Abstract
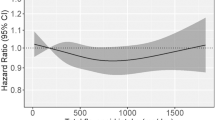
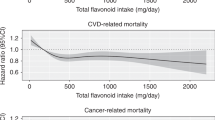
Using updated National Health and Nutrition Examination Survey (NHANES) follow-up data, and a large nationwide representative sample of adult U.S. citizens, the aim of this study was to explore the relationship between dietary flavonol intake, all-cause and cause-specific mortality risks. In this prospective cohort study based on NHANES (2007–2008, 2009–2010, and 2017–2018), a total of 11,679 participants aged 20 years and above were evaluated. The amount and type of food taken during a 24-h dietary recall were used to estimate dietary flavonol intake, which includes total flavonol, isorhamnetin, kaempferol, myricetin, and quercetin. Each analysis of the weighted data was dealt with in accordance with the NHANES reporting requirements' intricate stratification design. The Cox proportional risk regression model or Fine and Gray competing risks regression model were applied to evaluate all-cause and cause-specific mortality risks, respectively. The follow-up period was calculated using the time interval between the baseline and the death date or December 31, 2019 (whichever occurs first). Each data analysis was performed between October 1, 2023, and October 22, 2023. Dietary flavonol intake included total flavonol, isorhamnetin, kaempferol, myricetin, and quercetin. Up to December 31, 2019, National Death Index (NDI) mortality data were used to calculate mortality from all causes as well as cause-specific causes. A total of 11,679 individuals, which represents 44,189,487 U.S. non-hospitalized citizens, were included in the study; of these participants, 49.78% were male (n = 5816), 50.22% were female (n = 5, 863); 47.56% were Non-Hispanic White (n = 5554), 18.91% were Non-Hispanic Black (n = 2209), 16.23% were Mexican American (n = 1895), and 17.30% were other ethnicity (n = 2021); The mean [SE] age of the sample was 46.93 [0.36] years, with a median follow-up of 7.80 years (interquartile range, 7.55–8.07 years). After adjusting covariates, Cox proportional hazards models and fine and gray competing risks regression models for specific-cause mortality demonstrated that total flavonol intake was associated with all-cause (HR 0.64, 95% CI 0.54–0.75), cancer-specific (HR 0.45, 95% CI 0.28–0.70) and CVD-specific (HR 0.67, 95% CI 0.47–0.96) mortality risks; isorhamnetin intake was associated with all-cause (HR 0.72, 95% CI 0.60–0.86), and cancer-specific (HR 0.62, 95% CI 0.46–0.83) mortality risks; kaempferol intake was associated with all-cause (HR 0.74, 95% CI 0.63–0.86), and cancer-specific (HR 0.62, 95% CI 0.40–0.97) mortality risks; myricetin intake was associated with all-cause (HR 0.77, 95% CI 0.67–0.88), AD-specific (HR 0.34, 95% CI 0.14–0.85), and CVD-specific (HR 0.61, 95% CI 0.47–0.80) mortality risks; quercetin intake was associated with all-cause (HR 0.66, 95% CI 0.54–0.81), cancer-specific (HR 0.54, 95% CI 0.35–0.84), and CVD-specific (HR 0.61, 95% CI 0.40–0.93) mortality risks; there was no correlation observed between dietary flavonol intake and DM-specific mortality. According to the current study, all-cause, AD, cancer, and CVD mortality risks declined with increased dietary flavonoid intake in the U.S. adults. This finding may be related to the anti-tumor, anti-inflammatory, and anti-oxidative stress properties of flavonol.
Similar content being viewed by others
Introduction
Biologically active polyphenolic chemicals called flavonoids are present in a wide variety of common vegetables, fruits, and other plant-based meals1. Flavonoids can be divided into six main subclasses, namely flavonol, isoflavones, flavanones, flavonoid glycosides, flavonolignans, and flavones. Of these, flavonol is the primary representative of the flavonoid subclasses; it is also the flavonoid that is found the most widely in nature and is thought to be the most active1,2,3. Additionally, the primary flavonol components of tea, onions, and berries are quercetin, kaempferol, myricetin, and isorhamnetin4. Flavonoids are widely present in our customary diet and may produce numerous advantageous biological processes in the body when consumed. Previous research has produced strong and consistent evidence that flavonoids may improve endothelial function and maintain and enhance nitric oxide (NO) status5,6,7. What's more, there's evidence that these substances can impact lipid and glucose metabolism, platelet function and thrombosis, inflammation, oxidative damage, blood pressure, and inflammation8,9,10. Furthermore, by specifically targeting important molecules and signaling pathways in a range of tumor cells, flavonoids can cause apoptosis and prevent cell growth and metastasis11,12,13,14. The discovery that food rich in flavonoids has cardioprotective and anti-tumor effects may be explained by these effects15,16.
Since 1999, the National Health and Nutrition Examination Survey (NHANES), which is population-based research, has been published every two years. It provides a nationwide typical sample of the U.S. non-institutional population11. The relationship between flavonol intake (i.e., total flavonol, isorhamnetin, kaempferol, myricetin, and quercetin) and cause-specific mortality (i.e., AD, cancer, CVD, and DM) in appropriate models has not been thoroughly reported in any literature, much less analyses that account for significant confounders and include trend chi-square tests for flavonoid intake and mortality. Based on the latest and comprehensive data from NHANES, we aim to thoroughly demonstrate the relationship between flavonoid intake and all-cause and cause-specific mortality risks, as well as its potential as an independent prognostic indicator for risk assessment of various diseases.
Results
Basic characteristic of including participants
In the 2007–2008, 2009–2010 and 2018–2019 circles of NHANES, the overall 11,679 participants aged 20 years and above were evaluated, representing 44,189,487 U.S. non-institutionalized residents. Table 1 listed the minimum, 25th percentile, median, mean, 75th percentile, and maximum of each flavonol intake. Table 2 provided an overview for the basic characteristics of included participants. The outcome presented that subjects in the highest category of total flavonol intake had higher proportion in groups with the following characteristics: males (1682 [55.84%] vs. 1238 [44.16%]), tended to be younger (≥ 80 y, 107 [2.15%] vs. 3.76 [15.72%]), Non-Hispanic White (1542 [74.6%] vs. 429 [6.78%]), married or living with a partner (1933 [67.95%] vs. 987 [32.05%]), educational attainment ≥ HS (2306 [86.84%] vs. 614 [13.16%]), at or above poverty line (2433 [89.63%] vs 487 [10.37%]), having alcohol consumption (2619 [92.80%] vs. 301 [7.20%]), BMI 18.5–30.0 (1785 [63.50%] vs. 40 [1.29%]), having history of DM (8467 [72.50%] vs. 3212 [27.5%]), hypertension (6718 [57.52%] vs. 4961 [42.48%]), hyperlipidemia (3104 [26.58%] vs. 8575 [73.42%]), congestive heart failure (11,352 [97.20%] vs. 327 [2.80%]), coronary heart disease (11,207 [95.96%] vs. 472 [4.04%]), angina (11,390 [97.53%] vs. 289 [2.47%]), heart attack (11,165 [95.60%] vs. 514 [4.40%]), and stroke (11,206 [95.95%] vs. 473 [4.05%]). Further, participants with intakes of isorhamnetin, kaempferol, myricetin, and quercetin also showed similar characteristics. More detailed information about basic characteristics of participants and dietary flavonol intake was presented in Table 2.
Different flavonol intake level, survival status and cause of death in participants
Table 3 presented the general characteristics of flavonol intake in participants by survival status or cause of death. As the intake of total flavonol increases, there is a decreasing trend in all-cause mortality, AD-specific mortality, cancer-specific mortality, and CVD-specific mortality (all P values for trend < 0.050). When isorhamnetin intake increases, there is a declining tendency in all-cause mortality, AD mortality, cancer mortality, CVD mortality, DM mortality, and other cause mortality (all P values for trend < 0.050). As the intake of kaempferol increases, there is a decreasing trend in all-cause mortality, AD-specific mortality, cancer-specific mortality, CVD-specific mortality, and other cause mortality (all P for trend < 0.050). As the intake of myricetin increases, there is a decreasing trend in AD mortality (P for trend = 0.003). With increasing quercetin intake quartiles, there is a trend toward lowering all-cause mortality, cancer mortality, and other cause mortality (all P for trend < 0.050).
All-cause mortality by basic characteristics of sociodemographic variables and disease history
We applied Cox regression analysis to explore the relationship between basic characteristics (including sociodemographic variables, and disease history) and all-cause mortality in U.S. participants, and the detailed outcome was presented in Table 4. It is important to notice that HRs increased significantly every ten years of age. Female (HR 0.77; 95% CI 0.69–0.86), and Mexican American (HR 0.36; 95% CI 0.28–0.47), educational attainment ≥ high school (HR 0.49; 95% CI 0.43–0.57), being married or living with a partner (HR 0.64; 95% CI 0.56–0.72), having alcohol consumption (HR 0.60; 95% CI 0.46–0.78) were significantly related to a lower risk of all-cause mortality. What’s more, the covariates including BMI < 18.5 (HR 1.76; 95% CI 1.11–2.81), having disease history including DM (HR 2.99; 95% CI 2.55–3.51), hypertension (HR 4.25; 95% CI 3.62–5.00), hyperlipidemia (HR 4.25; 95% CI 3.62–5.00), congestive heart failure (HR 8.42; 95% CI 6.89–10.29), coronary heart disease (HR 5.35; 95% CI 4.58–6.24), angina (HR 3.70; 95% CI 2.86–4.79), heart attack (HR 4.67; 95% CI 3.85–5.67), heart attack (HR 5.54; 95% CI 4.25–7.21) were significantly related to higher risk of all-cause mortality.
Association between dietary flavonol intake and all-cause mortality
After adjustment for age, gender, race/ethnicity, marital status, education level, poverty income ratio, alcohol consumption, BMI, and disease history, multivariate Cox regression analysis showed a significant correlation between total flavonol intake in the highest category and all-cause mortality risk (HR 0.64, 95% CI 0.54–0.75, P for trend < 0.001 ), compared to the lowest quartile (Table 5). Further, we investigated the associations between intake of four types of flavonol and all-cause mortality risks. We observed that isorhamnetin (HR 0.72, 95% CI 0.60–0.86, P for trend < 0.001), kaempferol (HR 0.74, 95% CI 0.63–0.86, P for trend < 0.001), myricetin (HR 0.77, 95% CI 0.67–0.88, P for trend < 0.001) and quercetin (HR 0.66, 95% CI 0.54–0.81, P for trend < 0.001) intakes in the highest quartile were all related to all-cause mortality risks, compared to the lowest quartile (Table 5).
Association between dietary flavonol intake and cause-specific mortality
Of the 11,679 subjects in this study, 43 (0.03%) died of AD, 336 (2.88%) died of cancer, 486 (4.16%) died of CVD, 46 (0.40%) died of DM, and 457 (3.91%) died from other causes. After adjusting covariates, the multivariate cox regression analysis showed that total flavonol intake in the highest quartile was significantly associated with lower cancer-specific mortality (HR 0.45, 95% CI 0.28–0.70, P for trend = 0.003), CVD-specific mortality (HR 0.67, 95% CI 0.47–0.96, P for trend = 0.080) and other cause mortality (HR 0.64, 95% CI 0.51–0.81, P for trend = 0.003), compared to the lowest quartile; isorhamnetin intake in the highest quartile was significantly related to lower cancer-specific mortality (HR 0.62, 95% CI 0.46–0.83, P for trend = 0.010), and other cause mortality (Q4 vs. Q1, HR 0.66, 95% CI 0.47–0.92, P for trend = 0.010), compared to the lowest quartile; kaempferol intake in the highest category was significantly related to lower cancer-specific mortality (HR 0.62, 95% CI 0.40–0.97, P for trend = 0.020), compared to the lowest category; myricetin intake in the highest category was significantly related to lower AD-specific mortality risk (HR 0.34, 95% CI 0.14–0.85, P for trend = 0.090), cancer-specific mortality risk (HR 0.65, 95% CI 0.42–0.99, P for trend = 0.010), CVD-specific mortality risk (HR 0.61, 95% CI 0.47–0.80, P for trend < 0.001), and other cause mortality risk (HR 0.70, 95% CI 0.53–0.91, P for trend = 0.010), compared to the lowest category; quercetin intake in the highest quartile was significantly associated with lower cancer-specific mortality risk (HR 0.54, 95% CI 0.35–0.84, P for trend = 0.004), CVD-specific mortality risk (HR 0.61, 95% CI 0.40–0.93, P for trend = 0.130), and other cause mortality risk (HR 0.61, 95% CI 0.46–0.80, P for trend < 0.001), compared to the lowest quartile (Table 5).
Subgroup analysis stratified by race/ethnicity
After adjustment for age, gender, race/ethnicity, marital status, education level, poverty income ratio, alcohol consumption, BMI, and disease history, the subgroup analysis stratified by race/ethnicity showed that total flavonol (HR 0.59, 95% CI 0.49–0.72, P for trend < 0.001), isorhaminetin (HR 0.63, 95% CI 0.52–0.77, P for trend < 0.001), kaempferol (HR 0.62, 95% CI 0.50–0.76, P for trend < 0.001), myricetin (HR 0.97, 95% CI 0.81–1.17, P for trend < 0.001) and quercetin (HR 0.68, 95% CI 0.55–0.83, P for trend < 0.001) intake in highest quartile were significantly associatied with lower all-cause mortality risk in Non-Hispanic White, compared with lowest quartile. More detailed outcomes were presented in Table 6. Compared to Non Hispanic Black, Mexican American, and other race/enthnicity, dietary flavonol intake has a more significant protective effect on the Non Hispanic White population.
Sensitivity analysis
In our sensitivity analysis, we applied the final model (Cox proportional risk regression model and Fine and Gray competing risk regression model) including squared age in order to adjust the potential non-linear association between age and mortality. The observed results were consistent with those shown in the main analysis, which indicated our results were relatively robust (Table 5).
Discussion
Established the up-to-date NHANES follow-up data and NDI mortality data, we evaluated a nationally representative sample of 11,679 U.S. citizens aged 20 years old and above, representing 44,189,187 non-institutionalized U.S. residents, and we observed that flavonol intake was significantly related to lower all-cause mortality risk. What’s more, intakes of total flavonol, isorhamnetin, kaempferol and quercetin were associated with lower cancer-specific mortality risk. Meanwhile, intakes of total flavonol, quercetin and myricetin were associated with reduced CVD-specific mortality risk. We also observed that myricetin intake was related to decreased mortality risk for AD-specific causes. As a result, consumption of flavonoids is protective against all cause, cancer, and CVD-mortality in particular. Subgroup analysis based on age further revealed that flavonoid intake had a more significant protective effect on all-cause mortality reduction in those aged ≥ 40 years compared to participants aged < 40 years.
Compared to 2020, the global burden of cancer is estimated to increase by 47% in 2040, from 19.3 million cases to 28.4 million cases. Approximately one-third of cancer cases in affluent nations are linked to physical activity, nutrition, and food. It has been suggested that consuming an appropriate amount of fruits and vegetables high in flavonoids helps prevent cancer17. This study suggests an inverse association between flavonol intake and cancer mortality, which is consistent with the conclusions of previous epidemiological studies by Bondonno et al.18 and Zhou et al.19. The previous meta-analysis also highlighted the negative relationship between flavonoid intake and the incidence rate of some cancers, including breast cancer20, prostate cancer21, lung cancer, gastric cancer and colorectal cancer22, and cancers related to smoking22. There is evidence that flavonol inhibit to the development and spread of cancer in vitro. For example, by focusing on the phosphoinositide 3-kinase / protein kinase B signaling pathway, kaempferol can suppress epithelial mesenchymal transition and cause apoptosis and cell cycle arrest in the G2 / M phase23. By targeting cell cycle proteins, quercetin causes cell cycle arrest in the G1 phase24, which prevents cells from proliferating. Furthermore, a high quercetin content can prevent the cell cycle from moving from G0/G1 to G2/M25. Quercetin has an impact on the cell cycle, but it can also cause apoptosis by signaling the pro-apoptotic PI3K/Akt and mitogen-activated protein kinase pathways26,27. Similar to other flavonol, however, it should be noted that flavonoids can exhibit toxic effects at high doses28,29. After the phenolic ring containing flavonoids is oxidized by oxidative enzymes, cytotoxic phenoxyl radicals are generated; unsaturated lipids, nucleic acids, ascorbate, nicotinamide, adenine dinucleotide, and glutathione are all oxidized by CO, which produces reactive oxygen species and is harmful to mitochondria. In some circumstances, quercetin and other flavonoids have been shown to cause a notable frequency of sister chromatid swaps and micronuclei as well as to suppress cell growth by their pro-oxidant properties30. Thus, it is necessary to look at how dietary supplements containing flavonoids affect cancer survival.
It is noteworthy that, following numerous confounding factor adjustments, we discovered that the highest flavonoid intake was linked to a lower risk of CVD death in comparison to the lowest consumption. This outcome is in line with the findings of a previous meta-analysis performed by Huxley et al., which revealed that there may be a negative correlation between the death rate from coronary heart disease and flavonol intake31. In a meta-analysis including sixteen studies, Wang et al. found that a 20 mg/d increase in flavonol consumption was linked to a 14% reduction in the risk of stroke (RR, 0.86; 95% CI 0.77–0.96)32. The possible mechanisms by which flavonol reduce the risk of CVD may involve multiple pathways, which have been reported to be generally associated with their Vasodilatory, anti-inflammatory, and antioxidant properties33,34. Using flavonol as an example, a large body of research indicates that quercetin and other related flavonol may provide protection against the most prevalent CVD. By preventing one or more processes linked to the development of the disease, such as oxidative stress, endothelial dysfunction, and inflammation, flavonol protects against atherosclerosis3. By reducing endothelial dysfunction, hypertension, and atherosclerosis, flavonol can protect coronary arteries3. The majority of acute coronary events are caused by myocardial ischemia following the rupture of atherosclerotic plaque. On the other hand, quercetin can stabilize atherosclerotic plaques by decreasing matrix metalloproteinase expression35. Flavonoids may affect stroke at different stages. Flavonol can decrease excitatory toxicity, regulate oxidative stress, stop platelet aggregation and thrombosis, and enhance cerebral blood flow during the acute phase36. In the intermediate stage, flavonol can protect the integrity of endothelial cells and reduce inflammatory responses37,38. Flavonol may obstruct ischemia-induced cell death pathways such as necrosis and apoptosis in later stages39,40,41.
DM remains one of the major healthcare challenges in the world. Although there is a growing understanding of the pathophysiology of diabetes, currently available treatment methods can only provide a temporary hypoglycemic effect, and cannot completely prevent the development of these abnormalities42. Previous literature has reported that flavonol has the potential of anti- diabetes. For example, Yang et al. reported that kaempferol can regulate lipid metabolism, improve insulin resistance, improve insulin signal transduction, and restore the imbalance between autophagy and apoptosis to protect β cells43. Kalai et al. reviewed that isorhamnetin can lower the impact of diabetes related diseases by reducing glucose level, improving oxidation status, reducing inflammation, and regulating lipid metabolism and adipocyte differentiation44. Li et al. reported that long-term intake of myricetin can inhibit apoptosis of pancreatic islets β cells and regulation of glucose levels, and myricetin can induce glucose dependent insulin secretion45. According to Eid et al., quercetin regulates systemic glucose homeostasis by interacting with numerous molecular targets in the liver, skeletal muscle, adipose tissue, small intestine, and pancreas46. The anti -diabetes mechanism of quercetin involves inhibition of intestinal glucose absorption, insulin secretion, insulin sensitizing activity, and improvement of glucose utilization in peripheral tissues. However, our results did not indicate significant correlations between flavonol intake and DM-specific mortality (all P values > 0.05). Whether the flavonol intake can prevent the progress of diabetes and protect diabetic patients still needs to be further explored by longitudinal studies in the population.
Alzheimer's disease (AD) is one of the most prevalent types of age-related neurodegenerative illness. There is an urgent need for effective treatment and prevention strategies since the present therapy options are not able to appreciably modify the development of AD. Myricetin was related to a lower risk of AD-specific mortality in this investigation. Myricetin has been shown to be helpful in treating Alzheimer's disease (AD) in animal trials. Ramezani et al. showed that intraperitoneal injection of myricetin dramatically raised the number of hippocampal CA3 pyramidal neurons in Alzheimer's disease rats, improving learning and memory problems47. Myricetin treatment dramatically reversed the novel effects of polyamines in the AD mouse model, which included downregulating brain iron and inhibiting acetylcholinesterase (AChE). Furthermore, myricetin administration improves the activity of antioxidant enzymes and decreases oxidative damage in mice45. In summary, our research indicates that consuming more myricetin may help stop or slow the progression of AD.
Previous studies did not take into account the competing risks of death when calculating specific causes of death. In our approach, we tackled this methodological challenge by employing a multiple confounder-adjusted competing risks model. This allowed us to elucidate the significant association between dietary flavonol intake and all-cause and specific-cause mortality risks. Nevertheless, the study still includes the following limitations: first, more than half of the sample size lacks data on dietary flavonol intake in the NHANES database, and the remaining 11,679 participants may not be nationally representative. Second, only an estimate of the flavonol dose was made at baseline, therefore it may not precisely reflect the flavonol intake over the course of the investigation. Third, the NHANES collected the representative participants in the North America, the western dietary pattern is most prevalent, which has an impact on the dietary composition of flavonol and other nutrients. Due to the limitation of database, we were unable to give the main food sources for dietary flavonol intake. Further, subgroup analysis showed that flavonol intake had a significant protective effect on mortality in the Non-Hispanic White population, suggesting that dietary patterns of different race/ethnicity may play an important role. Fourth, since the adjusted confounding factors in the model do not include total energy intake, micronutrient/antioxidant supplement intake, vitamin intake, and other dietary factors like lycopene or green tea catechins, it may be impossible to exclude the influence of these factors on the observed effect of flavonol intake.
Conclusion
Through comprehensive updating NHANES records, this study concluded that dietary flavonol intake was significantly linked with overall, AD, cancer, and CVD-specific mortality risks. The outcome of our research elucidated the relationship between flavonol intake, all-cause, and cause-specific mortality risks in a sample representing the entire nation of non-hospitalized citizens in the United States, presenting evidence for flavonol intake as an independent, practical, quantitative, and reliable predictor of disease survival status, this means that it is suitable for the health and risk alert management of AD, CVD and cancer patients. Our findings have practical significance for public health, because flavonol can be supplemented by making daily dietary modifications and eating habits better.
Methods
Study population
Dietary Flavonol intake data in this study were collected from three cycles of the nationwide representative NHANES, corresponding to years 2007–2008, 2009–2010, and 2017–2018. The National Center for Health Statistics, which examined the health and nutritional status of U.S. citizens using a nationally representative sample, sponsored and supervised NHANES22. The three cycles NHANES provided a total of 111,066 participants. 84,841 participants were removed because they did not have dietary flavonol intake values from FNDDS, the percentage of missing values for dietary flavonol intake were 76.4%. Additionally, 14,546 individuals were excluded because they were who had no data on marital status, educational attainment, poverty income ratio, alcohol consumption, and body mass index (BMI), and disease history (Fig. 1). Finally, the present analyses were based on a total of 11,679 individuals who were aged ≥ 20 y and had completed a battery of questionnaires, in-person assessments, and laboratory tests were mandated for participants, either at the mobile examination center (MEC) or at home. The National Center for Health Statistics Research Ethics Review Board approved the NHANES programs, and all study participants gave written informed consent and followed the guidelines of the Helsinki's Declaration48. Relevant ethical certifications can be found in Supplement A.
Dietary flavonol intake assessment
The purpose of conducting a dietary interview through the 24-h recall method is to gather comprehensive information about the dietary intake of NHANES participants . This method involves obtaining detailed data on the types and quantities of foods and beverages (including all forms of water) consumed in the 24-h period preceding the interview (midnight to midnight). The collected information is then used to estimate nutrient intake and other food components from the reported foods and beverages. Each NHANES participant is eligible for two 24-h dietary recall interviews, with the first interview conducted in-person at the Mobile Examination Center (MEC), and the second interview conducted via telephone 3 to 10 days later. In our study, the data on dietary flavonol intake were sourced from the USDA Survey Food and Beverage Flavonoid Values database established in 2003–200449. This database contains flavonol values for various foods and beverages listed in the USDA Food and Nutrient Database for Dietary Studies (FNDDS), along with corresponding dietary data from NHANES50. The specific amounts of total flavonols, including isohamnetin, kaempferol, myricetin, and quercetin, measured in milligrams per 100 g, were determined for each food and beverage by the USDA Nutrient Data Laboratory51. The calculation of dietary flavonol intake was performed on a daily basis.
Mortality data
For this investigation, the National Death Index (NDI) file and the 2019 Public Access Link mortality dataset were utilized to generate mortality data using a probabilistic matching technique52. The personal identification information of all NHANES participants aged 18 and above, including name, gender, date of birth, etc., is matched between the NHANES and NDI databases. Codes C00-C97 were designated as cancer-specific causes of death under the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). Causes of mortality directly connected to cardiovascular disease (CVD) were identified as heart disease (ICD-10: I00-I09, I11, I13, I20-I51), and cerebrovascular disease (ICD-10: I60-I69). Codes E10-E14 in were used to identify the causes of mortality that were primarily related to diabetes mellitus (DM). Code G30 in ICD-10 was used to identify the cause of mortality that associated with Alzheimer's disease (AD). Other causes of death were considered for the remaining fatalities that were not categorized. The follow-up period was determined by taking time from the date of the baseline interview and death or the end date of the study review, which is December 31, 2019, whichever comes first.
Covariates
In this study, participants from NHANES were stratified by sociodemographic variables and disease history. Age was grouped as: 20–29 y, 30–39 y, 40–49 y, 50–59 y, 60–69 y, 70–79 y, and ≥ 80 y. Sex was coded as male or female. Race/ethnicity was classified as: non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, and other. Marital status was coded as: unmarried or other, married or living with a partner. Education level was coded as lower than high school (< HS), at or higher than high school (≥ HS). Poverty ratio was grouped as: below poverty line (< 1.00), at or above poverty line (≥ 1.00). Alcohol consumption was classified as: no, and yes. BMI, calculated as weight in kilograms divided by height in meters squared, was grouped as: < 18.5, 18.5–30.0, ≥ 30.0 kg/m2. Disease history (yes/no) included DM, hypertension, hyperlipidemia, congestive heart failure, coronary heart disease, angina, heart attack, and stroke.
Statistical analysis
Three NHANES cycles (2007–2008, 2009–2010, and 2017–2018) were integrated into one dataset, and all analyses were carried out using the design that the NHANES Analytics and Reporting Guide supplied. The intake of dietary flavonol including total flavonol, isorhamnetin, kaempferol, myricetin, and quercetin, was classified by quartiles, with quartile 1 (Q1) indicating the lowest intake, and quartile 4 (Q4) indicating the highest intake. The basic characteristics of each participant and their dietary flavonol intake distribution were described using numbers and weighted percentages for included categorical variables. These comprised the following: age, sex, race/ethnicity, marital status, educational attainment, poverty income ratio, alcohol consumption, and BMI. A chi-square test was applied for categorical variables. Then, we described the number and percentage of overall deaths and specific-cause deaths, corresponding to different intake levels of flavonol. And trend chi-square test was applied to determine the relationship between dietary flavonol intake and death outcomes in participants. To determine basic characteristics (sociodemographic variables and disease history) related to survival endpoints, we performed the Cox regression analysis adjusted age and sex to estimate the hazard ratio (HR) and 95% confidence interval (CI) for survival. Further, covariates significantly correlated with mortality and dietary flavonol intake were added to the Cox regression model, which calculated the HR and population attributed risk of dietary flavonol intake on mortality. We applied the Fine and Gray competing risks regression model to estimate specific-cause (e.g., AD, cancer, CVD, DM) mortality risks, given that deaths from other causes can be considered the competing risk event for one specific cause of death53, and this method has been used by previous studies with high quality54,55. Sensitivity analyses was perform by adjusting for age and squared age to evaluate the potential non-linear association between age and mortality. The Variance Inflation Factor (VIF) was used to examine the covariate effect, and all VIFs were less than 3.00. The P-values on both sides are less than 0.05, which is considered statistically significant. R software (version 4.2.1, http://www.R-project.org) was applied to analyze all the data, with packages "nhanesR", "reshape2 ", "survey", "do", "dplyr" and others.
Ethics statement
These studies involving humans have been approved by the Ethics Review Board of the National Center for Health Statistics. The studies were conducted in accordance with local legislation and institutional requirements. According to national legislation and institutional requirements, participants or their legal guardians/next of kin do not require written informed consent.
Data availability
The datasets generated during the current study are freely available without restriction in the NHANES repository (http://www.cdc.gov/nchs/nhanes.htm).
References
Erdman, J. W. Jr. et al. Flavonoids and heart health: Proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. J. Nutr. 137, 718S-737S. https://doi.org/10.1093/jn/137.3.718S (2007).
Cassidy, A. et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 93, 338–347. https://doi.org/10.3945/ajcn.110.006783 (2011).
Perez-Vizcaino, F. & Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects Med. 31, 478–494. https://doi.org/10.1016/j.mam.2010.09.002 (2010).
Holland, T. M. et al. Association of dietary intake of flavonols with changes in global cognition and several cognitive abilities. Neurology 100, e694–e702. https://doi.org/10.1212/WNL.0000000000201541 (2023).
Loke, W. M. et al. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 88, 1018–1025. https://doi.org/10.1093/ajcn/88.4.1018 (2008).
Schroeter, H. et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. U S A 103, 1024–1029. https://doi.org/10.1073/pnas.0510168103 (2006).
Karim, M., McCormick, K. & Kappagoda, C. T. Effects of cocoa extracts on endothelium-dependent relaxation. J. Nutr. 130, 2105S-2108S. https://doi.org/10.1093/jn/130.8.2105S (2000).
Hodgson, J. M. & Croft, K. D. Tea flavonoids and cardiovascular health. Mol. Aspects Med. 31, 495–502. https://doi.org/10.1016/j.mam.2010.09.004 (2010).
Al-Dashti, Y. A., Holt, R. R., Stebbins, C. L., Keen, C. L. & Hackman, R. M. Dietary flavanols: A review of select effects on vascular function, blood pressure, and exercise performance. J. Am. Coll. Nutr. 37, 553–567. https://doi.org/10.1080/07315724.2018.1451788 (2018).
Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 96, 305–312. https://doi.org/10.1016/j.biopha.2017.10.001 (2017).
Davatgaran-Taghipour, Y. et al. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 12, 2689–2702. https://doi.org/10.2147/IJN.S131973 (2017).
Abotaleb, M. et al. Flavonoids in cancer and apoptosis. Cancers 11, 28. https://doi.org/10.3390/cancers11010028 (2018).
Zhang, H. W. et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kgamma mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 8, 11255. https://doi.org/10.1038/s41598-018-29308-7 (2018).
Masuelli, L. et al. In vitro and in vivo anti-tumoral effects of the flavonoid apigenin in malignant mesothelioma. Front. Pharmacol. 8, 373. https://doi.org/10.3389/fphar.2017.00373 (2017).
Zhou, Z. G., Li, D. D., Chen, Y., Chen, X. & Man, R. J. Discussion on the structural modification and anti-tumor activity of flavonoids. Curr. Top. Med. Chem. 22, 561–577. https://doi.org/10.2174/1568026622666220308162049 (2022).
Syahputra, R. A., Harahap, U., Dalimunthe, A., Nasution, M. P. & Satria, D. The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: A review. Molecules 27, 1320. https://doi.org/10.3390/molecules27041320 (2022).
Wiseman, M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc. Nutr. Soc. 67, 253–256. https://doi.org/10.1017/S002966510800712X (2008).
Bondonno, N. P. et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort. Nat. Commun. 10, 3651. https://doi.org/10.1038/s41467-019-11622-x (2019).
Zhou, Y., Gu, K. & Zhou, F. Dietary flavonoid intake and cancer mortality: A population-based cohort study. Nutrients 15, 976. https://doi.org/10.3390/nu15040976 (2023).
Hui, C. et al. Flavonoids, flavonoid subclasses and breast cancer risk: a meta-analysis of epidemiologic studies. PLoS ONE 8, e54318. https://doi.org/10.1371/journal.pone.0054318 (2013).
Wu, S. H. & Liu, Z. Soy food consumption and lung cancer risk: A meta-analysis using a common measure across studies. Nutr. Cancer 65, 625–632. https://doi.org/10.1080/01635581.2013.795983 (2013).
Woo, H. D. & Kim, J. Dietary flavonoid intake and smoking-related cancer risk: A meta-analysis. PLoS ONE 8, e75604. https://doi.org/10.1371/journal.pone.0075604 (2013).
Imran, M. et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 24, 2277. https://doi.org/10.3390/molecules24122277 (2019).
Jeong, J. H., An, J. Y., Kwon, Y. T., Rhee, J. G. & Lee, Y. J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 106, 73–82. https://doi.org/10.1002/jcb.21977 (2009).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hebert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17, 1689–1696. https://doi.org/10.1017/S1368980013002115 (2014).
Ren, M. X., Deng, X. H., Ai, F., Yuan, G. Y. & Song, H. Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 10, 579–583. https://doi.org/10.3892/etm.2015.2536 (2015).
Deng, X. H., Song, H. Y., Zhou, Y. F., Yuan, G. Y. & Zheng, F. J. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp. Ther. Med. 6, 1155–1158. https://doi.org/10.3892/etm.2013.1285 (2013).
Galati, G. & O’Brien, P. J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 37, 287–303. https://doi.org/10.1016/j.freeradbiomed.2004.04.034 (2004).
Skibola, C. F. & Smith, M. T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 29, 375–383. https://doi.org/10.1016/s0891-5849(00)00304-x (2000).
Engen, A. et al. Induction of cytotoxic and genotoxic responses by natural and novel quercetin glycosides. Mutat. Res. Genet. Toxicol. Environ. Mutagen 784–785, 15–22. https://doi.org/10.1016/j.mrgentox.2015.04.007 (2015).
Huxley, R. R. & Neil, H. A. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur J Clin Nutr 57, 904–908. https://doi.org/10.1038/sj.ejcn.1601624 (2003).
Wang, Z. M. et al. Flavonol intake and stroke risk: A meta-analysis of cohort studies. Nutrition 30, 518–523. https://doi.org/10.1016/j.nut.2013.10.009 (2014).
Middleton, E. Jr., Kandaswami, C. & Theoharides, T. C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52, 673–751 (2000).
Fisher, N. D., Hughes, M., Gerhard-Herman, M. & Hollenberg, N. K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 21, 2281–2286. https://doi.org/10.1097/00004872-200312000-00016 (2003).
Motoyama, K. et al. Atheroprotective and plaque-stabilizing effects of enzymatically modified isoquercitrin in atherogenic apoE-deficient mice. Nutrition 25, 421–427. https://doi.org/10.1016/j.nut.2008.08.013 (2009).
Simonyi, A. et al. Polyphenols in cerebral ischemia: Novel targets for neuroprotection. Mol. Neurobiol. 31, 135–147. https://doi.org/10.1385/MN:31:1-3:135 (2005).
Patil, C. S. et al. Protective effect of flavonoids against aging- and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology 69, 59–67. https://doi.org/10.1159/000072357 (2003).
Kao, T. K. et al. Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci. 86, 315–321. https://doi.org/10.1016/j.lfs.2009.12.014 (2010).
Silva, B., Oliveira, P. J., Dias, A. & Malva, J. O. Quercetin, kaempferol and biapigenin from Hypericum perforatum are neuroprotective against excitotoxic insults. Neurotox. Res. 13, 265–279. https://doi.org/10.1007/BF03033510 (2008).
Mercer, L. D., Kelly, B. L., Horne, M. K. & Beart, P. M. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: Investigations in primary rat mesencephalic cultures. Biochem. Pharmacol. 69, 339–345. https://doi.org/10.1016/j.bcp.2004.09.018 (2005).
Echeverry, C. et al. Pretreatment with natural flavones and neuronal cell survival after oxidative stress: A structure-activity relationship study. J. Agric. Food Chem. 58, 2111–2115. https://doi.org/10.1021/jf902951v (2010).
Lotfy, M., Adeghate, J., Kalasz, H., Singh, J. & Adeghate, E. Chronic complications of diabetes mellitus: A mini review. Curr. Diabetes Rev. 13, 3–10. https://doi.org/10.2174/1573399812666151016101622 (2017).
Yang, Y. et al. Mechanisms of Kaempferol in the treatment of diabetes: A comprehensive and latest review. Front. Endocrinol. 13, 990299. https://doi.org/10.3389/fendo.2022.990299 (2022).
Kalai, F. Z., Boulaaba, M., Ferdousi, F. & Isoda, H. Effects of isorhamnetin on diabetes and its associated complications: A review of in vitro and in vivo studies and a post hoc transcriptome analysis of involved molecular pathways. Int. J. Mol. Sci. 23, 704. https://doi.org/10.3390/ijms23020704 (2022).
Wang, B., Zhong, Y., Gao, C. & Li, J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 490, 336–342. https://doi.org/10.1016/j.bbrc.2017.06.045 (2017).
Eid, H. M. & Haddad, P. S. The antidiabetic potential of quercetin: Underlying mechanisms. Curr. Med. Chem. 24, 355–364. https://doi.org/10.2174/0929867323666160909153707 (2017).
Ramezani, M., Darbandi, N., Khodagholi, F. & Hashemi, A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer’s disease. Neural Regen. Res. 11, 1976–1980. https://doi.org/10.4103/1673-5374.197141 (2016).
Bundy, J. D. et al. Social determinants of health and premature death among adults in the USA from 1999 to 2018: A national cohort study. Lancet Public Health 8, e422–e431. https://doi.org/10.1016/s2468-2667(23)00081-6 (2023).
USDA Special Interest Databases on Flavonoids. https://www.ars.usda.gov/northeast-area/beltsvillemd-bhnrc/beltsville-human-nutrition-research-center/methods-and-application-of-food-composition-laboratory/mafcl-site pages/flavonoids/ (accessed on 23 November 2022).
FNDDS Documentation and Databases. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsvillehuman-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/ (accessed on 23 November 2022).
Harnly, J. M. et al. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 54, 9966–9977. https://doi.org/10.1021/jf061478a (2006).
Loprinzi, P. D. & Addoh, O. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease-specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin. Proc. 91, 763–769. https://doi.org/10.1016/j.mayocp.2016.03.019 (2016).
Pfirrmann, M., Lauseker, M., Hoffmann, V. S. & Hasford, J. Prognostic scores for patients with chronic myeloid leukemia under particular consideration of competing causes of death. Ann. Hematol. 94(Suppl 2), S209-218. https://doi.org/10.1007/s00277-015-2316-0 (2015).
Gui, S. Y. et al. Association of retinopathy with risk of all-cause and specific-cause mortality in the National Health and Nutrition Examination Survey, 2005 to 2008. Front. Public Health 11, 1200925. https://doi.org/10.3389/fpubh.2023.1200925 (2023).
Zhu, Z., Wang, W., Keel, S., Zhang, J. & He, M. Association of age-related macular degeneration with risk of all-cause and specific-cause mortality in the National Health and Nutrition Examination Survey, 2005 to 2008. JAMA Ophthalmol. 137, 248–257. https://doi.org/10.1001/jamaophthalmol.2018.6150 (2019).
Funding
This research was funded by 2021 Anhui Provincial University Collaborative Innovation Project (No. GXXT-2021–015) and Clinical Research and Cultivation Plan of the Second Affiliated Hospital of Anhui Medical University (No. 2020LCYB13).
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.Q.Z. and X.C.; methodology, Z.Q.Z.; software, Z.Q.Z. and J.C.Q.; validation, Y.Y., J.Q.H., and F.F.L.; formal analysis, Z.Q.Z.; writing-original draft preparation, Z.Q.Z.and X.C; writing, review and editing, Y.Y., J.Q.H., and F.F.L.; visualization, Z.Q.Z.; supervision, J.Q.H., and F.F.L.; project administration, J.Q.H., and F.F.L.; funding acquisition, F.F.L. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zong, Z., Cheng, X., Yang, Y. et al. Association between dietary flavonol intake and mortality risk in the U.S. adults from NHANES database. Sci Rep 14, 4572 (2024). https://doi.org/10.1038/s41598-024-55145-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55145-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.