Abstract
Background
Behçet’s disease (BD) is a relapsing-remitting vasculitis, which can manifest in different organ systems including the eyes. There is currently limited published data describing the incidence of ophthalmic disease within the United Kingdom. The primary aim of this study was to survey the incidence and manifestations of ophthalmic BD prospectively, with a secondary aim of reviewing treatment modalities initiated in first-line therapy.
Methods
Using the British Ophthalmic Surveillance Unit reporting system between October 2016 and November 2018, we prospectively surveyed the number of cases of BD presenting to UK ophthalmologists. A total of 89 cases of ophthalmic manifestations of BD were reported and complete information was collected on 58 patients.
Results
93 eyes of 58 patients were affected. The median age of reported cases was 31 years (range 13–55 years) who were born in 15 different countries. Most cases (n = 35, 60%) had bilateral involvement. Vitritis was the most common ocular manifestation (68%; n = 63) followed by anterior uveitis (46%; n = 43). The greatest causes of visual morbidity were cystoid macular oedema, vitritis and retinal ischaemia. Most patients were prescribed either topical or oral corticosteroids (59%; n = 34), with some given intravitreal or intravenous corticosteroids. Five patients (8.6%) were initiated on disease-modifying anti-rheumatic drugs and one given an anti-TNF monoclonal antibody.
Conclusions
This is the first prospective study to analyse the incidence of ophthalmic involvement in BD over a 2-year period, finding an annual incidence of 0.04 per 100,000 individuals in the UK.
Similar content being viewed by others
Introduction
Behçet’s Disease (BD) is a relapsing-remitting vasculitis affecting vessels of all sizes. Manifestations of this multi-system inflammatory disease include oral and genital aphthae, erythema nodosum-like skin lesions, thrombophlebitis, and uveitis. The disease is most common in those whose ancestry is based in the Mediterranean Basin to the Far East, along the ancient Silk Road [1, 2], and was described separately by Benediktos Adamantiades and Hulusi Behçet in the 20th century [3]. In keeping with the current ICD-10 terminology by the World Health Organisation [4], the term BD is used in this article.
Typically, BD affects younger patients and infrequently develops before puberty or after the fifth decade. The frequency of ocular BD varies around the world. In some ethnic groups, inflammatory eye disease occurs in over 50% of patients [5]. Uveitis is the most common ocular manifestation and can be present in up to 90% of patients [6]. Behçet’s uveitis can be challenging to treat and even in those treated, up to 74% lose vision in the decade following onset of ocular symptoms [7]. One of the current epidemiological challenges in BD is to further our understanding of ophthalmic manifestations worldwide. There is currently very limited data describing the incidence and range of ophthalmic disease within the United Kingdom.
The main aim of this study was to survey the incidence and manifestations of ophthalmic BD in the UK prospectively. The secondary aim was to understand treatment modalities used to manage ophthalmic disease.
Method
The British Ophthalmic Surveillance Unit (BOSU) surveys rare ophthalmic in the UK, using a physical paper reporting card system. All ophthalmologists on the General Medical Council specialist register (approximately 1300) are sent a card on a monthly basis. The BOSU methodology has been previously described [8].
Reporting criteria can be seen in supplemental material (Supplementary Material 1). Briefly, clinicians were asked to report new cases of ophthalmic manifestations of BD. This could either be the first ophthalmic manifestation in a previously diagnosed patient with systemic BD, or a new diagnosis of BD, in which the ophthalmic findings play an integral part.
Cases were surveyed for a period of 24 months between October 2016 and November 2018. Data collected in the initial 12-month period was analysed and published separately as part of a PhD thesis [9].
Results
Over the two-year surveillance period, 89 cases were reported to the study, with 31 cases excluded; three cases were a reporting error (not the first episode of uveitis), six cases were a misdiagnosis and 22 questionnaires were incomplete or unreturned. Questionnaires were completed and returned by the reporting clinicians for 58 patients.
In total, 93 eyes of 58 patients were affected. The age distribution is shown in Fig. 1. The median age of reported cases was 31 years (range 13–55 years; IQR 16). Most cases (n = 35, 60%) had bilateral involvement and of those affected unilaterally, there was a slightly greater involvement of the right eye (n = 14, 24%; compared to the left eye n = 9, 16%). There were more males 60% (n = 35) than females 34% (n = 20); gender was not specified for 5% (n = 3) of individuals.
There were 44 patients who identified as White (76%), two patients Asian (3.5%), two patients North African, and two Other, with two patients’ ethnicities and countries of birth unknown.
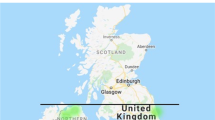
Table 1 details the country of birth of the individuals included in the study, and the map (Fig. 2) shows the postcode areas in which they currently live. Cases were born in at least 15 different countries, and primarily live in and around major cities in the UK in the South-East and the North-West.
Clinical presentation of ophthalmic Behçet’s disease
Sixty-seven percent (n = 39) of individuals were diagnosed with BD at the time or after they presented with ocular inflammation. The remaining 33% (n = 19) were reported to have their first ocular inflammatory event after they had a confirmed diagnosis of BD. These individuals had had a systemic manifestation of BD an average of 3.6 years (SD 6 years; range 1–36 years) prior to exhibiting ocular manifestations.
Ninety-three percent (n = 54) of cases had recurrent oral aphthous ulcers, 45% (n = 26) reported genital ulceration, 53% (n = 31) skin lesions, 2% (n = 1) systemic vascular occlusion and 7% (n = 4) inflammatory arthritis.
The different ocular manifestations of BD are shown in Fig. 3. There were no reported cases of episcleritis or ocular surface disease in any of the 93 affected eyes. Anterior uveitis was present in 46% (n = 43) and in conjunction with a hypopyon in a further 6% (n = 6). Posterior synechiae were seen in 5% (n = 4). Vitritis was reported in 68% (n = 63). Cystoid macular oedema (CMO) was present in 25% (n = 23) of eyes and retinal infiltrates in 28% (n = 26). Inflammatory retinal vascular occlusion was reported in 10% (n = 9). Retinal vasculitis was present in 9% (n = 8). One eye had neovascularization, and another eye had sclerokeratitis causing failure of two previous penetrating keratoplasties.
Respondents were asked to specify the cause of visual loss, if relevant. The top three causes of visual loss were cystoid macular oedema, vitritis and retinal ischaemia. The median best-corrected visual acuity (BCVA) in the affected eye for those presenting with cystoid macular oedema was 1.0 logMAR (IQR 0.5). Those with vitritis had a median LogMAR BCVA of 1.0 (IQR 0.9) and those with retinal ischaemia had a median LogMAR BCVA of 0.80 (IQR 0.71). The incidence of poor vision was not significantly different in those taking systemic corticosteroids alone, conventional immunosuppressants, a combination of the two or a biologic medication (p = 0.84). Best-corrected visual acuity in the worse affected eye from any cause was no better than 1.0 LogMAR in 28% percent of patients (n = 16). Median BCVA was 0.30 LogMAR in females (IQR 1.2) and 0.30 LogMAR in males (IQR 0.7).
Nineteen percent of patients (n = 11/58) were treated systemically with immunosuppressants prior to developing eye disease. Three of these patients (27%) had previously received a diagnosis of BD. The systemic medication the patients were receiving were: corticosteroids n = 4, disease-modifying anti-rheumatic drugs (DMARD) n = 1, corticosteroid plus DMARD n = 4, multiple DMARDs n = 1, and anti-TNF monoclonal antibody medication n = 1.
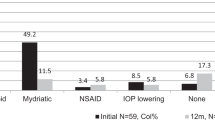
In determining the current treatment regimen for ocular Behçet’s disease, respondents were asked to specify which first-line therapy was given to patients.
Fifty-nine percent (n = 34) of patients were prescribed topical corticosteroids; of these, one patient was given an additional intraocular pressure reducing drop for both affected eyes. Fifty-nine percent (n = 34) of patients were prescribed oral corticosteroids. Two patients were prescribed intravitreal corticosteroids and four patients were prescribed intravenous corticosteroids. Five patients were initiated on a DMARD and one patient was prescribed an anti-TNF monoclonal antibody. Table 2 below shows the breakdown of first-line therapy for the top three causes of visual loss.
Of the five patients on DMARDs, three were on no previous treatment and the other two patients had already been on oral steroids. The patient commenced on anti-TNF monoclonal antibody medication had retinal infiltrates despite already being on oral corticosteroids.
Discussion
This is the first study to survey the incidence, manifestations and treatment of ophthalmic BD prospectively over a 2-year period. The data show the annual incidence of ocular inflammatory disease associated with BD in the UK to be 0.04 per 100,000 individuals. Previously published studies examining the incidence of BD have been retrospective in nature [10, 11] and have not specifically looked at the incidence of ocular manifestations of BD. With an estimated percentage of between 21 and 66% ophthalmic involvement in BD depending on ethnicity [10,11,12], it can be inferred that the incidence of ocular inflammatory disease associated with BD in the UK is lower than that in similar countries. There is currently BD incidence data available for Spain (0.66 per 100,000) [12], Sweden (0.2 per 100,000) [13], Italy (0.24 per 100,000) [14], and the Netherlands (1 per 100,000) [15], although it is important to note that these incidences are for BD and not ocular involvement in BD.
Ophthalmic disease in BD has been reported to be more common in males and to be associated with a more severe disease course [16]. Our findings confirmed males are affected more commonly, although visual outcomes were not significantly different between males and females. The prevalence of disease with regards to ethnicity was in keeping with the natural history of BD and its susceptible populations.
At the time of presentation with ocular disease, 33% already had a diagnosis of BD based on at least one other systemic manifestation. Ocular manifestations appeared between 7 days and 16 years after the original diagnosis of BD. An additional 67% had a diagnosis of BD made on the basis of presentation with ocular manifestations of BD.
In the patients with poor vision, respondents to the survey were asked to identify the major cause of visual morbidity; the three most common causes were macular oedema, vitritis and retinal ischaemia. Many patients presented with a some or all of these sight-threatening signs. The finding of macular oedema being the greatest cause of visual morbidity in the 12-month data [9] was reproduced in the larger cohort at 24 months.
Analysis of first-line treatment given for the reported cases showed topical and oral corticosteroids to be the mainstay of treatment for all manifestations. Intravenous corticosteroids were given to four patients. One had a retinal infiltrate and panuveitis, two had panuveitis with macular oedema, and another bilateral panuveitis with retinal vasculitis. Intravitreal corticosteroids were given to three eyes of two patients. One patient had bilateral injections for retinal vasculitis and panuveitis and one patient had a unilateral injection for macular oedema.
Second line agents were started in five patients. One had bilateral anterior uveitis, one bilateral vitritis and another bilateral panuveitis. One patient had retinal vasculitis with macular oedema, and another panuveitis with retinal infiltrates.
The European League Against Rheumatism (EULAR) guidance published in 2008 was updated in 2016 at the start of our data collection period. This revision was published in 2018 and recommended the use of systemic immunosuppressive agents such as azathioprine, infliximab or adalimumab, interferon-α or ciclosporin-A in conjunction with systemic corticosteroids in patients with posterior uveitis; corticosteroids alone are not recommended as a management strategy for ocular disease [17].
Of our five patients on DMARDs, three patients were started on dual therapy of DMARD and corticosteroid as a result of their eye disease. One patient was started on a DMARD who had already been on oral corticosteroids and another was started on a DMARD without any concurrent systemic corticosteroid therapy.
Eighty-four percent (n = 27/34) of patients given oral corticosteroids were given no additional DMARD as part of first-line treatment, and so may not have been managed in accordance with EULAR guidance. This could be due to the fact that a large number of ophthalmologists would most likely be requesting general medical input for these patients prior to starting systemic immunosuppression or referring onwards to BD centres of excellence for further specialist management and consideration of biologic medication.
Limitations
Prospective surveillance studies minimise recall bias but depend upon timely and accurate reporting of cases. Over 1300 ophthalmologists receive BOSU reporting cards monthly, with typically 70–75% of cards being returned each month (personal communication BF 2019; RCOphth). These cards either report a new case or state there is nothing to report, which are both equally important responses. It can be seen, however, that with rare conditions such as those monitored by BOSU, even a few unreported cases can significantly alter the incidence rate. Missed reporting can occur due to a ‘slip’ in busy clinics, due to patients being seen by ophthalmologists not on the specialist register and thus not receiving the reporting card, or due to reporting fatigue by those in centres where many of the surveyed conditions are being seen on a weekly basis and thus creating additional administrative work for the clinician.
In such studies, questionnaires should be sent out to reporting clinicians without delay to ensure clinical notes are available and correct information is obtained. We sent out all of our questionnaires within a week of notification of the reported case. In cases where we had received no reply after two weeks, a paper reminder was sent. Furthermore, this was followed up with a phone call or email one month later to promote higher return rates.
Twenty-two questionnaires were incomplete or not returned to us, with just over half (n = 12) of those contacted claiming not to have reported a case. Despite our best efforts to obtain the data from the remaining ten cases, we were unable to make direct contact with the relevant clinicians. The rate of return of questionnaires at 12 months and at 24 months were consistent.
Where a sizeable number of cases might be referred from district general hospitals to tertiary centres, there could be a potential for over-reporting through duplication. We ensured no duplicate reports in our study by cross-checking all reported cases.
Finally, in incidence studies, the denominator used is typically the population of the region surveyed. In the case of BD, as people over the age of 60 years are much less likely to be affected, the true incidence is likely to be higher. The Office of National Statistics estimated in 2018 that 63.7% of the population was aged between 16 and 64 years. This change in the denominator from 66.4 million down to 42.3 million people nearly doubles the incidence proportion of BD in the UK to 0.07 per 100,000 individuals over the two-year period.
Conclusion
This is the first prospective study to analyse the incidence of ophthalmic involvement in BD in a large cohort in the UK. The findings will be of use to clinicians in offering a more informed prognosis of ocular manifestations of BD, recommending more standardised treatment to these patients and in guiding clinical trials towards achieving the best systemic treatment for control of overall disease.
Summary
What was known before
-
The frequency of ocular Behçet’s disease varies around the world.
-
Uveitis is the most common ocular manifestation of BD.
What this study adds
-
The incidence of BD in the UK is 0.04 in 100,000.
-
The greatest causes of visual morbidity in BD arise from cystoid macular oedema, retinal ischaemia, and vitritis.
References
Sakane T, Takeno M, Suzuki N, Inaba G. Behçet’s disease. N Engl J Med. 1999;341:1284–91.
Verity DH, Marr JE, Ohno S, Wallace GR, Stanford MR. Behçet’s disease, the silk road and HLA-B51: historical and geographical perspectives. Tissue Antigens. 1999;54:213–20.
Zouboulis CC, Keitel W. A historical review of early descriptions of Adamantiades-Behçet’s disease. J Invest Dermatol. 2002;119:201–5.
International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2021. https://icd.who.int/browse10/2019/en#/M35.2.
Yazici H, Fresko I, Yurdakul S. Behçet’s syndrome: disease manifestations, management, and advances in treatment. Nat Clin act Rheumatol. 2007;3:148–55.
Yang P, Fang W, Meng Q, Ren Y, Xing L, Kijlstra A. Clinical features of Chinese patients with Behçet’s disease. Ophthalmology. 2008;115:312–8.e4.
Benezra D, Cohen E. Treatment and visual prognosis in Behçet’s disease. Br J Ophthalmol. 1986;70:589–92.
Foot B, Stanford M, Rahi J, Thompson J. The British Ophthalmological Surveillance Unit: an evaluation of the first 3 years. Eye. 2003;17:9–15.
Petrushkin H. Behçet’s disease in the United Kingdom: genetic risk factors and ophthalmic manifestations. PhD thesis. UK: Queen Mary University of London; 2019. https://qmro.qmul.ac.uk/xmlui/handle/123456789/55454.
Zouboulis CC, Kötter I, Djawari D, Kirch W, Kohl PK, Ochsendorf FR, et al. Epidemiological features of Adamantiades-Behçet’s disease in Germany and in Europe. Yonsei Med J. 1997;38:411–22.
González-Gay MA, García-Porrúa C, Brañas F, López-Lázaro L, Olivieri I. Epidemiologic and clinical aspects of Behçet’s disease in a defined area of Northwestern Spain, 1988-1997. J Rheumatol. 2000;27:703–7.
Shahram F, Mæhlen MT, Akhlaghi M, Davatchi F, Liao YJ, Weyand CM. Geographical variations in ocular and extra-ocular manifestations in Behçet’s disease. Eur J Rheumatol. 2019;6:199–206.
Mohammad A, Mandl T, Sturfelt G, Segelmark M. Incidence, prevalence and clinical characteristics of Behcet’s disease in southern Sweden. Rheumatology. 2013;52:304–10.
Salvarani C, Pipitone N, Catanoso MG, Cimino L, Tumiati B, Macchioni P, et al. Epidemiology and clinical course of Behçet’s disease in the Reggio Emilia area of Northern Italy: a seventeen-year population-based study. Arthritis Rheum. 2007;57:171–8.
Kappen JH, van Dijk EH, Baak-Dijkstra M, van Daele PL, Lam-Tse WK, van Hagen PM, et al. Behçet’s disease, hospital-based prevalence and manifestations in the Rotterdam area. Neth J Med. 2015;73:471–7.
Yazici H, Tüzün Y, Pazarli H, Yurdakul S, Ozyazgan Y, Ozdoğan H, et al. Influence of age of onset and patient’s sex on the prevalence and severity of manifestations of Behçet’s syndrome. Ann Rheum Dis. 1984;43:783–9.
Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis. 2018;77:808–18.
Author information
Authors and Affiliations
Contributions
SP: collection and analysis of data, literature review and drafting of manuscript and figures; HP: concept and design of study, literature review, critical review of manuscript; BF: BOSU support for study set up; MRS: Expert in ocular inflammatory disease; critical review of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Parvizi, S., Petrushkin, H., Foot, B. et al. Incidence of ophthalmic involvement in Behçet’s disease in the United Kingdom: a British Ophthalmic Surveillance Unit (BOSU) study. Eye 36, 1074–1079 (2022). https://doi.org/10.1038/s41433-021-01585-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01585-z