Abstract
Meiotic drive is widespread in nature. The conflict it generates is expected to be an important motor for evolutionary change and innovation. In this study, we investigated the genomic consequences of two large multi-gene meiotic drive elements, Sk-2 and Sk-3, found in the filamentous ascomycete Neurospora intermedia. Using long-read sequencing, we generated the first complete and well-annotated genome assemblies of large, highly diverged, non-recombining regions associated with meiotic drive elements. Phylogenetic analysis shows that, even though Sk-2 and Sk-3 are located in the same chromosomal region, they do not form sister clades, suggesting independent origins or at least a long evolutionary separation. We conclude that they have in a convergent manner accumulated similar patterns of tandem inversions and dense repeat clusters, presumably in response to similar needs to create linkage between genes causing drive and resistance.
Similar content being viewed by others
Introduction
A significant portion of eukaryote genomes consists of selfish or parasitic genetic elements. Such elements have a transmission advantage relative to other parts of the genome but are either neutral or detrimental to the fitness of the organism as a whole1,2. Despite such genomic conflicts being known since the early days of modern genetics, only recently has there been a clear awareness that genetic conflicts could be important engines for evolutionary change2,3. Meiotic drive elements (MDEs) constitute a class of selfish genetic elements that are located in the nuclear genome and mediate a skew in their own sexual transmission from parent to offspring, causing them to be recovered in more than half of the surviving meiotic products1,4. This phenomenon is referred to as meiotic drive, and with even minimal skews in transmission frequency, it can lead to quick fixation or stable polymorphisms of a driving allele5,6. Many MDEs require the interaction of multiple genes or genetic components to function, which is expected to lead to strong selection for linkage, especially in systems where unlinking distinct loci that confer killing and resistance would lead to self-killing. Accordingly, meiotic drivers are often associated with structural rearrangements that reduce recombination or are positioned in regions of low recombination, such as centromeres, heterochromatic regions, or non-recombining sex chromosomes1,5. This is the case for several of the most well-studied meiotic drive systems, including Segregation Distorter (SD) complex in Drosophila melanogaster7, the t haplotype8 in mice, and several so-called selfish sex chromosomes in different species of animals and plants9.
A multilocus MDE can be considered a supergene, the term used to refer to a group of functionally related genes that are linked together and, hence, segregate as a single unit10,11. In many supergenes, for instance sex chromosomes and fungal mating-type chromosomes, the reduced recombination has resulted in rapid divergence between the non-recombining haplotypes and a reduced ability to purge deleterious mutations through recombination12,13,14,15,16. This is also the case for many MDEs, which have accumulated recessive lethal mutations17, rearrangements such as segmental duplications and deletions8, and show an expansion of repetitive regions18. Furthermore, patterns of divergence between MDEs and non-driving individuals have been studied in several species8,19,20, inversions have been observed in cytological examinations17,20, and nucleotide polymorphism has been studied using markers and whole-genome single-nucleotide polymorphism (SNP) data8,19,21. However, the full extent of divergence can be challenging to study even in model organisms with high-quality genomic resources because discovery and mapping of complex rearrangements typically requires de novo genome assembly of the complete MDE.
In fungi, meiotic drive manifests itself as spore killing, where sexual spores carrying a spore killer genotype destroy spores that carry a sensitive genotype. Spore killing has so far only been observed in the ascomycete fungi, and several different killer systems are identified in Podospora anserina22,23, Schizosaccharomyces pombe24,25, and two species of Neurospora26. While the spore killers in P. anserina and S. pombe are single-gene systems, Spore killer-2 (Sk-2) and Spore killer-3 (Sk-3) are complex MDEs found in N. intermedia that map to a 30 cM region surrounding the centromere on chromosome 3 where recombination is suppressed between killer and sensitive strains27. A region necessary for killing (rfk-1) has been mapped in Sk-2 to the right edge of the region of suppressed recombination28,29 and a gene that confers resistance against both Sk-2- and Sk-3-based killing (rsk) has been identified at the left flank30. Depending on the allelic variant of the resistance gene a strain carries, it shows either resistance to Sk-2 or Sk-3 or sensitivity to killing30. The cause of the suppression of recombination is not known, but an inversion covering at least 200 kb was previously identified near the rfk-1 region in one Sk-2 strain28. Furthermore, the mechanism of killing in the Sk-2 and Sk-3 systems has not been elucidated, but Hammond et al.30 proposed a toxin–antitoxin model, in which the killer gene encodes a toxin that can kill a spore, and the resistance gene encodes an antitoxin that ensures the survival of the spore that produces it. Consequently, when two strains of the same spore killer are crossed, all spores will survive, while crosses of Sk-2 and Sk-3 result in the production of empty asci26, which has been interpreted as mutual killing by incompatible toxin–antitoxin systems30.
The purpose of this study was to investigate the consequences of multilocus meiotic drive on genome architecture in N. intermedia. We generated near-complete genome assemblies using PacBio long-read sequencing data from all five available spore killer strains (four Sk-2 and one Sk-3) and one sensitive strain of N. intermedia and assembled the complete non-recombining region in all of these. We also produced a detailed annotation of gene content, repeat content, and distribution of heterochromatin and euchromatin from one strain of each killer type and the sensitive strain. Short-read Illumina sequencing was used for phylogenetic and population genetic analysis of a large collection of N. intermedia strains from natural populations. Using this large and diverse dataset, we show that even though Sk-2 and Sk-3 are located in the same chromosomal region they do not cluster together in phylogenetic analyses, suggesting separate origins. Both the Sk-2 and Sk-3 haplotypes have accumulated a dense set of inversions that are interspersed with transposable elements (TEs). The inversions are unique for each killer type, further supporting a model with an ancient split. In the non-recombining region of Sk-2, we identified signs of relaxed selection, in agreement with the hypothesis that recombination suppression reduces the efficacy of selection in this region. For example, TEs have spread in the non-recombining regions of both Sk-2 and Sk-3 despite what appears to be a set of fully functional mechanisms to limit their spread.
In conclusion, our data show that at least two complex MDEs have established themselves in N. intermedia and, during this process, have induced independent and convergent structural changes in the same genomic region.
Results
Killer-specific inversions are found in the Sk regions
Neurospora intermedia is a close relative to the model species N. crassa, from which it diverged 3–4 million years ago (MYA), and it diverged from its closest relative N. metzenbergii around 2 MYA31. Spore killing was discovered in a small number of natural isolates of N. intermedia in south-east Asia in the 1970s, and most isolates were introgressed into different genetic backgrounds for increased tractability in the laboratory26. Today, only one natural isolate of Sk-2 is available at culture collections, while the other three Sk-2 isolates and the single Sk-3 isolate are available only as backcrosses to N. intermedia and N. crassa laboratory strains.
The analysis of the genomes in this study (Supplementary Table 1), including all currently available spore killer strains of N. intermedia, show that the chromosomal region containing Sk-2 and Sk-3 in N. intermedia represents an exception to the high degree of collinearity previously reported in Neurospora32. Specifically, we used the PacBio RSII platform to generate six high-quality genome assemblies with full or nearly full chromosome-length contigs (Table 1, Supplementary Fig. 1). In order to identify structural rearrangements, we aligned these assemblies against the N. crassa OR74 reference genome33 and against each other. These alignments show that the sensitive N. intermedia strain is collinear with N. crassa (as was previously shown for a different sensitive strain32), but the Sk-2 and Sk-3 strains carry clusters of large inversions on chromosome 3 in the region where recombination between killer and sensitive strains has previously been reported to be suppressed27 (Fig. 1, Supplementary Table 2). For both Sk-2 and Sk-3, the inversions are non-overlapping and situated next to each other, without any regions retaining the ancestral gene order interspersed between them (Fig. 1, Supplementary Table 2). The four sequenced Sk-2 genomes carry the same set of inversions in the inverted region (from here on referred to as the Sk region), but strain 7429 also carries an extra ~1.3 Mbp inversion on the right chromosome arm, outside of the region that showed suppressed recombination in previous studies (Supplementary Fig. 2). The Sk-3 genome carries several tandem inversions in the same region as the Sk-2 strains (Fig. 1), but the breakpoints of all Sk-3 inversions differ from those in Sk-2, indicating that they have accumulated independently (Fig. 1, Supplementary Table 2).
An alignment of chromosome 3 in Sk-2 and Sk-3 to sensitive Neurospora intermedia, which retains the ancestral gene order, shows a series of large inversions in the region between the killer and resistance loci. Blue color shows collinear regions and red inverted regions. All inversions are different between Sk-2 and Sk-3 and are interspersed with gene-poor and repeat-rich inserted regions. The location of the Sk-2 killer locus us marked with an orange circle and the rsk (resistance) locus and the centromeres are marked with green and purple circles, respectively. Tracks in gray show genes; GC content; repeat content; cytosine methylation; and H3K9me3, H3K27me3, and H3K4me2 histone methylation. H2K9me3 is a mark for constitutive heterochromatin, which in Neurospora is highly associated with repeats; H3K27me3 is a mark for facultative heterochromatin, which is primarily found in subtelomeric regions; and H3K4me2 is a mark for euchromatic regions. Cytosine methylation and the three different histone modifications were estimated using three biological replicates, and here the sum of the triplicates are presented
Sks include repetitive regions enriched for heterochromatin
Both Sk-2 and Sk-3 haplotypes are enriched for repetitive sequences (Fig. 1, Fig. 2, Table 2), which are mainly found in large, dense repeat clusters bordering the inverted regions (Fig. 1). The repeats are primarily the remains of TEs. Several TE families appear to have expanded preferentially (Supplementary Tables 3 and 4), although these correspond to no more than 20% of the repetitive content in the region (Table 2, Supplementary Table 4). TEs are enriched in the Sk haplotype both when compared to the rest of the genome and compared to the homologous region in the sensitive strains of N. intermedia and N. crassa (Fig. 2, Table 2).
Percentage of repetitive DNA in three strains of Neurospora intermedia with different spore killer phenotypes (Sk-3: 3194; Sk-2: 7426; sensitive: 8761). Blue bars show the whole genome, and yellow bars the non-recombining spore killer region. Error bars (95% confidence intervals) for the whole-genome data were calculated using sliding windows of the same size as the spore killer region, with a step size of 200 kb. Note that no formal statistical test has been performed to determine if the repeat content in the spore killer region is significantly different from the background genome, but the data point does not overlap with the 95% confidence interval from the whole genome
In N. crassa, a wide repertoire of mechanisms thought to defend against the expansion repetitive sequences are present34. Most notable is the Repeat-Induced Point mutation (RIP) system, which induces C-to-T mutations in any sequence appearing more than once in the genome. RIP is hypothesized to defend the genome from TEs35 and can inactivate these through mutations, lowers their average GC content, and also acts as a signal for heterochromatin formation36 and DNA methylation37, which both are expected to limit TE activity. Accordingly, the GC content of the repeat clusters in the Sk haplotypes is lower than that of genic regions (~30% vs 50%: Figs. 1 and 3). This is also the case in the whole genome of both the killer and sensitive strains, indicating that the expansion of repeats in the Sk-2 and Sk-3 haplotypes is not due to a less efficient RIP system in this region. In order to determine whether the heterochromatin structure is different from that found in N. crassa and whether a different heterochromatin structure could explain the Sk repeat expansions, we performed bisulfite sequencing and chromatin immunoprecipitation sequencing (ChIP-seq) of one Sk-2, one Sk-3, and one sensitive N. intermedia strain (Supplementary Tables 5 and 6). These analyses revealed that cytosine methylation and H3K9me3 (histone 3 lysine 9 tri-methylation) (which are both signatures of constitutive heterochromatin in N. crassa36) are enriched in repetitive regions (Figs. 1 and 3), and H3K4me2 and H3K27me3 methylation marks (signatures of euchromatin and facultative heterochromatin, respectively) are absent in the same regions38 (Fig. 1). In summary, we did not find a signal of reduced ability to defend against the expansion of TEs in the spore killer regions.
Repeat content, GC content, cytosine methylation, and H3K9me3 enrichment of the genome and the spore killer region in strains 3194 (Sk-3), 7426 (Sk-2), and 8761 (sensitive) of Neurospora intermedia. All values are calculated using 2 kb sliding windows with a step size of 2 kb. Windows consisting of >75% repetitive content are classified as “repeat windows” and those with <75% are classified as “non-repeat windows.” “Repeat content” plots indicate that most windows classified as repetitive contain close to 100% repeats, consistent with a clustered distribution of repeats. Error bars show 95% confidence intervals. GC content, cytosine methylation levels, and H3K9me3 levels are all significantly different between repeat and non-repeat windows (Student’s T test, p ≪ 0.001 for all comparisons). The Sk regions of the spore killers are also significantly different from the rest of the genome in all these characteristics, though the differences are less pronounced than when comparing repetitive and non-repetitive regions. These results are consistent with previous data from N. crassa, in which cytosine methylation and H3K9me3 histone methylation are both signatures of constitutive heterochromatin and primarily found in repetitive regions that have been mutated by the RIP mechanism, which also lowers the GC content
Novel gene content in the Sk regions
To evaluate whether the Sk regions have accumulated novel open reading frames (ORFs), the gene content of the spore killer strains and sensitive strains was compared. We gathered transcriptome data from vegetative tissue and from crosses involving four strains: one Sk-2, one Sk-3, and two sensitives (Supplementary Tables 7 and 8), and used these data to annotate the genome assemblies. Unique genes in the Sk-2 and Sk-3 regions were identified by comparing our gene annotations of the spore killer strains to N. crassa and N. intermedia sensitive strains and to each other (Supplementary Table 9, Supplementary Fig. 3). We identified 6, 4, and 5 unique ORFs in the Sk region of Sk-2, Sk-3, and sensitive N. intermedia, respectively (Supplementary Table 9), but the majority of these unique genes could not be assigned a known function. We also identified three genes that are found in both Sk-2 and Sk-3 but not in any of the sensitive strains (Supplementary Table 10, Supplementary Fig. 3). One of these genes is similar to the transposase in the DNA transposon Sly1-139 and a second one is found next to this gene in both Sk-2 and Sk-3, suggesting that they may form a single TE together. The third gene shows no homology to any genes with known function. Our data also suggest that the rate of gene gains in the spore killer region is not higher than in the homologous region of the sensitive strains of N. intermedia and N. crassa.
A region necessary for killing was recently identified in an Sk-2 killer strain28. This region contains an 11 kb insertion that is located between the inverted region and the collinear region at the right edge of the Sk-2 region (Fig. 1) and that is only found in the four Sk-2 strains. A smaller part of this insertion has recently been found to be the active killer factor29, and when using nucleotide blast to find homologous sequences in the Sk-3 genome, we were unable to find a similar sequence, neither at the homologous location nor anywhere else in the genome. However, by searching for the translated amino acid sequence, we identified a sequence on the left arm of chromosome 3 in both the Sk-3 strain and several sensitive N. intermedia strains that gave a weak hit (E-value 0.007). We performed a knockout of this sequence in a N. crassa strain carrying the Sk-3 region26,30, but no loss of killing was observed when crossing the knockout strain to a sensitive N. crassa strain (Supplementary Figs. 4 and 5). This result suggests that different genes are responsible for killing in Sk-3 and Sk-2 strains.
The population structure of N. intermedia
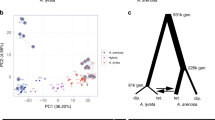
We sequenced 21 N. intermedia strains, including all known Sk-2 and Sk-3 strains and 5 N. metzenbergii strains (Supplementary Table 11) using the Illumina HiSeq platform. We then called SNPs, which were used to investigate the population structure of N. intermedia and to compare the evolutionary history of Sk region to that of the background genome (i.e., all chromosomes except chromosome 3). Previous studies of N. intermedia, based on four molecular markers, have suggested that the species is dominated by two major phylogenetic subgroups that are clearly diverged from each other and show low levels of diversity40. Among the 21 strains analyzed here, only 6 cluster with one of these 2 subgroups (Supplementary Fig. 6a), the remaining strains show long branches and weak clustering, indicating that the earlier studies failed to capture the diversity within N. intermedia. Three of the five N. metzenbergii strains were originally annotated as N. intermedia, but based on the clustering here we reclassify them as N. metzenbergii.
Except in the case of strain 7426, the original isolates of the spore killer strains are no longer available and 4 out of the 5 known spore killer strains instead remain as 1–3 times backcrosses to an N. intermedia tester strains from Taiwan (in this study represented by strain 8761)26 (Supplementary Table 1). This prevents a phylogenetic analysis of the natural genetic background of the spore killers, and only the signal of the backcrossing is apparent in the clustering (Supplementary Fig. 6a).
An old evolutionary separation of Sk-2 and Sk-3
The inversions present in the Sk region are expected to suppress recombination and potentially prevent gene flow between sensitive and killer strains, and accordingly, earlier studies have reported extremely low recombination rates within the spore killer regions26,27. We used a phylogenetic sliding window approach to investigate signatures of recombination between Sk-2 strains and sensitive strains occurring after the establishment of the inversions, searching for regions where the four Sk-2 strains do not show a monophyletic relationship. This analysis revealed several putative within-inversion, double-crossover events within the spore killer region (Fig. 4a). While inversions 3 and 4 (Supplementary Table 2) harbored no recombination events, Sk-2 strain 7429 shows signs of recombination throughout most of inversion 5 and in a large region of inversion 2. In inversion 1, recombination appears to be more common, with a noticeably weaker signal of monophyly as compared to the other inverted regions. Only inversions 3 and 4 and parts of inversions 1 and 2 show a signal of monophyly for all four Sk-2 strains, consistent with a shared phylogenetic history since the establishment of the suppression of recombination.
Phylogenetic signal over chromosome 3. SNPs from all 21 N. intermedia and 5 N. metzenbergii strains were split into non-overlapping windows each containing 50 variable sites, and a phylogenetic tree was inferred using the BioNJ algorithm in PhyML for each window. a The windows were then classified based on which of the four Sk-2 strains were grouping together in the tree. All Sk-2 strains share inversions 1–5 and in the absence of recombination the entire non-recombining region should form a monophyletic cluster (shown in red). When three or fewer Sk-2 strains group together, this indicates that recombination has happened (other colors). Top panel show the percentage of each monophyletic grouping in bins of 50 trees. The bottom panel shows the bootstrap support for the monophyletic group identified in each tree. Black bars show the borders of the five major inversions. In inversions 3 and 4 (as indicated by the numbers above the plot), the main signal is of all four strains grouping together, indicating no recombination. In inversion 5 and in a section of inversion 2, strain 7429 no longer groups with the other three (marked in green), consistent with a recombination event. The breakpoints of all inversion show the red grouping, indicating that the recombination events did not span several inversions. b Phylogenetic signal of monophyly of Sk-3 together with Sk-2. Top panel shows pairwise divergence between Sk-2 strain 7426 and Sk-3 strain 3194, in 10 kb sliding windows with a 2 kb step. Bottom panel show windows where Sk-2 and Sk-3 form a monophyletic group. Colors indicate which Sk-2 strains (7492, 7426, 7427, 7429) group with Sk-3 (3194) and the y axis indicates bootstrap support for that specific group. In the non-recombining region of chromosome 3, no strong signal for monophyly could be detected with only a smaller number of low bootstrap windows. The large clusters of windows on the right which show a signal of Sk-2-Sk-3 monophyly are the product of the backcrossing of the spore killer strains to the Taiwan genetic background
Recombination between the four Sk-2 strains was also evaluated. No larger crossover tracts could be identified but a couple of smaller regions, potentially corresponding to gene conversion events, showed signal of genetic exchange (Supplementary Fig. 7).
Using the same phylogenetic sliding window approach, we find no strong signal of monophyly between the Sk-2 and Sk-3 strains in the inverted region (Fig. 4b). Furthermore, Sk-3 groups on its own in a phylogenetic analysis of both the whole inverted region and the part where all Sk-2 strains are monophyletic (Supplementary Fig. 6b and 6c), indicating that Sk-3 and Sk-2 are of independent origin.
Finally, we estimated the age of Sk-2 based on the synonymous substitution rate. Pairwise synonymous divergence was calculated per gene between the Sk strains and all sensitive N. intermedia strains in the monophyletic regions. Since the sensitive strains are expected to be recombining freely, we could not identify a single sensitive strain that is the closest relative to the Sk strains, and instead we selected the gene copy that showed the least amount of divergence for each gene. This strategy allowed us to calculate a lower bound for the age of the non-recombining region to be between 255,000 and 428,000 years (Supplementary Table 12).
Reduced efficacy of selection in the Sk-2 region
In order to investigate the evolutionary trajectory of the Sk regions, we compared the diversity and divergence of non-synonymous and synonymous substitutions within the Sk region and in the rest of the genome. This analysis could only be performed for the sensitive and Sk-2 strains, since we only have a single Sk-3 strain in our dataset.
For the sensitive strains of N. intermedia, the nucleotide diversity varied little between chromosomes (πtotal, πN, and πS). The πN/πS ratio also showed marginal changes across chromosomes with values ranging from 0.18 to 0.21 (Supplementary Table 13). The estimates for the Sk region of the sensitive strain was not significantly different from the rest of the genome, and no significant difference in sequence divergence to the outgroup, N. metzenbergii, was found. The Neutrality Index (NI) quantifies the direction and degree of departure from neutrality and was calculated as the ratio of PN/PS over DN/DS. NI > 1 indicates negative selection while a value <1 indicates positive selection. In the sensitive strain, NI was close to 1 for the Sk region (1.04) as well as for the rest of genome (1.09), which suggests that the assumption of neutrality holds genome-wide. The summary statistics for nine sensitive strains gave similar results (Supplementary Table 14).
In contrast, the Sk region of the Sk-2 strains differed significantly from the rest of the genome, with respect to both diversity and divergence from N. metzenbergii (Table 3). Specifically, while all chromosomes of the Sk-2 strains showed similar diversity levels to the sensitive strains, the Sk-2 haplotype had only 40% of the average diversity at non-synonymous sites and even less at synonymous sites (πN = 0.0034, πS = 0.013, p value « 0.001). Because the reduction in the Sk region was more pronounced for synonymous sites, the πN/πs ratio rose to 0.27, a value significantly higher than in the rest of the genome (0.19) (p value « 0.001). The Sk-2 haplotype also had significantly more fixed divergent alleles than the rest of the genome (DN = 2985 vs 2216, DS = 7360 vs 4918, p values « 0.001). The rise of divergence in the Sk-2 haplotype had a slightly stronger effect on synonymous sites than on non-synonymous sites, which led to a slight decrease of DN/DS (p value = 0.14). The NI was higher but the difference was small and not significant (1.27, p value = 0.22). All measures of significance mentioned here were derived from comparisons of estimates from the Sk region to a null distribution bootstrapped from the rest of the genome (see Methods for further details).
In summary, the sensitive strains evolved neutrally and the region homologous to the non-recombining region in the Sk-2 strains did not depart in this respect from the rest of the genome. The increase in πN/πs ratio also suggests that purifying selection was less effective in the Sk-2 haplotype due to reduced recombination41.
An analysis of all variable sites was also performed (Supplementary Table 13, Supplementary Fig. 8). This supports the above analysis and shows that diversity is reduced in the Sk region. Linkage disequilibrium is elevated in the region but not consistently so, in agreement with occasional recombination between Sk-2 strains.
The rsk gene phylogeny
We performed a phylogenetic analysis of nearly 100 alleles of the rsk gene, previously shown to confer resistance against both Sk-2- and Sk-3-based killing29. rsk alleles from all N. intermedia strains included in this study and from several other Neurospora species were analyzed. The alleles form two major clusters (Fig. 5), which do not reflect the expected species relationship31. Strains of N. intermedia, N. crassa, and N. tetrasperma are found in both clusters, which indicates either ancestral polymorphism of rsk or extensive introgression. The most striking observation from our analysis is that strains that are resistant to Sk-3 but not Sk-2 have an rsk allele that is more similar to Sk-2-resistant strains. This observation contradicts the previous model30 predicting the existence of three major clades of rsk alleles (Sk-2-type alleles with Sk-2 resistance, Sk-3-type alleles with Sk-3 resistance, and Sk-sensitive alleles). An alignment of representative rsk alleles also shows that there are no polymorphisms in the gene that are unique for each resistance phenotype (Supplementary Fig. 9). Our findings suggest that rsk-based resistance to spore killing is more complicated than previously thought and that other genes may also influence the specificity of the resistance to each killer.
Maximum likelihood phylogeny of the resistance locus rsk. The alleles are split into two major groups, with Sk-2 (marked with red text) in one and Sk-3 (blue text) in the other. Sk-2-resistant strains (marked with red dots) cluster with Sk-2, but Sk-3-resistant strains (blue dots) are found in both groups. All Neurospora sitophila strains have similar alleles at the rsk locus, and there is no clustering depending on whether they are Sk-1 killers (orange text) or sensitives (black text). Most N. intermedia and N. crassa strains have been tested for resistance against Sk-2 and Sk-3, but this is not possible in most other Neurospora species, due to poor sexual compatibility. An exception is N. metzenbergii, where spore killing is observed in crosses with included strains
Discussion
Meiotic drive is often associated with genomic divergence, both on large and small scales1, but the size and complexity of MDEs has hitherto impeded detailed analyses of the genomic regions harboring them. Here we generated high-quality genomic and epigenomic data of two different MDEs in the fungus N. intermedia. This dataset allowed us to investigate a range of changes associated with meiotic drive, from large-scale rearrangements to gene content, repeat distribution, heterochromatin patterns, and signals of molecular divergence and degeneration. The genomic characteristics of the Sk-2 and Sk-3 haplotypes are similar to those observed in the SD complex7 and the t haplotype42, with both an accumulation of inversions and signs of degeneration, but our data have allowed us to study these changes in greater detail, and they have also revealed that similar changes have taken place independently in the two different spore killer systems.
Sk-2 and Sk-3 will both produce four-spored asci when crossed to a sensitive strain, and it is first when they are crossed to each other and mutual killing is observed that it becomes clear that they are functionally distinct. Despite being located in the same part of the genome, they are also genetically distinct and appear to have originated independently or at least have experienced a long evolutionary separation. This conclusion is drawn based on three main observations: (1) Sk-2 and Sk-3 do not share any inversions, (2) a phylogenetic analysis of the non-recombining region do not show a signal of common descent for the two spore killers, and (3) the sequence responsible for killing in Sk-2 cannot be found in the Sk-3 genome. If Sk-2 and Sk-3 had a shared evolutionary history, we would expect them to share at least some of the inversions and give a stronger signal of relatedness. Instead, our observations suggest convergent evolution of two distinct MDEs that have evolved independently in the same genomic region.
A clue to why both Sk-2 and Sk-3 are located in the same region may be that they share the resistance gene, at which they carry different alleles that confer specific resistance to their respective killing mechanisms. The resistance gene is of unknown function, but specific alleles are thought to be able to neutralize the toxic activity of the spore killer genes30. The different alleles are highly diverged and show signs of ancestral polymorphism, which may have allowed distinct mutations causing spore killing to independently pair up with already existent resistance alleles that only confer resistance specifically to them. These mutations could have happened in different genes in different parts of chromosome 3, after which modifiers that suppress recombination between the killer and the resistance genes, such as inversions43, have evolved, generating the genome structure that we see in our data. An alternative hypothesis for the evolution of Sk-2 and Sk-3 is that they have evolved from a common, ancestral, spore killer haplotype, where recombination suppression was achieved through non-structural means, which would relax selection against inversions. Such large-scale suppression of recombination without structural rearrangements was recently shown for the mating-type chromosomes in N. tetrasperma, and it was concluded that the large inversions found in these regions are derived and not the cause of the suppression32. We cannot with our data formally exclude this hypothesis, but the weak signal of monophyly of Sk-2 and Sk-3 and the fact that no homologous sequence to the Sk-2 rfk-1 killer sequence can be found in Sk-3 indicates that this is a less likely scenario.
A complete understanding of the molecular basis for killing in Sk-2 and Sk-3 is not yet available. A sequence required for killing, called rfk-1, was mapped to the right flank of Sk-228, corresponding to positions ~2,700,000–2,745,000 in the Sk-2 7426 haplotype. This region has been carefully dissected with gene deletion and complementation experiments, and the existence of a genetic element required for killing has been confirmed29. Utilizing our comparative transcriptomic approach, we were able to annotate the killer haplotypes and by doing so reveal a small number of novel ORFs in the region. Future functional analyses will reveal whether any of these genes are critical components of the killing and resistance mechanism or auxiliary modifiers of the meiotic drive system.
The inversions identified in the Sk-2 and Sk-3 haplotypes may be the cause of the suppression of recombination, and the fact that the inversions in the Sk-2 strains cover the exact region from the resistance gene to the killer sequence is suggestive of this. Indeed, the fact that Sk-2 and Sk-3 both carry inversions suggests that these are beneficial in linking together the killer and resistance genes. It is at this point not possible to determine whether the recombination suppression is caused by the inversions or by non-structural means as in N. tetrasperma32, but since any odd number of crossovers between the killer and the resistance loci will be highly deleterious, selection for reducing recombination is likely to be strong and both structural and non-structural mechanisms may act additively to reduce the recombination rate as much as possible.
It is possible that the inversions were facilitated by repetitive sequences, which also have accumulated in the Sk haplotypes. We see a strong increase of TEs in the Sk regions and this can be explained by several processes. Genome defence mechanisms such as RNA interference, heterochromatin, and RIP are known to limit the spread of TEs44, as is the ability to purge TEs through recombination with non-carrier strains45 or through recombination-associated unequal crossovers46. If these defence mechanisms are impaired or if recombination is reduced, the total TE content of a genome may increase, as is the case if the genome is invaded by new TE families that can evade the genome defence. We took advantage of the knowledge of the broad and efficient genome defence systems and repeat content in Neurospora34,47 to evaluate the relative importance of the different processes and detected no signs of either weakened genome defence mechanisms or an influx of new TE types. Specifically, a comparable GC distribution in repetitive and non-repetitive regions between spore killer strains and other Neurospora strains indicates a conserved RIP machinery35, while ChIP- and bisulfite sequencing data suggest that patterns of heterochromatin are normal. Instead the suppression of recombination of the region may allow repeats to accumulate due to a weaker efficiency of selection, i.e., slightly deleterious repeats cannot be purged because they are completely linked to the killing and resistance genes48. This hypothesis is consistent with the molecular evolution data and reduced DN/DS values of the Sk-2 haplotype, for which population-level analyses were possible. Because this region shows suppression of recombination with sensitive strains and because killer strains (with which they can freely recombine) appear to be rare, our results on reduced diversity and elevated πN/πS ratio of the spore killer region are in line with expectations of a reduced efficiency of selection in this region41. These findings are consistent with the recessive lethal mutations identified in the t haplotype42, except that the mutations accumulated in Sk-2 and Sk-3 would directly affect viability, due to the fact that N. intermedia grows vegetatively in the haploid state. Fitness assays of isogenic strains with and without the Sk haplotypes are needed in order to determine whether there is a more subtle negative fitness effect of these mutations.
Similar patterns of molecular evolution as found herein are found in other supergene systems, where coadapted gene complexes inhabit regions of suppressed recombination. For instance, molecular degeneration and large expansions of repetitive sequences are seen in animal and plant sex chromosomes49 and in many fungal mating-type chromosomes14,45. In many of these cases, degeneration has gone much further, with extensive loss of both genes and synteny12,50, but the conserved synteny and limited signs of gene loss found for Sk-2 is consistent with it being comparatively young (250–430 thousand years). We also see parallels between our MDEs and supergenes in convergent evolution for rearrangements and suppression of recombination11,51, supporting the idea that the need to link co-evolving genes may be an important driver of genome structure. On the other hand, both Sk-2 and Sk-3 have only very rarely been found in nature, whereas the single-gene spore killers in P. anserina and S. pombe appear to be much more common23,24,25. This distribution pattern suggests that either multilocus systems are more complex to evolve or that they fail to reach high frequencies quickly enough as the degeneration associated with suppressing recombination prevent them from spreading in the population.
Our dataset has a number of limitations. First, having only a single Sk-3 strain available means that we cannot detect signals of molecular degeneration in the non-recombining region of this spore killer, and we cannot test if it has experienced recent recombination. Furthermore, the backcrossing of four out of five spore killers to a laboratory strain means that we have a reduced ability to study the background genome of these strains and determine whether it differs from that of sensitive strains. For instance, Sk-2 could potentially form its own subpopulation of N. intermedia that only rarely interacts with sensitive strains. However, the fact that we detected evidence of recombination between Sk-2 and sensitive strains but limited signs of recombination among Sk-2 strains argues against this hypothesis.
In summary, this study is the first example of a complete assembly of a complex MDE that spans a large section of a chromosome, allowing the evaluation of the association between meiotic drive and genome architecture at an unprecedented resolution. Our data show a strong local impact of meiotic drive on chromosome architecture. It also suggests that TEs may be working in concert with meiotic drive in that they facilitate inversions and that suppression of recombination allows them to accumulate in the region, resulting in the killer haplotype having a different evolutionary trajectory than the rest of the Neurospora genomes.
Methods
Strains included in the study
All strains used in this study were ordered from the Fungal Genetics Stock Center (http://www.fgsc.net)52 and are listed in Supplementary Table 1. All strain used in this paper are referred to by their FGSC identification number. The reported phenotypes (i.e., Sk-2, Sk-3, sensitive or resistant) were confirmed by crossing them to N. intermedia spore killer tester strains. Crosses were performed on SC medium53 and each strain was crossed to an Sk-2 (7427 mat A, 7428 mat a), an Sk-3 (3193 mat A, 3194 mat a), and a sensitive (1766 mat A, 1767 mat a) strain of the opposite mating type. Only strains that showed the proper phenotype in all three crosses were included in the study.
Generation of genomic data using PacBio sequencing
Whole genomes of six strains (Table 1) were sequenced using the PacBio RSII platform (Pacific Biosciences). The strains were cultured by inoculating 500 ml Erlenmeyer flasks containing 200 ml of liquid malt extract (3%) medium with conidia (asexual spores). The flasks were incubated at 30 °C on a rotary shaking table for 3–4 days, and the cultures were then harvested by removing the mycelium from the flask and squeezing it between filter papers to remove excess liquid. The harvested mycelium was cut into small pieces and approximately 1 g was allotted into 2 ml tubes with screw-on caps, after which the tubes were stored at −20 °C until extraction. To extract the DNA, tissue of two tubes of each strain were freeze-dried overnight and macerated using a TissueLyzer II bead-beater (Qiagen). Two 2 mm metal beads were placed in each tube, which were then shaken at 25 Hz for 20–40 s, until no larger fragments remained. DNA was extracted using Genomic Tip G-500 columns (Qiagen) and cleaned using the PowerClean DNA Clean-Up Kit (MoBio Labs). The extracted DNA was sent to the Uppsala Genome Center (Science for Life Laboratory, Uppsala, Sweden), where libraries were prepared and sequenced on a PacBio RSII system, using four SMRT cells per sample and the C4 chemistry and P6 polymerase (Pacific Biosciences). Raw PacBio sequence data was filtered and assembled using the SMRT Analysis package and HGAP 3.0 assembler (Pacific Biosciences, https://github.com/PacificBiosciences/).
Generation of genomic data using Illumina HiSeq sequencing
Whole genomes of 91 strains were sequenced with Illumina HiSeq technology (Supplementary Table 1). For these samples, conidia were inoculated into 50 ml plastic culture tubes containing 10 ml of liquid malt extract (3%) medium. The tubes were incubated at 30 °C on a rotary shaker for 2–3 days before harvesting, which was done by removing the mycelium from the culture tubes and squeezing it between filter paper to remove excess liquid. The mycelium was then cut into small pieces and approximately 100 µg of tissue was allotted into 1.5 ml Eppendorf tubes, which were stored at −20 °C until extraction. Whole-genome DNA was extracted using the Fungal/Bacterial Microprep Kit (Zymo, www.zymo.com) and sent to the SNP&SEQ Technology Unit (SciLifeLab, Uppsala, Sweden), where libraries were prepared and sequenced in four lanes on an Illumina HiSeq 2500 system. Illumina reads were assembled de novo using ABySS54, with the following parameters: kmer size = 64, bubble size = 3, and minimum contig size = 200.
Fungal tissue for RNA-, bisulfite-, and ChIP-sequencing
We gathered transcriptome, bisulfite, and ChIP-seq data from three strains of N. intermedia, an Sk-2 strain, an Sk-3 strain, and a sensitive strain (Supplementary Table 1). For tissue collection during the vegetative development, strains were grown on solid Vogels medium N (Vogel 1956), on 90 mm Petri dishes covered with cellophane. After 2 days of growth at 25 °C, under 12:12 light–dark conditions, hyphal tissue was harvested from the surface of the cellophane with a sterile scalpel. Tissue from each plate was divided into three parts: One part of the tissue was stored at −20 °C for DNA extraction, a second part was immediately frozen in liquid nitrogen and stored at −80 °C for RNA extraction, and the third part of the tissue was immediately crosslinked with formaldehyde for the ChIP-seq experiment and stored at −80 °C. Three biological replicates were included by growing each strain on three plates.
In addition, transcriptome data were gathered from crosses. Crosses were performed on a single layer of Miracloth (50 × 50 mm2, MilliporeSigma, 475855-1 R) over 30.0 ml of synthetic crossing medium (pH 6.5, 1.5% sucrose; Westergaard and Mitchell 1947) in 100 mm diameter petri dishes. FGSC 1767 (sensitive a) was inoculated to the center of each sheet of Miracloth and cultured for 6 days before fertilization with FGSC 1766 (sensitive A), FGSC 7426 (Sk-2 A), or FGSC 3193 (Sk-3 A). Fertilizations were performed by transferring approximately 500 µl of a freshly prepared conidial suspension to each culture of FGSC 1767 (SkS a). Crosses were incubated at room temperature (RT) on laboratory shelves under ambient light conditions until 36 h after the first ascospores appeared on the undersides of the lids of the petri dishes. At this time point, which occurred at 11 days post fertilization (dpf) for FGSC 1767 × FGSC 1766, 12 dpf for FGSC 1767 × FGSC 7426, and 13 dpf for FGSC 1767 × FGSC 3193, perithecia and surrounding vegetative tissue were harvested from the sheets of Miracloth with razor blades, squished between filter paper to remove excess moisture, and ground to a fine powder under liquid nitrogen with a mortar and pestle. Total RNA was first isolated from ground tissue with TRIzol Reagent (Invitrogen) following the manufacturer’s guidelines. Total RNA was then purified with the miRNeasy Kit (Qiagen) following the manufacturer’s protocol for total RNA isolation with an on-column DNAase digestion. For each cross, we performed three biological replicates.
Bisulfite sequencing
For whole-genome bisulfite DNA sequencing, DNA was extracted using the ZR Fungal/Bacterial DNA MiniPrep™ Kit according to the manufacturer’s recommendations. Sequencing libraries were prepared from 100 ng of DNA using the TruSeq Methylation Kit (Illumina Inc., EGMK91324) according to the manufacturer’s protocol (#15066014). Unmethylated lambda DNA (Promega, D152A) was spiked-in at 0.1% to the samples in the library preparation procedure and sequencing was performed on Illumina HiSeq 2500 platform (SciLifeLab Uppsala) to generate 125 bp paired-end reads. All steps in the procedure were performed separately for the biological replicates.
ChIP and sequencing
For histone modification ChIP, 2-day-old mycelia tissue was crosslinked using 1% formaldehyde in 1× phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4) with gentle shaking at RT for 30 min. The reaction was quenched by adding glycine to final concentration of 125 mM and incubation at RT with gentle shaking for 5 min. The collected mycelia were washed with PBS, and for each biological replicate, 100 mg was homogenized by pestle in 600 µl ChIP lysis buffer (16.7 mM Tris-HCl - pH 8.0, 167 mM NaCl, 1.2 mM EDTA, 1.1% Triton X-100, 1× cOmplete EDTA-free protease inhibitor coctail (Roche)). The homogenized tissue was disrupted by sonication with submerged probe for 5 cycles, 20 s ON/40 s OFF, 5% intensity. ChIP lysis buffer was added to samples to 1000 μl and chromatin was then sheared by 60 cycles of sonication of 30 s ON/30 s OFF using high mode on a Bioruptor® (Diagenode) to reach median DNA fragment size of 0.5–1 kb. The sheared chromatin suspension was cleared by centrifugation (14,000 rpm, 5 min, 4 °C). In all, 15 μl of chromatin was set aside as input control and 150 μl of chromatin were mixed with 2 μl of antibody for each immunoprecipitation reaction. The following antibodies were used: IgG from rabbit serum (1 mg/ml, Sigma, cat. no. I5006), anti-Histone H3 (N-terminal) (Sigma, cat. no. H9289), anti-H3K4me2 (Active Motif, cat. no. 39141), anti-H3K9me3 (Active Motif, cat. no. 39161), and anti-H3K27me3 (Millipore, cat. no. 07-449). After overnight incubation with rotation at 4 °C, the immunoprecipitated complexes were collected using 15 μl of Dynabeads® Protein A (Thermo Fisher Scientific) per reaction after incubation for 1.5 h with rotation at 4 °C. Beads were washed once in 150 μl of following buffers at 4 °C: ChIP lysis buffer (without protease inhibitor) for 10 min, high-salt ChIP lysis buffer (as described above but supplied with 0.5 M NaCl) for 10 min, LiCl buffer (20 mM Tris-HCl—pH 8, 1 mM EDTA, 1% sodium deoxycholate, 1% NP-40 and 0.25 M LiCl) for 5 min, followed by 5 min in TE buffer (10 mM Tris-HCL, pH 8.0, 1 mM EDTA). Chromatin reverse crosslinking and DNA elution from input and immunoprecipitated samples were performed using iPure Kit (Diagenode, C03010015) following the manufacturer´s instructions. Three immunoprecipitation reactions from independent biological replicates were processed for next-generation sequencing library preparation. Each library was made from 5 ng of DNA using the MicroPlex Library Preparation Kit (Diagenode, C05010014) following the manufacturer’s instructions. Pools of libraries mixed in equimolar amounts were loaded onto 1% agarose electrophoresis gel for size selection. Fragments of 250–750 bp were excised from the gel and DNA was purified using the QIAquick Gel Extraction Kit (Qiagen). All steps were performed separately for the three biological replicates.
RNA sequencing
RNA was extracted from both vegetative and crossing tissue, prepared as outlined above. Traces of DNA were removed by DNase I treatment (Fermentas). The RNA concentration and quality was determined spectrophotometrically using Nano-Drop (Thermo Scientific), and RNA quality was assessed after electrophoresis on an Agilent Bioanalyzer using the RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s instructions. Sequencing libraries for mRNA were constructed by following the standard protocols of Illumina TruSeq mRNA library preparation kits, respectively (Cat# RS-122-2101/2102, Illumina Inc). Sequencing was performed on Illumina HiSeq 2500 platform at SciLifeLab Uppsala, to generate 125 bp paired-end reads. All steps were performed separately for the biological replicates.
Deletion of RFK-1 homolog in Sk-3
The genome of Sk-3 strain 3194 was searched with tblastn (BLAST 2.7.1+) for homologs of a protein required for Sk-2-based spore killing (RFK-1, 39 amino acid model). The most significant match to Sk-2 RFK-1 was found between positions 187,891 and 187,783 of chromosome 3. Two deletions vectors were constructed to delete this region (and flanking sequences) from RDGR170.3, an Sk-3-harboring strain of N. crassa55. Deletion vectors were constructed by Double-Joint PCR56. Specifically, they were designed to replace the following positions with a hygromycin selectable marker: 185,356–188,445 (Vector 207) and 187,617–188,445 (Vector 208). Deletion strains were obtained by electroporation of washed conidia as previously described57. Homokaryotic transformants were isolated by the microconidium method of Ebbole and Sachs (http://www.fgsc.net/fgn37/ebbole1.html) and confirmed by PCR.
Whole-genome alignments
To study structural variation in the PacBio assemblies, the MUMmer whole-genome aligner was used58. All genomes were aligned to the N. crassa OR74 assembly and to each other with the following parameters: nucmer –c 200 –b 2000. MUMmer alignment files were then visualized using a custom Python script.
Analysis of methylated DNA
Paired-end Illumina HiSeq reads were mapped to PacBio-based de novo assemblies of three N. intermedia strains: 3194 (Sk-3), 7426 (Sk-2), and 8761 (sensitive). The reads were trimmed using trimGalore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) (parameters: trim_galore–paired–trim1), and then mapped to the genomes using Bismark (http://www.bioinformatics.babraham.ac.uk/projects/bismark/) (parameters: bismark -X 700 –un). The reads where then deduplicated using the deduplicate bismark tool and finally methylated sites were extracted using bismark_methylation_extractor (parameters: bismark_methylation_extractor–paired-end–comprehensive–cytosine_report–CX–bedGraph–report–gzip–ignore 12–ignore_r2 12–ignore_3prime 3–ignore_3prime_r2). Whole-chromosome methylation patterns were visualized by merging the triplicate samples and calculating the percentage of methylated sites in 2 kb windows using a custom python script.
Analysis of ChIP-seq data
ChIP-seq reads were cleaned from adapter contamination and trimmed using CutAdapt59 and Trimmomatic60. The reads were then mapped to the corresponding PacBio assemblies using BWA61, deduplicated with Picard (http://broadinstitute.github.io/picard/), and mapping coverage was finally extracted using bedtools (https://bedtools.readthedocs.io/). Chromatin density was calculated as fold increase of coverage in immunoprecipitated samples compared to input control samples.
Analysis of repetitive DNA
Repetitive DNA and TEs were called using RepeatMasker (http://www.repeatmasker.org) and a library of Neurospora-specific repetitive elements47. Repeat enrichment in the Sk haplotypes was calculated using a one-sided binomial test, with Bonferroni corrected significance thresholds. Repeat clusters were identified using a sliding window approach. Percentage of repetitive sequence was determined in 2 kb non-overlapping windows, and windows consisting of more than 75% repetitive sequence were classified as repetitive. DNA methylation, H3K9me3 coverage, and GC content were then also calculated per window, and the average levels of these characteristics among windows classified as repetitive, non-repetitive, and unclassified (all windows) was plotted.
Analysis of transcriptome data
The raw reads from Illumina HiSeq were cleaned from adapter contamination and trimmed using CutAdapt and Trimmomatic as above. The trimmed reads were mapped to the high-quality PacBio assemblies using STAR62 (parameters: STAR–alignIntronMax 20000–alignMatesGapMax 20000–alignSJoverhangMin 10–outSAMtype BAM Unsorted–outFilterIntronMotifs RemoveNoncanonical–twopassMode Basic). The mapped reads were then assembled into transcripts using cufflinks63 (parameters:–library-type fr-firststrand). The data from the crosses were mapped to hybrid assemblies, where the non-recombining region of 7426 (Sk-2) or 3194 (Sk-3) was added to the 8761 (sensitive) assembly. The use of a hybrid assembly makes it possible to separate gene expression during meiosis in the non-recombining region of the spore killer strains from the gene expression of the homologous region in the sensitive strain. The called transcripts were then used to annotate the PacBio assemblies using MAKER64, which generated gene models that could be used to compare gene content between the sequenced species.
Comparisons of gene content
The gene content of strains 3194, 7426, and 8761 and N. crassa OR74 were compared by using OrthoMCL65 to infer orthological relationships. Protein sequences were extracted from the MAKER genome annotations of strains 3194, 7426, and 8761 and protein sequences of the N. crassa OR74 assembly was downloaded from FungiDB66. These were compared and orthologous relationships were inferred by performing an all-to-all protein BLAST search and clustering the genes with OrthoMCL. Genes that were unique for each strain were extracted and the ones located in the Sk region were further blasted back to the genomic sequences of all four strains using genBlastG67, in order to verify that they had not been missed in the annotation process of the other genomes. Gene function for these was inferred using HMMer (https://www.ebi.ac.uk/Tools/hmmer/) and searching FungiDB (http://www.fungidb.org).
An ORF that is necessary for killing in Sk-2 has been identified by the Hammond laboratory29: it is located at positions 2,744,002–2,744,118 on chromosome 3 in the genome assembly of strain 7426. We searched the genome of the Sk-3 strain 3194 and the sensitive strains 8761 and 8807 for homologous sequences using NCBI blastn and tblastn.
Calling of SNPs
Whole-genome raw Illumina HiSeq reads were cleaned from adapter contamination and trimmed using CutAdapt and Trimmomatic as above. The trimmed reads were then mapped to the PacBio assembly of the sensitive N. intermedia strain 880732 using BWA. This strain was selected as the reference genome since all seven chromosomes were assembled into complete contigs. The BAM file produced by BWA was deduplicated with picard, and complex regions were realigned with GATK IndelRealigner68. SNPs were called using GATK (first using -T HaplotypeCaller -bamWriterType CALLED_HAPLOTYPES -stand_emit_conf 10.0 -stand_call_conf 20.0 -gt_mode DISCOVERY–emitRefConfidence BP_RESOLUTION, then merging all vcf files using -T GenotypeGVCF–sample_ploidy 1–includeNonVariantSites). Variants from regions that had been annotated as repetitive with RepeatMasker were removed together with sites with missing data, using VCFtools69. Finally, the VCF files were converted to fasta files using GATK VariantsToTable and a custom Python script. The final FASTA file contained all called sites.
Phylogenetic analysis
The SNP data was used to infer phylogenetic relationships using RAxML70 with parameters: raxmlHPC-HYBRID-AVX -T 16 -m GTRCAT -x 45345 -p 22455 -# 100 -f a. Phylogenies for each chromosome were generated separately and were then merged together (excluding chromosome 3, which contains the Sk region) to form a network using Splitstree71. A phylogenetic network of the Sk region was also inferred by creating individual phylogenies for each inversion in the Sk-2 strains individually and merging these with Splitstree.
The phylogeny of the resistance (rsk) gene was also inferred with RAxML using the same parameters. The rsk sequence was extracted from de novo assemblies of all strains included in this study, together with 50 N. sitophila assemblies, that have also been generated from Illumina HiSeq data and four N. tetrasperma genomes from ref. 14, using GenBlastG. The rsk gene sequence from the N. tetrasperma72 reference genome was also added to the dataset and four alleles sequenced by Hammond et al. (2012)30.
Calculation of diversity and divergence in coding sequences
We calculated pairwise nucleotide difference on non-synonymous and synonymous sites (πN and πS) for all protein-coding genes in both N. intermedia sensitive and Sk-2 strains. Values were averaged across each chromosome except for chromosome 3, which was split into spore killer (Sk) and non-Sk regions. The πN/πS ratio was then calculated as an indicator of purifying selection. We performed McDonald–Kreitman tests on the Sk region and on the rest of the genome. Owing to limited number of polymorphism and high linkage disequilibrium in the Sk region, we treated the Sk region as a whole chromosome and counted the total number of polymorphism and divergence at non-synonymous and synonymous sites (PN, PS, DN, DS). The neutrality index (NI = (PN/PS)/(DN/DS) was used as an indicator of positive/negative selection. For comparison, we performed the same analysis on each chromosome. To control for difference in gene number and sequence length, we carried out bootstraps on all statistics by randomly sampling the same number of genes as in the Sk region and rescaled by the sequence length. For all analyses, we chose two Sk-2 strains (7427 and 7426) to reduce inbreeding effects introduced by several rounds of backcrossing to the Taiwan background26. To control for the variance introduced by sample size differences, we also carried out all analyses on a subsample of two randomly picked sensitive strains (1785 and 1787). We used two N. metzenbergii strains (6795 and P4149) as outgroup for calculation of divergence. All calculations were performed using the software dNdSpiNpiS_1.0 (http://kimura.univ-montp2.fr/PopPhyl).
Analyzing recombination patterns
Recombination between Sk-2 strains and other N. intermedia strains were assessed by creating phylogenetic trees from sliding windows of SNP data. The VCF files generated above were split up into sliding windows with a length of 50 variable sites, using the script phyml_sliding_windows.py downloaded from https://github.com/simonhmartin/genomics_general/ and phylogenies were generated using the BioNJ algorithm in PhyML (http://www.atgc-montpellier.fr/phyml/). Recombination was inferred by determining whether the four Sk-2 were monophyletic or whether one or more Sk-2 strain instead were grouping with other N. intermedia strains in the trees generated by PhyML. All trees were parsed and categorized using the Python library ETE Toolkit73 and the distribution over chromosome 3 was plotted.
Recombination between the four Sk-2 strains was assessed using LDhat (https://github.com/auton1/LDhat), with the interval algorithm and theta = 0.001.
Code availability
Scripts for analyzing recombination patterns and for calculating association between repetitive regions and heterochromatin are available at https://github.com/johannessonlab/intermedia_spore_killer/. Other scripts are available upon request.
Data availability
All raw sequencing reads generated in this study have been deposited at the Sequence Read Archive as BioProject PRJNA486257. Genome assemblies based on PacBio reads and of Illumina HiSeq read, SNP data in VCF format, predicted genes, and the alignment of the rsk gene have been deposited at Figshare (https://doi.org/10.6084/m9.figshare.c.4202669)74. All other relevant data are available upon request.
References
Burt, A. & Trivers, R. Genes in Conflict: The Biology of Selfish Genetic Elements (Harvard University Press, Cambridge, MA, 2009).
Werren, J. H. Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc. Natl. Acad. Sci. USA 108, 10863–10870 (2011).
Rice, W. R. Nothing in genetics makes sense except in light of genomic conflict. Annu. Rev. Ecol. Evol. Syst. 44, 217–237 (2013).
Sandler, L. & Novitski, E. Meiotic drive as an evolutionary force. Am. Nat. 91, 105–110 (1957).
Lindholm, A. K. et al. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol. Evol. 31, 315–326 (2016).
Lyttle, T. W. Segregation distorters. Annu. Rev. Genet. 25, 511–581 (1991).
Larracuente, A. M. & Presgraves, D. C. The selfish segregation distorter gene complex of Drosophila melanogaster. Genetics 192, 33–53 (2012).
Kelemen, R. K. & Vicoso, B. Complex history and differentiation patterns of the t-haplotype, a mouse meiotic driver. Genetics 208, 365–375 (2018).
Jaenike, J. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 32, 25–49 (2001).
Wang, J. et al. A Y-like social chromosome causes alternative colony organization in fire ants. Nature 493, 664–668 (2013).
Branco, S. et al. Multiple convergent supergene evolution events in mating-type chromosomes. Nat. Commun. 9, 2000 (2018).
Bachtrog, D. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 14, 113–124 (2013).
Charlesworth, B., Morgan, M. T. & Charlesworth, D. The effect of deleterious mutations on neutral molecular variation. Genetics 134, 1289–1303 (1993).
Corcoran, P. et al. Introgression maintains the genetic integrity of the mating-type determining chromosome of the fungus Neurospora tetrasperma. Genome Res. 26, 486–498 (2016).
Whittle, C. A., Sun, Y. & Johannesson, H. Degeneration in codon usage within the region of suppressed recombination in the mating-type chromosomes of Neurospora tetrasperma. Eukaryot. Cell 10, 594–603 (2011).
Fontanillas, E. et al. Degeneration of the nonrecombining regions in the mating-type chromosomes of the anther-smut fungi. Mol. Biol. Evol. 32, 928–943 (2015).
Artzt, K. Gene mapping within the T/t complex of the mouse. III: t-lethal genes are arranged in three clusters on chromosome 17. Cell 39, 565–572 (1984).
Didion, J. P. et al. A multi-megabase copy number gain causes maternal transmission ratio distortion on mouse chromosome 2. PLoS Genet. 11, e1004850 (2015).
Brand, C. L., Larracuente, A. M. & Presgraves, D. C. Origin, evolution, and population genetics of the selfish Segregation Distorter gene duplication in European and African populations of Drosophila melanogaster. Evolution 69, 1271–1283 (2015).
Dyer, K. A., Charlesworth, B. & Jaenike, J. Chromosome-wide linkage disequilibrium as a consequence of meiotic drive. Proc. Natl. Acad. Sci. 104, 1587–1592 (2007).
Pieper, K. E. & Dyer, K. A. Occasional recombination of a selfish X-chromosome may permit its persistence at high frequencies in the wild. J. Evol. Biol. 29, 2229–2241 (2016).
Dalstra, H. J. P., Swart, K., Debets, A. J. M., Saupe, S. J. & Hoekstra, R. F. Sexual transmission of the [Het-s] prion leads to meiotic drive in Podospora anserina. Proc. Natl. Acad. Sci. 100, 6616–6621 (2003).
Grognet, P., Lalucque, H., Malagnac, F. & Silar, P. Genes that bias Mendelian segregation. PLoS Genet. 10, e1004387 (2014).
Nuckolls, N. L. et al. wtf genes are prolific dual poison-antidote meiotic drivers. eLife 6, e26033 (2017).
Hu, W. et al. A large gene family in fission yeast encodes spore killers that subvert Mendel’s law. eLife 6, e26057 (2017).
Turner, B. C. & Perkins, D. D. Spore killer, a chromosomal factor in neurospora that kills meiotic products not containing it. Genetics 93, 587–606 (1979).
Campbell, J. L. & Turner, B. C. Recombination block in the spore killer region of Neurospora. Genome 29, 129–135 (1987).
Harvey, A. M. et al. A critical component of meiotic drive in Neurospora is located near a chromosome rearrangement. Genetics 197, 1165–1174 (2014).
Rhoades, N. A. et al. Identification of a genetic element required for spore killing in Neurospora. Preprint at https://www.biorxiv.org/content/early/2018/08/29/404004 (2018).
Hammond, T. M., Rehard, D. G., Xiao, H. & Shiu, P. K. T. Molecular dissection of Neurospora spore killer meiotic drive elements. Proc. Natl. Acad. Sci. USA 109, 12093–12098 (2012).
Corcoran, P. et al. A global multilocus analysis of the model fungus Neurospora reveals a single recent origin of a novel genetic system. Mol. Phylogenet. Evol. 78, 136–147 (2014).
Sun, Y., Svedberg, J., Hiltunen, M., Corcoran, P. & Johannesson, H. Large-scale suppression of recombination predates genomic rearrangements in Neurospora tetrasperma. Nat. Commun. 8, 1140 (2017).
Galagan, J. E. et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422, 859–868 (2003).
Rountree, M. R. & Selker, E. U. in Epigenomics (eds Ferguson-Smith, A. C., Greally, J. M. & Martienssen, R. A.) 321–341 (Springer, Dordrecht, 2009).
Galagan, J. E. & Selker, E. U. RIP: the evolutionary cost of genome defense. Trends Genet. 20, 417–423 (2004).
Lewis, Z. A. et al. Relics of repeat-induced point mutation direct heterochromatin formation in Neurospora crassa. Genome Res. 19, 427–437 (2009).
Selker, E. U. et al. The methylated component of the Neurospora crassa genome. Nature 422, 893–897 (2003).
Jamieson, K., Rountree, M. R., Lewis, Z. A., Stajich, J. E. & Selker, E. U. Regional control of histone H3 lysine 27 methylation in Neurospora. Proc. Natl. Acad. Sci. 110, 6027–6032 (2013).
Wang, Y., Smith, K. M., Taylor, J. W., Freitag, M. & Stajich, J. E. Endogenous small RNA mediates meiotic silencing of a novel DNA transposon. G3 (Bethesda) 5, 1949–1960 (2015).
Villalta, C. F., Jacobson, D. J. & Taylor, J. W. Three new phylogenetic and biological Neurospora species: N. hispaniola, N. metzenbergii and N. perkinsii. Mycologia 101, 777–789 (2009).
Castellano, D., James, J., Eyre-Walker, A. & Hernandez, R. Nearly neutral evolution across the Drosophila melanogaster genome. Mol. Biol. Evol. https://doi.org/10.1093/molbev/msy164 (2018).
Lyon, M. F. Transmission ratio distortion in mice. Annu. Rev. Genet. 37, 393–408 (2003).
Kirkpatrick, M. How and why chromosome inversions evolve. PLoS Biol. 8, e1000501 (2010).
Johnson, L. J. The genome strikes back: the evolutionary importance of defence against mobile elements. Evol. Biol. 34, 121–129 (2007).
Idnurm, A., Hood, M. E., Johannesson, H. & Giraud, T. Contrasted patterns in mating-type chromosomes in fungi: hotspots versus coldspots of recombination. Fungal Biol. Rev. 29, 220–229 (2015).
Lee, S.-I. & Kim, N.-S. Transposable elements and genome size variations in plants. Genomics Inform. 12, 87–97 (2014).
Gioti, A., Mushegian, A. A., Strandberg, R., Stajich, J. E. & Johannesson, H. Unidirectional evolutionary transitions in fungal mating systems and the role of transposable elements. Mol. Biol. Evol. 29, 3215–3226 (2012).
Hill, W. G. & Robertson, A. The effect of linkage on limits to artificial selection. Genet. Res. 8, 269–294 (1966).
Wright, A. E., Dean, R., Zimmer, F. & Mank, J. E. How to make a sex chromosome. Nat. Commun. 7, 12087 (2016).
Badouin, H. et al. Chaos of rearrangements in the mating-type chromosomes of the anther-smut fungus Microbotryum lychnidis-dioicae. Genetics 200, 1275–1284 (2015).
Purcell, J., Brelsford, A., Wurm, Y., Perrin, N. & Chapuisat, M. Convergent genetic architecture underlies social organization in ants. Curr. Biol. 24, 2728–2732 (2014).
McCluskey, K., Wiest, A. & Plamann, M. The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research. J. Biosci. 35, 119–126 (2010).
Westergaard, M. & Mitchell, H. K. Neurospora V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34, 573–577 (1947).
Simpson, J. T. et al. ABySS: a parallel assembler for short read sequence data. Genome Res. 19, 1117–1123 (2009).
TurnerB. C. & PerkinsD. D. Strains for studying spore killer elements in four Neurospora species. Fungal Genet. Rep. 40, 30 (1993).
Yu, J.-H. et al. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981 (2004).
Margolin, B., Freitag, M. & Selker, E. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Rep. 44, 34–36 (1997).
Kurtz, S. et al. Versatile and open software for comparing large genomes. Genome Biol. 5, R12 (2004).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Cantarel, B. L. et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18, 188–196 (2008).
Li, L., Stoeckert, C. J. & Roos, D. S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13, 2178–2189 (2003).
Stajich, J. E. et al. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res. 40, D675–D681 (2012).
She, R. et al. genBlastG: using BLAST searches to build homologous gene models. Bioinformatics 27, 2141–2143 (2011).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Huson, D. H. & Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267 (2006).
Ellison, C. E. et al. Massive changes in genome architecture accompany the transition to self-fertility in the filamentous fungus Neurospora tetrasperma. Genetics 189, 55–69 (2011).
Huerta-Cepas, J., Serra, F. & Bork, P. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 33, 1635–1638 (2016).
Svedberg, J. et al. Datasets deposited for Svedberg et al. (2018). ‘Convergent evolution of complex genomic rearrangements in two fungal meiotic drive elements’. Figshare https://dx.doi.org/10.6084/m9.figshare.c.4202669 (2018)
Acknowledgements
We would like to thank David J. Jacobson for fruitful discussions throughout the project, Markus Hiltunen for help with phenotyping strains, and Douglas Scofield for bioinformatics assistance. We also acknowledge support of the National Genomics Infrastructure (NGI)/Uppsala Genome Center and UPPMAX for providing assistance in massive parallel sequencing and computational infrastructure. Work at Illinois State University was funded by a grant from the National Science Foundation to T.M.H. (Award# 1615626). Work performed at NGI/Uppsala Genome Center was funded by RFI / VR and SciLifeLab, Sweden. The project was supported by a European Research Council grant under the program H2020, ERC-2014-CoG, project 648143 (SpoKiGen) (to H.J.)
Author information
Authors and Affiliations
Contributions
J.S. and H.J. planned the study. J.S. performed DNA extractions. S.H., P.M., and T.M.H. performed RNA extractions. S.H. prepared samples for bisulfite sequencing. S.H. and I.M. prepared samples for ChIP-seq. L.H. supervised work on ChIP-seq. A.A.V. performed experimental work and A.A. generated the deletion mutant. J.S. and J.C. performed bioinformatical analyses. J.S., J.C., M.L., and H.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Svedberg, J., Hosseini, S., Chen, J. et al. Convergent evolution of complex genomic rearrangements in two fungal meiotic drive elements. Nat Commun 9, 4242 (2018). https://doi.org/10.1038/s41467-018-06562-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-06562-x
This article is cited by
-
Allorecognition genes drive reproductive isolation in Podospora anserina
Nature Ecology & Evolution (2022)
-
Comparative genomics reveals the origin of fungal hyphae and multicellularity
Nature Communications (2019)
-
A cross-eyed geneticist’s view IV. Neurospora genes and inversions collude to cheat Mendel
Journal of Biosciences (2019)
-
Convergent evolution of complex genomic rearrangements in two fungal meiotic drive elements
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.