exception of being stronger among older men and those with lower serum total cholesterol. The effect sizes of these observed risk estimates were relatively small, however.
The present investigation is the largest to examine the association of vitamin A biochemical concentrations with all-cause and cause-specific mortality, and our data suggests mortality declines with increasing retinol concentration, with a risk nadir for overall and CVD mortality in older men with serum values of 600–700 µg/L, and excess mortality among men with retinol below 500 µg/L (n = 7,321, which included two and 21 participants that exhibited severe [≤98 µg/L] or subclinical [98 to ≤196 µg/L] vitamin A deficiency, respectively). Humans cannot synthesize vitamin A and derive this essential nutrient from animal product-based retinyl esters and plant-based pro-vitamin A carotenoids (e.g., alpha- and beta-carotene)16,17. For example, the primary dietary sources for dietary vitamin A in this population (as mean percentage of total daily dietary vitamin A) were liver (33.9%), butter (16.4%), egg (9.2%), milk (8.6%), cheese (3.5%) and other food items combined (28.4%). Preformed retinol is absorbed from the intestine, esterified, and transported in chylomicrons to the liver. For healthy, well-nourished individuals, it is estimated that 60–95% of vitamin A is stored in the liver which plays a central role in its metabolism and homeostasis through hydrolysis of stored retinyl esters, complexing of retinol with retinol-binding protein (RBP), and release of this “holo-RBP” into systemic circulation for uptake and use by other organs16,17,18,19. Previous studies raised the possibility that both insufficient and excess vitamin A status may be associated with increased risk of adverse health outcomes8,20, including as a result of direct toxic properties of hypervitaminosis A17. We did not observe excess mortality among men with high serum retinol, however, possibly because of a limited range of concentrations in our study population. The threshold of toxic concentrations of retinol, as well as whether there is a positive association of mortality risk with excess concentrations of serum retinol, can be evaluated in further studies of populations with available data of excessive serum retinol values. Given the fact that vitamin A is metabolized and stored in the liver17, a positive association between liver consumption and serum retinol is biologically plausible. However, along with previous findings12,13,14,21,22, our data showed a negligible association between serum retinol and dietary liver consumption (Pearson correlation coefficient = 0.05, P < 0.0001), and a weak association with alcohol consumption (Pearson correlation coefficient = 0.20, P < 0.0001), with negligible and weak correlations being defined as correlation coefficients that range from 0.00 to 0.10 and 0.10 to 0.39, respectively15. Additionally, our data showed that the inverse association between serum retinol and risk of overall mortality was not changed by additional adjustment for liver consumption (Q5 versus Q1: HR = 0.84, 95% CI: 0.80, 0.87, P for trend < 0.0001). This suggests that a vitamin A-enriched diet was not sufficient to modify the associations appreciably.
Findings from prior studies regarding vitamin A status and overall mortality have been conflicting, with several investigations showing no associations, dose-response or otherwise4,5,6,7,8. The NHANES III Study (The Third National Health and Nutrition Examination Survey) of 16,008 participants with 4,225 deaths showed a U-shaped association between serum retinol and total mortality such that individuals with concentrations in either the quintile 3 or 4 category (but not in quintile 5) experienced 18% reduced total mortality compared with those in the lowest quintile (HRs 95% CIs = 0.82 [0.68, 0.98] and 0.82 [0.69, 0.97], respectively; Ptrend = 0.93)7. Using the same NHANES III Study with 6,069 participants aged 50 years or older, a U-shaped overall mortality association was found with HRs of 2.9 and 1.2 for participants with vitamin A status that was deficient (<30 µg/dL) or excessive (>80 µg/dL) when compared to those with normal retinol concentrations (30–80 µg/dL)8, respectively. In a cohort of 379 renal transplant recipients with a median of 1,739 days follow-up, serum retinol concentration was related to a decreased risk of all-cause mortality9. For established mortality risk factors, our mediation analysis suggests that only a small, <3% role is played by serum retinol for the association of overall mortality with the duration of smoking and diabetes mellitus. Whether retinol mediates portions of other risk factor-mortality associations (e.g., lipid impairment) can be investigated in future studies.
Retinol biochemical status and cause-specific mortality, particularly CVD mortality, has been only sporadically studied, with conflicting findings and several null associations reported4,5,6,7. By contrast, serum retinoic acid was inversely associated with overall and CVD mortality among 1,499 patients with coronary artery disease, with those in the highest serum quartile experiencing 35 and 40% lower mortality, respectively, as compared with those in the lowest quartile category10. In 1,530 acute ischemic stroke patients, lower retinoic acid concentrations were related to higher risk of overall and CVD mortality, independent of other risk factors23, results that were essentially consistent with the present findings. Case-control studies have also documented inverse associations of plasma retinol with risk of CVD including coronary heart disease13, stroke12, and CVD mortality24. Such protective vitamin A-CVD mortality associations are supported by several plausible biological mechanisms. Retinol, its derivate retinoids, and their receptors exhibit potent inhibition of the atherosclerotic disease through cholesterol efflux from macrophages, enhanced reendothelialization, and reduced proinflammatory response. Cholesterol efflux capacity has been characterized as reverse cholesterol transport and shown prospectively to be inversely related to CVD events including mortality25,26,27. 9-Cis-retinoic acid also suppresses lipoprotein-derived cholesterol accumulation in macrophages and inhibits foam cell formation28 and the development of atherosclerosis29. Retinoids also exhibit anti-inflammatory, immune-modulatory, anti-angiogenic and anti-thrombotic properties, and they enhance vasodilatory biochemical synthesis, suppress vasoconstriction, reduce injury-reactive intimal thickening, and enhance nitric oxide pathway-related endothelial function30,31,32,33,34,35,36. In addition, the primary vitamin A carrier in circulation, retinol-binding protein 4 (RBP4), has been related to cardiometabolic and inflammatory markers, and higher RBP4 concentrations are associated with metabolic syndrome and cardiovascular disease37,38,39,40.
In contrast to CVD, there are relatively few studies of circulating vitamin A and respiratory disease mortality. The British National Diet and Nutrition Survey study of 1054 participants observed a non-significant inverse association for respiratory disease mortality with an age- and sex-adjusted 12% lower risk per SD increment in plasma vitamin A6. Previous case-control studies showed significantly increased risk of both chronic obstructive pulmonary disease (COPD)41 and nontuberculous mycobacterial pulmonary disease42 for persons with lower plasma vitamin A, and a cross-sectional study of 50 COPD patients and controls reported essentially the same43. Whether vitamin A inhibition of inflammatory responses and suppression of host respiratory infections can account for the respiratory mortality associations will require further study. The present finding of a weak and non-significant retinol-cancer mortality association after excluding the first 5 years of follow-up, indicating some potential reverse causality, is consistent with the lack of associations reported in previous studies5,6,7,44.
The major strength of our study is its basis in a large prospective cohort with complete follow-up up to 31 years and identification of cause-specific mortality via linkage with Finnish national registries. Retinol serological status was determined independently through a high-quality isocratic high-performance liquid chromatography platform for more than 29,000 men, which provided an accurate and objective measurement of retinol biochemical concentrations. Additionally, the large sample size enabled us to examine associations for the less common causes of mortality and test whether other factors such as age, alcohol consumption, or smoking intensity modified the vitamin A-mortality associations. Our findings are also subject to some limitations that should be noted. The cohort was a relatively homogenous population of male Finnish smokers which limits generalizability of our findings to other populations. There was only a single assessment of baseline serum retinol, and the physiological status of some participants may have changed during the long follow-up period. Other biomarkers relevant to vitamin A status, including retinol-binding protein, retinoic acid receptor expression, and C-reactive protein (CRP, a marker of inflammation, that the inflammation status may affect retinol homeostasis and serum retinol concentrations), that would have afforded a deeper evaluation of the mortality associations, were not available for the cohort. Underestimated mortality associations towards the null may result from biases attributed to nondifferential misclassification such as measurement error. However, we found the baseline and year-3 retinol concentrations were highly correlated (Spearman correlation coefficient = 0.69, P-value < 0.0001), indicating its relative stability during the post-randomization period. Despite having controlled for a large number of potential confounding factors, the possibility of findings being subject to residual confounding from other unmeasured factors cannot be ruled out.
In conclusion, our data indicate that higher vitamin A status is associated with lower overall and cause-specific mortality in men. The associations were based on over 30 years of follow-up and remained unchanged after adjusting for potential confounding risk factors and were largely stable across a range of population subgroups. The study provides evidence supporting the importance of vitamin A biochemical sufficiency for long-term health and longevity. Reexamination of these associations in populations that include women, nonsmokers, and other races/ethnicities is warranted.
Methods
Study cohort
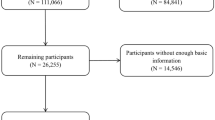
The present analysis is nested within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study cohort, details of which have been documented (ClinicalTrials.gov Identifier: NCT00342992)45. This randomized, double-blind, placebo-controlled trial evaluated whether alpha-tocopherol or beta-carotene could decrease cancer incidence (particularly lung cancer). The study enrolled 29,133 male smokers, aged 50–69 years from 1985 to 1988 in southwestern Finland. The participants were assigned to one of four supplementation groups—alpha-tocopherol (50 mg/day), beta-carotene (20 mg/day), both vitamins, or placebo—for 5–8 years until the intervention ended on April 30, 1993. At the baseline visit, questionnaires obtained data for demographics, medical and smoking histories, and lifestyle characteristics, including a modified food frequency questionnaire and color picture booklet that queried consumption frequency and portion sizes for 203 food items and 73 mixed dishes for the previous 12 months. Height, weight, and blood pressure were measured by professional nurses, and pre-supplementation fasting blood samples were processed to serum and stored at −70 °C. The ATBC Study was conducted in accordance with the requirement by the Declaration of Helsinki. All participants provided written informed consent, and the study was reviewed and approved by the Institutional Review Boards at the US National Cancer Institute and the Finnish National Public Health Institute.
Laboratory measurements
Serum concentrations of retinol, alpha-tocopherol, and beta-carotene were measured for 29,104 of the participants (99.9%) using reversed-phase high-performance liquid chromatography, and the coefficient of variation for serum retinol was 3.1%45,46.
Prospective follow-up and causes of death
Cohort participant person-time was calculated from randomization date during 1985–1988 until date of death or the end of follow-up (December 31, 2015), whichever occurred first. Specific causes of death were obtained through linkage with the Finnish Death Registry of Statistics Finland (Supplemental Fig. 2). Codes from three revisions of International Classification of Diseases (the eighth, ninth, and tenth revisions: ICD-8, ICD-9, ICD-10) were used to define underlying causes of death: CVD mortality (ICD-8 = 390–458; ICD-9 = 390–459; ICD-10 = I00–I99), heart disease mortality (ICD-8 and ICD-9 = 390–398, 401–404, 410–429, and 440–448; ICD10 = I00–I13, I20–I51, and I70–I78), stroke mortality (ICD-8 and ICD-9 = 430–438; ICD10 = I60–I69), cancer mortality (ICD-8 and ICD-9 = 140–239; ICD10, C00-D48), respiratory disease mortality (including influenza, pneumonia, COPD, and other related conditions; ICD-8 = 470–474, 480–486, 490–493, and 518; ICD-9 = 480–487 and 490–496; ICD-10 = J10–J18 and J40–J47), diabetes mortality (ICD-8 and ICD-9 = 250; ICD-10 = E10–E14), injury and accident mortality (ICD-8 = 800–978; ICD-9 = E800–E978; ICD-10 = U01–U03, V01–X59, X60–X84, X85–Y09, Y35, Y85–Y86, Y87.0, Y87.1, and Y89.0), and other causes of mortality combined (including 47 subjects with missing cause of death).
Statistical analysis
Cox proportional hazard regression models with attained age as the time metric were used to estimate hazard ratios (HRs) and two-sided 95% confidence intervals (CIs) to examine the association between serum retinol (quintiles and per one SD) and mortality. The proportional hazard assumption was not violated and was tested using model interaction terms of the dichotomized indicator of follow-up years and serum retinol quintiles, with a likelihood ratio test comparing models with and without the interaction terms. A set of a priori covariables were chosen for the risk models based on the existing knowledge and research regarding potential confounding of the serum retinol-mortality risk associations. In the multivariable analysis, we adjusted for age at baseline (continuous), body mass index (BMI, continuous), serum total and serum high-density lipoprotein (HDL) cholesterol (continuous), cigarettes smoked per day (continuous), years of smoking (continuous), alcohol intake (categorical), intervention assignment (alpha-tocopherol supplementation: yes or no; beta-carotene supplementation: yes or no), systolic and diastolic blood pressure (continuous), history of CVD (yes or no), and history of diabetes (yes or no). For each HR from the primary Cox regression model, the adjusted absolute risk difference (ARD) was calculated for each one SD increment of serum retinol for observation periods of 10, 20, and 30 years, with the 95% CIs for ARD being computed based on 500 bootstrap samples. In terms of missing values for any individual covariate (<5%), we generated a missing value indicator in the models.
Restricted cubic splines with four knots were applied to flexibly model the possible nonlinear association between serum retinol levels (continuous) and overall and cause-specific mortality, with the selected change-points being at the 5th, 25th, 75th, 95th percentiles of retinol concentration. Likelihood ratio tests compared differences between the linear term model and the model with both linear and cubic spline terms, with a P-value of < 0.0056 indicating nonlinearity [nine tests]. We further used multivariable fractional polynomials to model the potential nonlinear association between serum retinol and overall and cause-specific mortality (by using R [version 3.5.2] Package “mfp”). Kaplan−Meier survival plots and log-rank tests assessed survival differences across serum retinol quintiles both for all participants and (separately) men without a baseline history of CVD or diabetes mellitus.
In order to evaluate potential interactions and the robustness of our findings, stratified analyses were constructed based on a priori categories of baseline age (<54, 54–<59, or ≥59 years), daily cigarettes (<16, 16–20, or ≥20 cigarettes per day), alcohol consumption (grams of ethanol per day, <5.3, 5.3–<20.4, or ≥20.4), BMI ( <25, 25–<28, or ≥28 kg/m2), intervention group (alpha-tocopherol or no alpha-tocopherol, beta-carotene or no beta-carotene), serum alpha-tocopherol (low: <10.4 mg/L, medium: 10.4–<12.8 mg/L, or high: ≥12.8 mg/L), serum beta-carotene (low: <130 μg/L, medium: 130−<223 μg/L, or high: ≥223 μg/L), serum total cholesterol (low: 5.7 mmol/l, medium: 5.7–<6.6 mmol/l, or high: ≥6.6 mmol/l), self-reported CVD history (no, or yes), and years of follow-up (<13, 13–<23, or ≥23 years). Potential effect modification was examined through likelihood ratio tests that entered interaction terms for serum retinol (one SD) and each stratified factor into the multivariate-adjusted model and compared regression models with and without the interaction terms. Secondary analyses were restricted to participants without a history of CVD or diabetes mellitus (n = 12,488 men excluded). In a lag analysis, we further excluded the first 5 years of follow-up to minimize the potential bias due to reverse causation (n = 2,727 men excluded). In a sensitivity analysis, we examined serum retinol concentrations measured from blood collected in year three in relation to subsequent overall and cause-specific mortality (n = 22,312 men included). Baseline and year 3 serum retinol were highly correlated (Spearman correlation coefficient = 0.69).
We also performed a mediation analysis to quantify what portion of the association between overall mortality and known risk factors (including obesity, intensity of smoking, duration of smoking, diabetes mellitus, alcohol drinking and physical activity) was mediated through serum retinol concentration. Using the above-mentioned Cox proportional regression model, we estimated the HRs and 95% CIs, and adjusted for age, obesity (no: BMI < 30; yes: BMI ≥ 30), BMI (continuous), years of smoking (quintile), cigarettes per day (quintile), history of diabetes (no or yes, self-reported at baseline), alcohol consumption (quintile), physical activity (light or moderate physical activity: no or yes), serum total and HDL cholesterol (continuous), intervention assignment, systolic and diastolic blood pressure (continuous), and history of CVD (no or yes, self-reported at baseline). The traditional risk factors examined were mutually adjusted. We used the %MEDIATE SAS macro to estimate the mediation proportion and 95% CIs of serum retinol for the traditional risk factor-mortality associations47.
All statistical tests were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC) and the R statistical language (version 3.5.2; Vienna, Austria). All reported P-values are two-sided at type I error rate of 0.05. The Bonferroni correction threshold was applied to control for multiple tests (0.05/9 = 0.0056 for primary and secondary analyses [nine tests], and 0.05/10 = 0.005 for ten effect modification tests in subgroups).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Because of previously enacted EU Data Protection Regulation (GDPR) privacy rules and an existing data use agreement between Finland and the U.S. National Cancer Institute, the ATBC Study data, and materials described in the manuscript may not be made indiscriminately publicly available for the purposes of reproducing the findings. Please contact the ATBC Study Principal Investigators for appropriate specific data requests (https://atbcstudy.cancer.gov/). Data requests are subject to approval by the study review committees. For example, the review committee will check if the data requests containing information that could compromise research participant privacy or participant informed consent. The response time to data requests may be affected during the COVID-19 pandemic.
Code availability
The analytical methods for the current study are available from the corresponding author upon appropriate request. Data analysis packages using R and SAS software are listed in the Reporting summary.
References
Blomhoff, R. & Blomhoff, H. K. Overview of retinoid metabolism and function. J. Neurobiol. 66, 606–630 (2006).
Napoli, J. L. Retinoic acid: its biosynthesis and metabolism. Prog. Nucleic Acid Res. Mol. Biol. 63, 139–188 (1999).
Lengerke, C. et al. Interactions between Cdx genes and retinoic acid modulate early cardiogenesis. Dev. Biol. 354, 134–142 (2011).
Fletcher, A. E., Breeze, E. & Shetty, P. S. Antioxidant vitamins and mortality in older persons: findings from the nutrition add-on study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Am. J. Clin. Nutr. 78, 999–1010 (2003).
Ito, Y. et al. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac. J. Cancer Prev. 7, 533–546 (2006).
Bates, C. J., Hamer, M. & Mishra, G. D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 105, 123–132 (2011).
Goyal, A., Terry, M. B. & Siegel, A. B. Serum antioxidant nutrients, vitamin A, and mortality in U.S. Adults. Cancer Epidemiol. Biomark. Prev. 22, 2202–2211 (2013).
Min, K. B. & Min, J. Y. Relation of serum vitamin A levels to all-cause and cause-specific mortality among older adults in the NHANES III population. Nutr. Metab. Cardiovasc Dis. 24, 1197–1203 (2014).
Connolly, G. M., Cunningham, R., Maxwell, A. P. & Young, I. S. Decreased serum retinol is associated with increased mortality in renal transplant recipients. Clin. Chem. 53, 1841–1846 (2007).
Liu, Y. et al. Association of serum retinoic acid with risk of mortality in patients with coronary artery disease. Circ. Res. 119, 557–563 (2016).
Jayedi, A., Rashidy-Pour, A., Parohan, M., Zargar, M. S. & Shab-Bidar, S. Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Adv. Nutr. 9, 701–716 (2018).
Yu, Y. et al. Plasma retinol and the risk of first stroke in hypertensive adults: a nested case-control study. Am. J. Clin. Nutr. 109, 449–456 (2019).
Gey, K. F. et al. Low plasma retinol predicts coronary events in healthy middle-aged men: the PRIME Study. Atherosclerosis 208, 270–274 (2010).
Woodside, J. V. et al. Factors associated with serum/plasma concentrations of vitamins A, C, E and carotenoids in older people throughout Europe: the EUREYE study. Eur. J. Nutr. 52, 1493–1501 (2013).
Schober, P., Boer, C. & Schwarte, L. A. Correlation coefficients: appropriate use and interpretation. Anesthesia Analgesia 126, 1763–1768 (2018).
Carazo A. et al. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use, and toxicity. Nutrients 13, 1703 (2021).
Tanumihardjo, S. A. et al. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J. Nutr. 146, 1816S–1848S (2016).
Yee, M. M. F., Chin, K. Y., Ima-Nirwana, S. & Wong, S. K. Vitamin A and bone health: a review on current evidence. Molecules 26, 1757 (2021).
Borel, P. & Desmarchelier, C. Genetic variations associated with vitamin A status and vitamin A bioavailability. Nutrients 9, 246 (2017).
Michaelsson, K., Lithell, H., Vessby, B. & Melhus, H. Serum retinol levels and the risk of fracture. N. Engl. J. Med. 348, 287–294 (2003).
Moran, N. E., Mohn, E. S., Hason, N., Erdman, J. W. Jr. & Johnson, E. J. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 9, 465–492 (2018).
Feart, C. et al. Plasma retinol and association with socio-demographic and dietary characteristics of free-living older persons: the Bordeaux sample of the three-city study. Int J. Vitam. Nutr. Res. 80, 32–44 (2010).
Tu, W. J. et al. Lower serum retinoic acid level for prediction of higher risk of mortality in ischemic stroke. Neurology 92, e1678–e1687 (2019).
Brazionis, L., Walker, K. Z., Itsiopoulos, C. & O’Dea, K. Plasma retinol: a novel marker for cardiovascular disease mortality in Australian adults. Nutr. Metab. Cardiovasc. Dis. 22, 914–920 (2012).
Rohatgi, A. et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371, 2383–2393 (2014).
Liu, C. et al. Cholesterol efflux capacity is an independent predictor of all-cause and cardiovascular mortality in patients with coronary artery disease: a prospective cohort study. Atherosclerosis 249, 116–124 (2016).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364, 127–135 (2011).
Zhou, W. et al. Retinoic acid induces macrophage cholesterol efflux and inhibits atherosclerotic plaque formation in apoE-deficient mice. Br. J. Nutr. 114, 509–518 (2015).
Glass, C. K. & Witztum, J. L. Atherosclerosis. the road ahead. Cell 104, 503–516 (2001).
Camacho, M. et al. Retinoic acid induces PGI synthase expression in human endothelial cells. J. Lipid Res. 49, 1707–1714 (2008).
Yokota, J. et al. Retinoic acid suppresses endothelin-1 gene expression at the transcription level in endothelial cells. Atherosclerosis 159, 491–496 (2001).
Haxsen, V., Adam-Stitah, S., Ritz, E. & Wagner, J. Retinoids inhibit the actions of angiotensin II on vascular smooth muscle cells. Circ. Res. 88, 637–644 (2001).
Barstad, R. M., Hamers, M. J., Stephens, R. W. & Sakariassen, K. S. Retinoic acid reduces induction of monocyte tissue factor and tissue factor/factor VIIa-dependent arterial thrombus formation. Blood 86, 212–218 (1995).
Miano, J. M. et al. All-trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation 98, 1219–1227 (1998).
Neuville, P. et al. Retinoic acid regulates arterial smooth muscle cell proliferation and phenotypic features in vivo and in vitro through an RARalpha-dependent signaling pathway. Arterioscler Thromb. Vasc. Biol. 19, 1430–1436 (1999).
Achan, V. et al. All-trans-retinoic acid increases nitric oxide synthesis by endothelial cells: a role for the induction of dimethylarginine dimethylaminohydrolase. Circ. Res. 90, 764–769 (2002).
Zabetian-Targhi, F., Mahmoudi, M. J., Rezaei, N. & Mahmoudi, M. Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Adv. Nutr. 6, 748–762 (2015).
Graham, T. E. et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354, 2552–2563 (2006).
Ingelsson, E. & Lind, L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care 32, 733–735 (2009).
Chavarria, N. et al. Increased levels of retinol binding protein 4 in patients with advanced heart failure correct after hemodynamic improvement through ventricular assist device placement. Circ. J. 76, 2148–2152 (2012).
Lin, Y. C., Wu, T. C., Chen, P. Y., Hsieh, L. Y. & Yeh, S. L. Comparison of plasma and intake levels of antioxidant nutrients in patients with chronic obstructive pulmonary disease and healthy people in Taiwan: a case-control study. Asia Pac. J. Clin. Nutr. 19, 393–401 (2010).
Oh, J., Park, H. D., Kim, S. Y., Koh, W. J. & Lee, S. Y. Assessment of vitamin status in patients with nontuberculous mycobacterial pulmonary disease: potential role of vitamin A as a risk factor. Nutrients 11, 343 (2019).
Caram, L. M. et al. Serum vitamin A and inflammatory markers in individuals with and without chronic obstructive pulmonary disease. Mediators Inflamm. 2015, 862086 (2015).
Ito, Y. et al. Cancer mortality and serum levels of carotenoids, retinol, and tocopherol: a population-based follow-up study of inhabitants of a rural area of Japan. Asian Pac. J. Cancer Prev. 6, 10–15 (2005).
The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann. Epidemiol. 4, 1–10 (1994).
Milne, D. B. & Botnen, J. Retinol, alpha-tocopherol, lycopene, and alpha- and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin. Chem. 32, 874–876 (1986).
Hertzmark, E., Pazaris, M. & Spiegelman, D. The SAS MEDIATE Macro (Harvard TH Chan School of Public Health, 2012).
Acknowledgements
We appreciate all participants in the ATBC Study cohort for their contribution to this research. The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health. The funder of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
J.H. analyzed and drafted the manuscript. J.H. and D.A. conceived and designed the study. K.Y. checked the accuracy of data analysis. J.H., S.J.W., K.Y., S.M., and D.A. interpreted the results, critically revised the manuscript, and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J.H. and D.A. are the study guarantors and accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Corresponding authors
Ethics declarations
Competing interests
All author declares no competing interests.
Additional information
Peer review information Nature Communications thanks Catherine Feart, Heiko Becher, and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Weinstein, S.J., Yu, K. et al. Association between serum retinol and overall and cause-specific mortality in a 30-year prospective cohort study. Nat Commun 12, 6418 (2021). https://doi.org/10.1038/s41467-021-26639-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-26639-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.