Abstract
Designing and synthesising new metastable compounds is a major challenge of today’s material science. While exploration of metastable oxides has seen decades-long advancement thanks to the topochemical deintercalation of oxygen as recently spotlighted with the discovery of nickelate superconductor, such unique synthetic pathway has not yet been found for chalcogenide compounds. Here we combine an original soft chemistry approach, structure prediction calculations and advanced electron microscopy techniques to demonstrate the topochemical deintercalation/reintercalation of sulfur in a layered oxychalcogenide leading to the design of novel metastable phases. We demonstrate that La2O2S2 may react with monovalent metals to produce sulfur-deintercalated metastable phases La2O2S1.5 and oA-La2O2S whose lamellar structures were predicted thanks to an evolutionary structure-prediction algorithm. This study paves the way to unexplored topochemistry of mobile chalcogen anions.
Similar content being viewed by others
Introduction
Topochemistry enables a step-by-step control of chemical composition and atomic arrangements of materials by introducing, removing, or replacing specific atoms/molecules of host lattices without drastic modification of the overall crystal structure. It is one of the most versatile ways to design phases attainable only in mild synthesis conditions, i.e., at low temperature. Reactions aiming at the intercalation and the deintercalation of cationic species (mainly Li, Na, and other alkali metals) from an inorganic host have been extensively investigated since the sixties1. These topochemical reactions recently highlighted by the 2019 Nobel Prize in Chemistry led to the stabilization of numerous metastable phases2,3,4,5,6 and also gave rise to major applications in Li-ion batteries7. In 1999, Hayward and co-workers opened up a seminal topochemistry route based on the deintercalation of oxygen anions. Namely, they succeeded in extracting oxygen atoms of the perovskite-type oxide LaNiO3 at low temperature (T = 200 °C) using NaH as reducing reagent and oxygen getter to form the layered nickelate LaNiO2 with Ni+ cations in square planar coordination8. This synthetic pathway received much interest, as extraordinary changes in physical properties (e.g., magnetism, electrical conductivity) could be expected thanks to the stabilization of unusual oxidation states and chemical environment of cations while maintaining the low dimensionality of the inorganic framework9,10,11. In that respect the stunning discovery of the superconducting nickelate, Nd0.8Sr0.2NiO2, has recently spotlighted the benefit of this topochemical approach12. So far, such reactions were mainly limited to oxides and no topochemical deintercalation of sulfur at low temperature leading to metastable phases was reported.
To bring this situation to an end, we explore the intercalation/deintercalation of sulfur anions in lanthanum oxysulfides via an original soft chemistry route. We use the evolutionary structure prediction algorithm USPEX13,14, and 3D electron diffraction15 to confirm and characterize the topochemical deintercalation of sulfur and the formation of new metastable compounds. Return very recently, we demonstrated the formation of layered transition metal chalcogenides by insertion of transition metals in layered precursors containing chalcogenide dimers or trimers16,17. We took advantage of the ability of chalcogen oligomers to be easily reduced to form chalcogenide anions. In the case of sulfide dimers or trimers, the reductive cleavage (S2)2- + 2e- → 2 S2- or (S3)2- + 4e- → 3 S2- induced by contact with micrometric copper triggers the spontaneous intercalation of the metal into the precursor at low temperature. As a result, La2O2Cu2S2 and BaCu4S3 were obtained from the reaction of La2O2S2 or BaS3 with elemental copper at 250 °C. Our work also revealed that in the case of the reaction of BaS3 with Ni at 340 °C, sulfur could be extracted from the (S3)2- containing precursor to form a (S2)2- containing compound (e.g., BaS2) and a binary transition metal chalcogenide (e.g., NiSx)17. This observation suggests that the reduction of the oligomer by zero-valent metal can either lead to the intercalation of metal species or to the partial removal of chalcogen depending on the difference in free energies between the two antagonist reactions. It promoted us to explore more deeply the possible topochemical deintercalation of chalcogenides in layered precursors containing oligomers with various reducing reagents.
Results and discussion
Topochemical conversion from La2O2S2 to oA-La2O2S
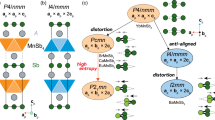
La2O2S2 appears as the ideal precursor to test the possibility to extend such a concept of anionic topochemistry from oxides to chalcogenides (Fig. 1a). Its structure consists of fluorite-type 2/∞[La2O2]2+ infinite layers separated from each other by discrete (S2)2- sulfur dimers aligned in parallel to these 2D blocks18. The deintercalation of one sulfur atom per dimer with metal should lead a priori to a La2O2S compound (Fig. 1b) whose structure should be inherited from the layered structure of the precursor La2O2S2 and that may come with potentially interesting optical properties as found in other oxysulfides19,20. To confirm this hypothesis, we started our investigation by scanning the low-energy structures of La2O2S compound using a designed crystal structure prediction (CSP) methodology detailed in Methods section. The combination of USPEX structure searching evolutionary algorithm with first-principles calculations makes it possible to locate two polymorphs, namely hP and oA crystal structures that are, respectively, stable and metastable (see Fig. 1c and S1–2 for details). Both phases are dynamically stable, justifying their respective location at global and local minima on the potential energy surface of La2O2S (see their computed phonon dispersion curves in Figs. S3–4, as well as DFT details in SI). The most stable candidate exhibits a hexagonal layered structure with 2/∞[La2O2] fluorite-type (111) slab alternating with sulfur atoms in octahedral environment of lanthanum (P\(\bar{3}\)m1 space group). Note that, this is the exact structure of the La2O2S compound already reported in the literature21, and commonly prepared at high temperature (800–1200 °C). This is interesting as it gives a direct validation of the modeling approach. In the following, this structure will be denoted hP−La2O2S according to the Pearson notation (h for hexagonal and P for primitive cell). The second low-energy phase lies at 72 meV/atom above ground-state hP-La2O2S (GGA PBE level of theory, see SI). Its structure is fully reminiscent to the one of the La2O2S2 precursor: it is built upon the stacking of 2/∞[La2O2] fluorite-type (001) slabs alternating with sulfur atoms in prismatic environments. In the same way with hP-La2O2S, this metastable polymorph with orthorhombic Amm2 space group is named hereafter oA-La2O2S. The thermal and kinetic stability of these two structures were further confirmed by ab initio molecular dynamics (AIMD) simulation in which both hP- and oA−La2O2S retained their main structural framework after 10 ps at temperatures up to 600 K (Fig. S5–S6). Consequently, the theoretical calculations clearly anticipate the possible existence of metastable oA−La2O2S besides the already known phase hP-La2O2S.
a Structure of La2O2S2 reported by Ostorero et al. (SG: Cmca)18. Lanthanum, oxygen, and sulfur atoms are represented by white, red, and yellow balls, respectively. b Conceptual scheme of S–S bond cleavage under the donation of one electron per elemental metal M0 that triggers subsequently the deintercalation of half sulfur atom of the S2 dumbbell that possibly enables topochemical conversion of La2O2S2 into the new polymorph of La2O2S. c The two low-energy dynamically stable phases of La2O2S predicted by USPEX that are separated by 72 meV/atom.
The topochemical deintercalation of sulfur in the layered precursor La2O2S2 was subsequently attempted by reaction with alkali metal Rb0 at low-temperature in evacuated and sealed pyrex tubes. Once excess Rb (and its salts) was washed out by dry ethanol (see synthetic procedure in Methods section), the powder X-ray diffraction (XRD) patterns were collected on products synthesized at 200 °C and 350 °C. They are very similar and could not be indexed with any known phases (Fig. 2a). The energy dispersive X-ray (EDX) spectra of the bulk product powder clearly revealed the absence of rubidium and a molar ratio La / O / S of 2.0(3) / 2.0 (4) / 0.98(11) (see Fig. S7). These results indicate the formation of a La2O2S phase without incorporation of Rb in the structure. The hP-La2O2S XRD peaks were not detected at all in the X-ray pattern but the existence of the polymorph oA-La2O2S predicted by USPEX could be readily established via Rietveld refinement (Fig. 2a) with goodness of fit χ2 = 1.33 and Bragg reliability factor R(obs) = 1.68% (see Table S3 for details). Scanning Transmission Electron Microscopy (STEM) also support the conclusion that the newly synthesized phase is oA-La2O2S. The stacking of 2/∞[La2O2] infinite sheets with the fluorite-type (001) slab structure is clearly visible on the High Angle Annular Dark Field (HAADF) STEM image (Fig. 2b, c). In contrast, the fluorite-type (111) slabs characteristic of the stable polymorph hP-La2O2S (Fig. S8) could not be found in the experiment STEM image. The EDX spectrum of a nanosized single crystal indicated, similarly to the EDX analysis of the bulk powder, a La / S ratio of 2.0(3) / 1.0(2) consistent with the composition La2O2S (see Fig. S9). The structural arrangement of the new oA-La2O2S compound is directly inherited from La2O2S2. This observation definitely supports the topochemical nature of the deintercalation process. Indeed, the sulfur deintercalation process does not modify at all the integrity of the 2/∞[La2O2] slab but entails a shift of every second 2/∞[La2O2] layer along the ½(b + c) direction of the La2O2S2 structure (SG: Cmca). Raman spectroscopy confirmed the complete loss of the sulfur dimers along the topochemical reduction. Namely, the band associated to the S–S stretching modes located at 487 and 498 cm−1 in La2O2S2 have totally disappeared after the deintercalation of one sulfur from La2O2S2 (Fig. S10), confirming the conclusion made from the XRD pattern that the reaction of La2O2S2 towards oA-La2O2S was complete. Finally, the diffuse-reflectance spectra also support the cleavage of (S2)2- dimers (Fig. S11). The absorption thresholds move from 2.56 eV in La2O2S2, a value characteristic of a π*–σ* electronic transition of sulfur pairs22,23, to 3.88 eV in oA-La2O2S, a value significantly lower than the one observed in the hP-La2O2S (4.13 eV). Our DFT calculations with the HSE06 functional fully confirmed the expected increase of the bandgap going from oA-La2O2S to hP-La2O2S (calculated gaps of 4.1 and 4.3 eV, respectively). Based on previous experimental and theoretical studies on hP-La2O2S24,25, their optical bandgaps can be associated to a S-3p → La-6s/La-5d transition. Therefore, the difference of the bandgap values between the metastable and stable La2O2S varieties is likely ascribed to changes in the local environment of sulfur and lanthanum atoms.
a Experimental XRD pattern of oA-La2O2S obtained by the reaction of La2O2S2 with Rb0 metal at 350 °C. The Rietveld refinement was conducted using as input the structure predicted by USPEX. b Simulated HAADF-STEM images viewed along [010] and [100] directions obtained using the refined structure of oA-La2O2S. In the projected structure lanthanum, oxygen, and sulfur atoms are represented by white, red, and yellow balls, respectively. c The experimental HAADF-STEM image of oA-La2O2S superimposed by each of the simulated images. Only La atoms were observed as bright spheres (ADF signal is proportional to the nth power of the atomic number, with n~2).
As a whole, we can conclude that during the reaction between La2O2S2 and elemental rubidium, the alkali metal activates a redox reaction with (S2)2- dimers that triggers the fracture of the S–S bonds. However, contrary to Cu0 metals that intercalate into the La2O2S2 host lattice16, Rb0 led to the topochemical deintercalation of sulfur to afford the unprecedented oA-La2O2S metastable phase. Preliminary calculations suggest that the intercalation of metal versus the deintercalation of sulfur is dictated by thermodynamic considerations and that the choice of reducing agents is the decisive factor for the outcome of the reaction. Even though, complete theoretical investigations are needed to ascertain this assertion, some experimental work further highlight the decisive role of the reducing power. Indeed, no reaction occurred when La2O2S2 was treated at 200–300 °C under reducing atmosphere, i.e., 5% H2/Ar flow (Fig. S12). The reduction was activated at 350 °C, but it led to the thermodynamically stable hP-La2O2S, where the original fluorite (001) slab was deformed into the fluorite (111) slab. This result highlights the contrast between the common reducing agent such as H2 atmosphere and the more powerful reducing agent Rb0, which favored, even at the same reaction temperature (i.e., 350 °C), topochemical reduction to oA-La2O2S.
Partial (de)intercalation of sulfur
At this stage, we might naturally wonder whether the topotactic deintercalation of La2O2S2 is reversible or not at low temperature. To test this possibility, a portion of oA-La2O2S was mixed with one molar equivalent of sulfur and heated at 200 °C (Fig. 3a) and the product was analyzed by XRD (Fig. 3b). The original La2O2S2 material could be fully recovered with no other reaction product, confirming the full reversibility of the temperature-assisted intercalation/deintercalation processes based on the formation/rupture of sulfur dimers within the La2O2S/La2O2S2 layered oxychalcogenides. To gain more insight into the reaction mechanism of the sulfur intercalation, we also tested the reactivity of oA-La2O2S with only half molar equivalent of sulfur at low temperature to reach the target La2O2S1.5 compound, i.e., a mid-composition between La2O2S2 and La2O2S. The XRD pattern of the product obtained at 200 °C (Fig. 3b) revealed the conversion of oA-La2O2S into an unknown intermediate phase along with a small amount of La2O2S2. The XRD pattern of this intermediate phase was similar to the one of oA-La2O2S but shifted to lower diffraction angles, suggesting the existence of an intercalated oA-La2O2Sx phase (1 < x < 2.0). The same XRD pattern was observed in the attempt to deintercalate 0.5 S from La2O2S2 using 1.0 equiv. of Rb0, 1.0 equiv. of Ag, and 0.5 equiv. of Ni0 (Fig. 3c). These diffraction patterns could be refined with the same space group as oA-La2O2S (Amm2) and cell parameters of ~8.5 Å, ~4.0 Å, and ~12.9 Å without any superstructure peak. This clearly proved the existence of an intermediate phase with a strong reminiscence of the oA-La2O2S structure. One of the reasonable assumptions is that this new phase replaced one half of monoatomic S2- with dimeric (S2)2- anions retaining the main structural framework of oA-La2O2S. This partial dimerization should lead to the expected La2O2S1.5 composition. To confirm this hypothesis, we analyzed the elemental composition of the nanocrystal (≤100 nm) found in the sample after the thermal treatment of oA-La2O2S + 0.5 S. Its EDX spectrum acquired on STEM indicated the La / O / S ratio of 2.0(3) / 2.0(3) / 1.5(3), supporting the hypothetical composition La2O2S1.5 (see Fig. S13). Furthermore, both intercalation of 0.5 S and deintercalation of 0.5 S using metal species gave similar Raman spectra that featured a single intense peak at 413–417 cm−1 while Raman peaks from oA-La2O2S and La2O2S2 were absent (Fig. S14–S15). Since an intense peak around 400–500 cm−1 is characteristic of S–S stretching mode26, these Raman spectra support the formation of La2O2S1.5 through the partial cleavage of S–S bonds.
a Schematic representation of incomplete intercalation of sulfur into oA-La2O2S and deintercalation of sulfur from La2O2S2 that leads to an intermediate compound La2O2S1.5. b Intercalation experiments of sulfur into oA-La2O2S. Experimental XRD patterns of pure oA-La2O2S and of the products of its mixture with sulfur (0.5 or 1 equiv. of S) after thermal treatments at 150 or 200 °C. The new XRD peaks emerging after the thermal treatment with 0.5 S are marked by *. c Deintercalation of sulfur from La2O2S2. Experimental XRD patterns of La2O2S2 and of the product of its mixture with Rb0, Ag0, and Ni0 after the thermal treatments at 200 or 350 °C. The XRD peaks of minor by-products are marked as follows: ● = Ag2S37, ■ = α-NiS38.
To solve the crystal structure of this novel phase, we analyzed it by precession electron diffraction tomography (PEDT) (Fig. 4a, see Fig. S16 and Table. S4 for details)27,28. This emerging technique allows the solution of complex structures using single nanocrystals. PEDT data were collected on several nanocrystals of the novel phase. All data sets were analyzed using the computer programs PETS2.029, Superflip30, and Jana200631. Figure 4a shows the reconstruction of the reciprocal lattice planes hk0, h0l, and 0kl which are consistent with an orthorhombic unit cell a = 8.348 Å, b = 3.961 Å, and c = 12.645 Å (V = 418.1 Å3) and a non-centrosymmetric space group Amm2 (see SI). The structure was subsequently solved and refined using the program Jana2006 on the basis of electron diffraction data using the dynamical theory of diffraction32,33. The structure analysis converged with electron Bragg reliability factor R(obs) = 10.1%, revealing the layered structure displayed in Fig. 4b with the composition La2O2S1.5. This structure consists of 2/∞[La2O2] fluorite-type (001) infinite slab alternating with sulfur layers containing one third and two thirds of sulfur atoms in the S2- and (S2)2- anionic forms, respectively. Using this oA-La2O2S1.5 structure model, both powder XRD patterns from sulfur intercalation and deintercalation, i.e., from oA-La2O2S + 0.5 S and La2O2S2 + 0.5 Ni reaction mixtures (Fig. 3), were successfully refined (Fig. S17 and Tables S5–S6). Large strain parameters had to be considered to reach satisfactory fitting. This can be interpreted as the signature of a stacking disorder occurring, as expected, during the intercalation or deintercalation processes in relation with the 2D structure of the host lattice and possible co-existence of different stages of (de)intercalation. The structure analysis was based on data collected on the most ordered crystals. However, in most of the electron diffraction data, diffuse scattering features along the [001] axis signified the presence of stacking faults in the crystal. Fig. 4c shows the high-resolution image obtained on a well-organized domain of oA-La2O2S1.5. The experimental contrast in the HAADF-STEM image (Fig. 4c) asserts the stacking of 2/∞[La2O2] fluorite-type (001) infinite slabs. A similar structure was predicted independently by the evolutionary algorithm USPEX for the composition La2O2S1.5. Indeed, the structure prophesied to be the most stable accorded well with the experimental structure obtained by the PEDT analysis (Fig. S18). The 2nd and 3rd most stable structures displayed intergrowth structures made of, respectively, corrugated or hexagonal (fluorite-type (111)) 2D [La2O2] slabs alternating with (quasi-)2D arrays of sulfur dimers/atoms. However, none of them could be found in our experiments.
a Projection of the reciprocal space obtained from PEDT data following the indexing of the first crystal (green). The second visible lattice (blue) corresponds to a second adjacent crystal and was measured in the same data set. b The structure of oA-La2O2S1.5 solved and refined from PEDT data set. The right side of the panel represents the simulated HAADF-STEM image along [010] on the basis of the oA-La2O2S1.5 structure model. c The experimental HAADF-STEM image acquired in a well-organized domain oA-La2O2S1.5 superimposed with the simulated one.
To conclude, this work demonstrates the deintercalation and reintercalation of sulfur in a layered oxychalcogenide compound using an original topochemical approach. Alkali or transition metals may be used as reducing agent to trigger the reduction of the chalcogenide oligomers and breaking of the chalcogen–chalcogen bond. In the case of La2O2S2, the low-temperature deintercalation of sulfur atoms proceeds in two steps to form two new metastable phases La2O2S1.5 and oA-La2O2S that both retain the layered feature of the precursor (Table 1). This is a fully reversible topotactic process as the sulfur atoms may be re-intercalated at low temperature to form back the precursor La2O2S2 (illustrated schematically in Fig. 5). This work gives therefore a glimpse of what could be the richness of the topochemistry of chalcogenide compounds and may open up an avenue to forthcoming batteries built upon mobile sulfur ions as well as to promising electronic or optical materials19,23,24,25.
Methods
Topochemical conversion from La2O2S2 into oA-La2O2S
The initial precursor La2O2S2 was synthesized following the procedure described in our previous report16. La2O2S2 and Rb (Aldrich, 98 + %) were weighed in a 1: 2 molar ratio and introduced into a silica tube whose bottom was protected by a carbon coating (cracking of acetone in the flame). All these preparations were done under argon atmosphere, then the silica tube was evacuated (~10−3 torr) and sealed. The mixture was heated to 200 °C at a rate of 20 °C h−1 and held at that temperature for 2 h. Finally, the sealed tube was gradually cooled to room temperature. The obtained powder turns to be pale greyish-blue. The silica tube was opened under argon atmosphere and the content was washed with ethanol, which rendered the powder colorless. The colorless precipitate was contaminated by tiny flakes of carbon, which were removed by repetitive decantation with mechanical agitation. The precipitate was then washed with ethanol, water, and acetone, followed by dryness in vacuo to obtain the pure colorless powder of oA-La2O2S. The product was stable under ambient atmosphere. The same reaction performed at 350 °C gave identical results, i.e., pure oA-La2O2S without any trace of hP-La2O2S.
Intercalation of sulfur anions into oA-La2O2S
The colorless powder of oA-La2O2S (ca. 200 mg) was combined with S flakes (Aldrich, 99.99 + %) in oA-La2O2S: S = 1: 0.5 molar ratio and ground in an agate mortar under argon atmosphere. Then the mixture was pelletized and sealed in an evacuated (~10−3 torr) silica tube. The sealed mixture was heated to 150 or 200 °C at a rate of 100 °C h−1 and held at the same temperature for 4 h (T = 200 °C) and 48 h (T = 150 °C, see results in Fig. 3b), respectively, followed by gradual cooling in a furnace to obtain a pale yellow pellet. To complete the intercalation, the obtained pellet was ground with additional 0.5 equiv. of S under argon atmosphere. The mixture was again subject to the thermal treatment at 200 °C in the evacuated silica tube. After 160 h of annealing, the mixture was fully converted into the pale yellow pellet of the pure La2O2S2.
Partial deintercalation of sulfur from La2O2S2 to get oA-La2O2S1.5
To prepare oA-La2O2S1.5, either 0.5 equiv. of Ni (Aldrich, <100 nm, 99%) or 1.0 equiv. of Ag (Aldrich, 2–3.5 μm, ≥99.9%) was added to 1.0 equiv. of La2O2S2 (ca. 150–250 mg). The mixture was ground together under argon atmosphere until the powder became greyish and sticky. Then the powdered sample was pelletized, sealed in an evacuated (~10−3 torr) silica tube, and heated to 350 °C at a rate of 300 °C h−1.The plateau temperature was maintained for 2 or 4 h before gradual cooling at RT for Ni and Ag, respectively (see Fig. 3c for XRD). Repeated thermal treatments did not lead to further consumption of La2O2S2 even when excess Ag was added (i.e., using 1.1 equiv. of Ag). In the same way, when using Ni prolonged and repeated thermal treatments did not improve the yield of oA-La2O2S1.5.
Characterizations
Powder X-ray diffraction (XRD) patterns were recorded at room temperature on a Bruker D8 Advance Diffractometer (Bragg-Brentano geometry, θ−2θ), which employs Cu Kα1 radiation (λ = 1.540598 Å) produced through Ge (111) monochromator and a LynxEye detector. Rietveld refinements of these XRD patterns were performed with Jana2006 package31 using the fundamental parameter approach34. XRD peaks from the known phases were fitted on the basis of the previously reported structure models (see Table S3 and S5–6 for the details). Anisotropic microstrain was considered by refining anisotropic peak broadening employing Stephen’s tensor method35. The chemical composition of oA-La2O2S was inspected by energy-dispersive X-ray (EDX) spectroscopy on a JEOL 5800LV scanning electron microscope (SEM) operating at 20 keV. The flat-polished specimens were prepared by impregnation of powder samples with the epoxy resin and subsequent grinding with ethanol/diamond grit suspensions. The detailed procedures of Raman spectroscopy and diffuse-reflectance spectroscopy are described in the supporting information.
Scanning Transmission Electron Microscopy (STEM) imaging
Samples were prepared by dispersing the powder in ethanol and depositing the solution obtained on a holey-carbon-coated copper grid. Z-contrast imaging was performed on a Cs-probe corrected STEM Themis Z G3 (Thermo Fisher Schientific) equipped with a High Angle annular Dark Field (HAADF) detector (Fischione) operating at 80 or 300 kV accelerating voltage, with a 21.4 mrad convergence angle and 63–200 mrad collection angle. It is equipped with the 4-SDD detectors Super-X system allowing EDS analysis. Simulated images were obtained using the Dr Probe program36.
Precession Electron Diffraction Tomography (PEDT) of oA-La2O2S1.5
The black powder from the reaction of La2O2S2 with Ni (vide supra) was crushed in ethanol. A drop of the suspension was deposited and dried on a gold grid with a thin film of holey amorphous carbon. 3D electron diffraction data (3D ED)15 were collected using the precession electron diffraction tomography (PEDT) technic on a Philips CM120 electron transmission microscope (TEM) (Vacc = 120 kV, LaB6) with the precession device Nanomegas Digistar and a side-mounted CCD camera Olympus Veleta with 14 bit dynamic range. PEDT data sets were collected at the ambient temperature on several crystals using a 1-degree precession angle and a 1-degree tilt step (see Fig. S16 and Table. S4). 3D ED data were analyzed using the computer programs PETS2.029, Superflip30, and Jana200631. The structure analysis was performed from one data set that included the diffraction signal from two crystals (Fig. S16). For each data set, two hkl-type files are obtained: one for structure solution and kinematical refinement, and another file suitable for the dynamical refinement, where each ED frame is considered independent32,33. The refinement procedure using the dynamical theory of diffraction (called “dynamical refinement”) implemented in JANA2006 was used . For the analysis, the data coming from the two adjacent crystals were combined to increase the data coverage and the statistic of the refinement. The data indicate an orthorhombic unit cell a = 8.348 Å, b = 3.961 Å, and c = 12.645 Å (V = 418.1 Å3) and a non-centrosymmetric space group Amm2 (k + l = 2n on hkl). The initial structure can be described with 8 atoms forming an alternation of infinite fluorite-type layers [La2O2] and sulfuric layers stacked along c-axis (Fig. S16e). The model was validated by the dynamical refinement following the procedure suggested in Palatinus et al.32,33. The refinement leads to satisfying values with R(obs)/wR(obs) = 10.12%/11.42% for 3523/4246 observed/all reflections. The refinement and crystallographic parameters are presented in Table S4.
Structure predictions by the evolutionary algorithm
USPEX (Universal Structure Predictor: Evolutionary Xtallography) works on the EA (evolutionary algorithm) to perform a global search of crystal structures. USPEX is developed by the A.R. Oganov laboratory since 2004 and its detail is described in the articles from his group13,14. In this work, this EA code was interfaced with VASP (Vienna Ab initio Simulation Package) for DFT structure relaxation (shape, volume, atomic positions are optimized by VASP). The EA development allows the prediction of stable and metastable structures knowing only the chemical composition, i.e., 2 La and 2 O and 1 S here, to search 3D La2O2S bulk phases. An initial population of 80 randomly created structures was considered as the first generation. Then the structures considered as worst were discarded. A structure was considered bad according to its fitness, that was the computed free enthalpy derived from ab initio total energy calculations (VASP). The remaining structures form the parent structures participated in producing the next generation. A new candidate structure was produced from parent structures using one of four operators: (i) heredity (50%), (ii) permutation (10%), (iii) lattice mutations (10%), and (iv) soft mutation (10%). 80% of the new candidates were generated from evolutionary operators, while 20% were produced randomly. We verified that our results were not sensitive to these parameters (initial population size, ratio between the four operators), by performing multiple runs with them varying from 10% around the nominal value indicated here. The USPEX search was terminated when the global structure minimum was found in the last 10 generations. Each USPEX job was run at least twice to ensure the convergence to a global minimum. As mentioned above, structure relaxations and energy calculations were done by the external code VASP (5 INCAR files, 5 steps per phase). The total number of atoms in the primitive cell is up to 40.
Data availability
All data are available within the Article and Supplementary Files, or available from the corresponding authors on reasonable request.
References
Lerf, A. Storylines in intercalation chemistry. Dalton Trans. 43, 10276–10291 (2014).
Lévy, F. (ed) Intercalated Layered Materials (Reidel Publishing Company, 1979).
Vivanco, H. K. & Rodriguez, E. E. The intercalation chemistry of layered iron chalcogenide superconductors. J. Solid State Chem. 242, 3–21 (2016).
Motter, J. P., Koski, K. J. & Cui, Y. General strategy for zero-valent intercalation into two-dimensional layered nanomaterials. Chem. Mater. 26, 2313–2317 (2014).
Cassidy, S. J. et al. Layered CeSO and LiCeSO oxide chalcogenides obtained via topotactic oxidative and reductive transformations. Inorg. Chem. 58, 3838–3850 (2019).
Ranmohotti, K. G. S., Josepha, E., Choi, J., Zhang, J. & Wiley, J. B. Topochemical manipulation of perovskites: low-temperature reaction strategies for directing structure and properties. Adv. Mater. 23, 442–460 (2011).
Goodenough, J. B. & Park, K.-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Hayward, M. A., Green, M. A., Rosseinsky, M. J. & Sloan, J. Sodium hydride as a powerful reducing agent for topotactic oxide deintercalation: synthesis and characterization of the nickel(I) oxide LaNiO2. J. Am. Chem. Soc. 121, 8843–8854 (1999).
Tsujimoto, Y. et al. Infinite-layer iron oxide with a square-planar coordination. Nature 450, 1062–1065 (2007).
Romero, F. D. et al. SrFe0.5Ru0.5O2: square-planar Ru2+ in an extended oxide. J. Am. Chem. Soc. 135, 1838–1844 (2013).
Kageyama, H. et al. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 9, 772 (2018).
Li, D. et al. Superconductivity in an infinite-layer nickelate. Nature 572, 624–628 (2019).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: principles and applications. J. Chem. Phys. 124, 244704 (2006).
Oganov, A. R., Lyakhov, A. O. & Valle, M. How evolutionary crystal structure prediction works –and why. Acc. Chem. Res. 44, 227–237 (2011).
Gemmi, M. et al. 3D electron diffraction: the nanocrystallography revolution. ACS Cent. Sci. 5, 1315–1329 (2019).
Sasaki, S. et al. A topochemical approach to synthesize layered materials based on the redox reactivity of anionic chalcogen dimers. Angew. Chem. Int. Ed. 57, 13618–13623 (2018).
Sasaki, S. et al. Unexplored reactivity of (Sn)2− oligomers with transition metals in low-temperature solid-state reactions. Chem. Commun. 55, 6189–6192 (2019).
Ostoréro, J. & Leblanc, M. Room-temperature structure of La2O2S2. Acta Cryst. C46, 1376–1378 (1990).
Fan, B., Chlique, C., M.-Conanec, O., Zhang, X. & Fan, X. Near-infrared quantum cutting material Er3+/Yb3+ doped La2O2S with an external quantum yield higher than 100%. J. Phys. Chem. C. 116, 11652–11657 (2012).
Larquet, C. & Carenco, S. Metal oxysulfides: from bulk compounds to nanomaterials. Front. Chem. 8, 179 (2020).
Morosin, B. La2O2S structure refinement and crystal field. Acta Cryst. B29, 2647–2648 (1973).
Driss, D. et al. Crystal structure and chemical bonding in the mixed anion compound BaSF. Dalton Trans. 46, 16244–16250 (2017).
Xu, G. et al. Synthesis and optical properties of Eu3+ ion-doped La2O2S2 via a solid state reaction method using La2O2SO4 as a raw material. Ceram. Int. 44, 19070–19076 (2018).
Li, Y. F., Xiao, B., Gao, Y. M. & Cheng, Y. H. Theoretical study of anisotropic structural, electronic, mechanical and thermodynamic properties of rare-earth (R = Y, La) oxysulfides. Comp. Mater. Sci. 125, 154–167 (2016).
Dai, Q. et al. Size and concentration effects on the photoluminescence of La2O2S:Eu3+ nanocrystals. J. Phys. Chem. C. 112, 19399–19404 (2008).
Steudel, R. Properties of sulfur-sulfur bonds. Angew. Chem. Int. Ed. Engl. 14, 655–720 (1975).
Vincent, R. & Midgley, P. A. Double conical beam-rocking system for measurement of integrated electron diffraction intensities. Ultramicroscopy 53, 271–282 (1994).
Kolb, U., Gorelik, T., Kübel, C., Otten, M. T. & Hubert, D. Towards automated diffraction tomography: part I—data acquisition. Ultramicroscopy 107, 507–513 (2007).
Palatinus, L. et al. Specifics of the data processing of precession electron diffraction tomography data and their implementation in the program PETS2.0. Acta Cryst. B75, 512–522 (2019).
Palatinus, L. & Chapuis, G. SUPERFLIP–a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 40, 786–790 (2007).
Petříček, V., Dušek, M. & Palatinus, L. Crystallographic computing system JANA2006: general features. Z. Krist. – Cryst. Mater. 229, 345–352 (2014).
Palatinus, L. et al. Structure refinement using precession electron diffraction tomography and dynamical diffraction: tests on experimental data. Acta Cryst. B71, 740–751 (2015).
Palatinus, L., Petříček, V. & Corrêa, C. A. Structure refinement using precession electron diffraction tomography and dynamical diffraction: theory and implementation. Acta Cryst. A71, 235–244 (2015).
Cheary, R. W. & Coelho, A. A. Axial divergence in a conventional X-ray powder diffractometer. I. Theoretical foundations. J. Appl. Cryst. 31, 851–861 (1998).
Stephens, P. W. Phenomenological model of anisotropic peak broadening in powder diffraction. J. Appl. Cryst. 32, 281–289 (1999).
Barthel, J. Dr. Probe: a software for high-resolution STEM image simulation. Ultramicroscopy 193, 1–11 (2018).
Frueh, A. J. The crystallography of silver sulfide, Ag2S. Z. F.ür. Krist. – Cryst. Mater. 110, 136–144 (1958).
Trahan, J., Goodrich, R. G. & Watkins, S. F. X-ray diffraction measurements on metallic and semiconducting hexagonal NiS. Phys. Rev. B 2, 2859–2863 (1970).
Acknowledgements
S.S. is financially supported by JSPS Overseas Research Fellowships. The electron diffraction experiments were performed within CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110) and supported by Operational Programme Research, Development and Education financed by European Structural and Investment Funds and the MEYS CR (Project No. SOLID21 CZ.02.1.01/0.0/0.0/16_019/0000760). TEM images were collected in the CIMEN Electron Microscopy Center in Nantes funded by the French Contrat Plan État-Région and the European Regional Development Fund of Pays de la Loire. We also acknowledge the Jean Zay/IDRIS GENCI (France) under project n° A0080911512 and Jean Zay Challenge for allocation of computing time. Louis-Béni Mvélé Eye’a is thanked for is help in the chemical synthesis work.
Author information
Authors and Affiliations
Contributions
S.S. performed the synthetic work and characterization of the products; M.T.C., C.G.-D., and E.G. conducted TEM analyses; G.S. and L.P. studied the structure of oA-La2O2S1.5 by 3D electron diffraction; I.B. and G.F. performed the computational study; E.J., B.C., L.C., and S.J. supervised the study. S.S., L.C., and S.J. wrote the initial draft and all authors contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Jingbao Lian, Krzysztof Zberecki and other, anonymous, reviewers for their contributuions to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sasaki, S., Caldes, M.T., Guillot-Deudon, C. et al. Design of metastable oxychalcogenide phases by topochemical (de)intercalation of sulfur in La2O2S2. Nat Commun 12, 3605 (2021). https://doi.org/10.1038/s41467-021-23677-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-23677-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.