Abstract
Aims
To estimate the incidence of childhood uveitis not associated with juvenile idiopathic arthritis (JIA) in the United Kingdom.
Methods
Children under 16 years who presented with a new diagnosis of uveitis from November 2014 to October 2015 were identified prospectively through the British and Scottish Ophthalmological Surveillance Unit reporting card system. Incident questionnaires were sent to reporting ophthalmologists at presentation and 12 months.
Results
From 1st November 2014 to 31st October 2015, 119 cases were reported. Thirty-nine cases were excluded. The estimated minimum annual incidence of non-JIA uveitis in children younger than 16 years is 0.66 per 100,000 (95% CI 0.52–0.82). Median age at presentation was 10 years. 73% had bilateral uveitis. Median (IQR) BCVA in the worse eye was 0.3 (IQR 0.1–0.66) logMAR. The location of uveitis was: anterior 36%, intermediate 24%, posterior 6.8% and panuveitis 30%. 70% of cases were idiopathic. Most children were started on topical corticosteroids at presentation (86%, n = 51). At presentation, 31% (n = 19) were on started on systemic corticosteroids. At 1 year only 13% (n = 7) remained on corticosteroids, with the majority transitioned to steroid-sparing agents: methotrexate (30.8%, n = 16), mycophenolate (5.8%) and anti-TNF agents 5 (9.6%). At 1 year, 46% had ongoing intraocular inflammation despite treatment. The most common ocular adverse event was raised intraocular pressure (13.5%, n = 7).
Conclusion
Our study provides the first national population-based data of non-JIA childhood uveitis. Most children remain on treatment at 1 year, but visual acuity improves and none were eligible for sight-impairment registration.
Similar content being viewed by others
Background
Uveitis in children is a rare condition, with an incidence of 4.85/100,000 children age 0–16 years per year reported by a regional five-centre study in the United Kingdom. 47% of cases occurred in the context of juvenile idiopathic arthritis (JIA) [1]. In children and young people uveitis tends to be more severe and aggressive than in adults, and the rate of irreversible loss of vision, including blindness is high at 17–30% [2, 3].
National registries for JIA in Germany and Italy include data on JIA-associated uveitis and have produced reports on treatment outcomes [4,5,6], yet epidemiological data for the UK are lacking, especially for non-JIA-associated uveitis. This can make service planning and client-centred commissioning of uveitis services difficult, as basic data about incidence and regional variations in management are not known. For patients and families, the lack of clear pathways and information is frustrating.
In 2003 Edelsten et al. described the incidence of childhood uveitis in one region of the UK [1]. While this study provided useful data it may not necessarily have been representative of the whole of the UK, and the incidence may possibly have changed over the last 15 years, with improved screening and referral pathways. In addition, most of the available data on childhood uveitis are for cases associated with JIA. We therefore conducted this study to identify all new cases of non-JIA-associated childhood uveitis over a 12-month period, with the aim of estimating its minimum incidence. We also aimed to deliver information about clinical findings at presentation, management, systemic associations, visual outcomes and adverse outcomes from uveitis or from its treatment. These findings will help us establish the scale of the problem, with a view to future clinical studies and planning of patient care. This work will also provide clinicians and families with data on current practice, management and outcomes.
Materials and methods
This study had research approval from the South West Bristol Research Ethics Committee (14/SW/1094). The study was conducted according to the Tenets of the Declaration of Helsinki.
Children aged 0–16 years with a new diagnosis of uveitis were identified prospectively by nationwide active surveillance through the British Ophthalmological Surveillance Unit (BOSU) and Scottish Ophthalmological Surveillance Unit (SOSU) monthly reporting card system.
All consultants or associate specialist ophthalmologists in the United Kingdom form the reporting base for BOSU and SOSU, and are sent a reporting card each month. This card contains a list of rare eye conditions under surveillance, and ophthalmologists are requested to report if they have seen a patient with any of these conditions in the preceding month. Whenever a positive notification is made, BOSU and SOSU inform the respective study investigators, who then contact the reporting ophthalmologists to gather more information.
We collected incident cases over a 12-month period from November 2014 to October 2015. Our case definition was any child aged 0–16 years with newly diagnosed uni- or bilateral uveitis, without a known diagnosis of JIA or ocular trauma.
Following a positive notification, an incident questionnaire was sent to reporting ophthalmologists to determine the demographics, clinical findings at presentation and initial management. At 12 months, a follow-up questionnaire was sent to determine the aetiology of uveitis, management, visual outcomes and adverse events. Ophthalmologists who did not return the questionnaire were sent reminder letters and emails to increase the response rate.
Data were recorded in a Microsoft Access database, and analysed using Microsoft Excel and SPSS. Where visual acuity was recorded as Snellen acuity it was converted to LogMAR equivalents. Using mid-2015 population estimates for the 12 UK regions (Office of National Statistics, www.statistics.gov.uk), incidence figures were calculated.
Uveitis was classified according to the International Uveitis Study Group criteria based on the anatomical location of the inflammation; anterior, intermediate, posterior or panuveitis [7].
Results
Incidence
Between 1 November 2014 and 31 October 2015, 119 cases of non-JIA childhood uveitis were reported, of which 39 were excluded. Twenty-nine were outside the study date range (initial presentation of uveitis not within the defined study period), two had associated JIA, four were reported in error (reporting ophthalmologist did not report a case), and four were unidentifiable (reporting ophthalmologist could not recall patient). Our return rates were 74% for incident questionnaires, and of these, we were able to obtain follow-up data for 52/59 (88%) cases.
The total number of incident cases over the 12-month study period was therefore 80. This gives an estimated annual incidence of non-JIA-associated uveitis in under-16s of 0.66 per 100,000 (95% CI 0.52–0.82).
Patient demographics
Median age at presentation was 10 years (range 3–16 years). The male: female ratio was 31(52%)/25(43%), and in three cases (5%) gender were not recorded on the questionnaire.
Symptoms and clinical findings
72.9% (n = 43) had bilateral uveitis, 10% (n = 6) had right-sided uveitis and 17% (n = 10) had left-sided uveitis. The most common symptoms at diagnosis were: red eye 54% (n = 32), painful eye 42% (n = 25), poor vision/reduced vision 39% (n = 23), photophobia 12% (n = 7), abnormal appearance of the eye 7% (n = 4) and watery eye three cases 5% (n = 3). Of note, 7% (n = 4) of children were asymptomatic and the uveitis was diagnosed on routine eye examinations.
Median best corrected visual acuity (BCVA) in the worse eye was 0.3 (IQR 0.1–0.66, range −0.2 to 3) logMAR at presentation, improving to 0.1 (IQR 0–0.35, range −0.2 to 2.7) logMAR at 1 year. No children were eligible for certification of visual registration, as the children with very poor vision had unilateral uveitis. Median (IQR) intraocular pressure was 14 (12–16) mmHg at presentation and 15.5 (12–19) mmHg at 1 year.
The location of uveitis was: 35% anterior uveitis, 23% intermediate uveitis, 9% posterior uveitis and 33% panuveitis. Table 1 shows a breakdown of clinical findings in the cases.
Underlying medical conditions
In the majority of cases (70%) no underlying cause was identified; these were classified as “idiopathic”. 10% were infective (herpes simplex virus, Lyme disease, toxocara), 9% noninfective systemic (sarcoidosis, Behçet’s disease), 5% noninfective nonsystemic (Fuchs heterochromic cyclitis, phacolytic uveitis), and 4% unknown (no aetiology stated by reporting clinician on both incident and follow-up questionnaires).
No underlying systemic medical problem was identified at diagnosis in 83% of children. The remaining children had: preceding upper respiratory tract infection (n = 2), Adams Oliver syndrome (n = 1), TINU (n = 1), asthma (n = 1), demyelinating neuropathy (n = 1), epilepsy (n = 1), skin problems (n = 2) and coeliac disease (n = 1).
Management
Most (87%) children were treated with topical corticosteroids following the initial diagnosis of uveitis. 10% (n = 6) of children were not started on any treatment—of these four had intermediate uveitis, and one had anterior uveitis with vitritis. All had good visual acuities. Topical nonsteroidal medications were used in 3.4% (n = 2). Figure 1 shows the breakdown of treatment.
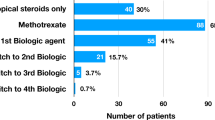
Systemic treatment was started in 32% (n = 19) of children. This included corticosteroids, steroid-sparing agents, antibiotics and antiviral agents. Of those who were started on systemic immunosuppression, 11 had panuveitis, 4 intermediate uveitis, 2 anterior uveitis and 1 posterior uveitis. Of note, three children were started on steroid-sparing agents early, all of whom had panuveitis. At 1 year, there was a shift towards the use of steroid-sparing agents such as methotrexate and the anti-TNF agent adalimumab. Figure 2 shows the distribution of systemic treatment at diagnosis, and at 1 year.
16% of children underwent invasive procedures: intravitreal bevacizumab (5.8%), Baerveldt tube (3%), diagnostic tap (3%), surgical iridotomy (2%) or examination under anaesthesia (2%).
Visual outcomes and adverse effects
At 1 year, 52/59(88%) remained under follow-up. Median (Interquartile range, IQR) BCVA in the worse eye improved from 0.3 (0.1–0.66) logMAR at diagnosis to 0.1 (0–0.33) logMAR at 1 year. No child required sight-impairment certification, as the few children with loss of vision due to uveitis were unilateral cases. Figure 3 shows BCVA at baseline and 1 year in the worse-affected eye.
Four children had worse vision at 1 year compared to baseline. This was primarily due to the development of posterior segment pathology: pigment epithelial detachment (n = 1, logMAR 0.00 at baseline, 0.5 at 1 year); multifocal choroiditis (n = 1, logMAR 0.3 at baseline, 2.7 at 1 year); and macular oedema (n = 1, logMAR 0.8 at baseline, 2.7 at 1 year). One child had a mild reduction of vision from logMAR 0.2 to 0.35 at 1 year due to development of lens opacity. One child was reported to have vision of logMAR 2.7 at 1 year due to band keratopathy, unfortunately visual acuity for this child was not recorded in the incident questionnaire.
At 1 year, 73% of children remained on topical treatment and 65% remained on systemic treatment. 46% had ongoing intraocular inflammation despite treatment.
Ocular adverse events were: raised intraocular pressure (11.5%, n = 6), cystoid macular oedema (5.8%, n = 3), macular oedema (3.8%, n = 2), band keratopathy (3.8%, n = 2), pigment epithelial detachment (1.9%, n = 1) and reduced vision (1.9%, n = 1). Of those with raised intraocular pressure, one child had a surgical iridotomy, two had tube surgery, and seven were untreated. Two children were given an intravitreal injection of bevacizumab to treat macular oedema and pigment epithelial detachment.
Adverse effects from systemic medication occurred in 11% (n = 3). These were all due to methotrexate, and included nausea, abnormal liver function and neutropenia.
At 1 year, approximately half the children remained under follow-up under their initial hospital unit. Figure 4 shows the breakdown of where follow-up was conducted.
Discussion
The principal finding of this study is that in the UK, the incidence of childhood uveitis not associated with JIA is 0.66 per 100,000.
This figure is lower than the previously reported 4.85 per 100,000 for all types of childhood uveitis in a single region of the UK [1]. The discrepancy may arguably relate to clustering of cases in a region where there is an expert subspeciality practice. However, both figures are in keeping with previous reports that 14–47% of childhood-onset uveitis cases are attributable to JIA [1, 8].
Most children in our study had bilateral uveitis at presentation, where the anatomical locations were 35% anterior uveitis, 23% intermediate uveitis, 9% posterior uveitis and 33% panuveitis. This is similar to previously reported percentages of anterior uveitis (13.4–44.6%), intermediate uveitis (27.7–41%), posterior uveitis (14–23.7%) and panuveitis (13–30%) [8,9,10].
The most frequent diagnosis was idiopathic uveitis, in 70%. This was based on the data provided in the 1-year follow-up questionnaire, where clinicians were asked to provide a diagnosis. This diagnosis would arguably have been reached following full investigation to rule out an underlying systemic disorder or infective cause, although given the nature of our study we were not able to determine what tests were performed for each child.
Idiopathic cases were the most common type of anterior and intermediate uveitis, whilst toxoplasmosis was the most common cause of posterior uveitis and all infectious uveitis.
It has previously been reported that noninfectious uveitis accounts for between 69 and 95% of childhood uveitis [11]. Our study showed that only 10% of cases were due to an infective cause, which is within this range. Despite the relatively low percentage of such cases, we feel that it is important for children to be screened for infectious causes to ensure appropriate treatment can be administered.
87% of children were started on topical corticosteroids at the point of diagnosis of uveitis. 10% were not started on any treatment—of these four had intermediate uveitis, and one had anterior uveitis with vitritis. All had good visual acuities, which may be why no treatment was used.
The use of systemic immunosuppression at 1 year was high, with 30.8% children receiving methotrexate, 5.8% receiving mycophenolate and 9.6% receiving a biologic agent. This is not unsurprising as it has been shown that the use of immunosuppressive medications decreases the risk of vision loss in JIA related uveitis [12]. Other reports have also suggested that up to 40% of children with JIA uveitis have intraocular inflammation that cannot be controlled with topical treatment alone [13].
Of the children started on methotrexate, only four required biologic therapy. This suggests that methotrexate was beneficial in this cohort, which is consistent with previous evidence that up to 73% of children experience improved intraocular inflammation with methotrexate [14]. Interestingly a more recent report from the United Kingdom suggested that up to 47.9% of children on methotrexate required third line therapy in the form of one or more biologic agent [11]. However, this cohort included children with JIA, which may explain the higher rates of biologic therapy use.
Adalimumab has been shown to improve control of intraocular inflammation and lower the rate of treatment failure in children with active JIA-associated uveitis who were taking a stable dose of methotrexate [15]. In the UK, it is now approved for the use in children with sight threatening uveitis with or without JIA who have an inadequate response to topical steroids eye drops and methotrexate, who would otherwise require prolonged high doses of systemic steroids to control their disease [16]. A large multinational trial in adults with noninfectious uveitis showed that the use of adalimumab was effective in reducing inflammation and risk of uveitic flare ups, even after discontinuation of systemic corticosteroids [17]. Given the growing evidence base supporting the effectiveness of biologic agents in achieving disease control in uveittis, the trend towards using biologic therapy (especially anti-TNF agents) is likely to increase.
This study provides the first UK-wide data on childhood uveitis not associated with JIA. With this prospective ascertainment study, the reported incidence rate is an estimate of the minimum incidence, as there is likely to be underreporting in addition to non-returned questionnaires with the BOSU system. Similar BOSU studies have reported ascertainment levels of 75–100% [18]. Questionnaires are completed by reporting ophthalmologists retrospectively, which increases the risk of bias and missing data. In addition, not all clinicians may have the same criteria for diagnosing a particular associated underlying systemic condition.
There is also the possibility of overestimating the incidence of non-JIA uveitis. Two of the cases were diagnosed with JIA within 12 months of the diagnosis of uveitis, and were therefore excluded from analysis. We were however not able to identify any cases of JIA with a diagnosis interval of more than 12 months from uveitis onset with the study design.
Conclusions
In summary, this study provides the first national population-based data of non-JIA childhood uveitis in the UK. Most children still have uveitis at 1 year and remain on treatment, but median visual acuity usually improves. Following initial systemic corticosteroid administration, children are commenced on steroid-sparing agents such as methotrexate and anti-TNF agents to control ongoing inflammation.
Ultimately the aim is to prevent or minimise ocular inflammation, to reduce the risks of permanent visual impairment. All children with noninfectious uveitis should have a full paediatric rheumatology review to ensure that there is no concurrent joint involvement before making a diagnosis of non-JIA uveitis.
Summary
What was known before
-
Uveitis in children is a rare condition, with a previously reported incidence of 4.85 per 100,000.
-
Approximately half the cases of childhood uveitis occur in the context of juvenile idiopathic arthritis (JIA).
-
Uveitis in children tends to be more severe and aggressive than in adults, with a higher risk of irreversible vision loss.
What this study adds
-
The estimated minimum annual incidence of non-JIA uveitis in children younger than 16 years is 0.66 per 100,000.
-
Most children remain on treatment at 1 year, but visual acuity improves and none were eligible for sight-impairment registration.
References
Edelsten C, Reddy MA, Stanford MR, Graham EM. Visual loss associated with pediatric uveitis in english primary and referral centers. Am J Ophthalmol. 2003;135:676–80.
Heiligenhaus A, Heinz C, Edelsten C, Kotaniemi K, Minden K. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm. 2013;21:180–91.
Moorthy LN, Petersen MG, Hassett AL, Lehman TJA. Burden of childhood-onset arthritis. Pediatr Rheumatol Online J. 2010;8:20.
Heiligenhaus A, Michels C, Schumacher C, Kopp I, Neudorf U, Niehues T, et al. Evidence-based, interdisciplinary guidelines for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatol Int. 2012;32:1121–33.
Minden K, Niewerth M, Listing J, Mobius D, Thon A, Ganser G, et al. The economic burden of juvenile idiopathic arthritis-results from the German paediatric rheumatologic database. Clin Exp Rheumatol. 2009;27:863–9.
Zannin ME, Birolo C, Gerloni VM, Miseocchi E, Pontikaki I, Paroli MP, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian Registry. J Rheumatol. 2013;40:74–9.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005;89:444–8.
Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111:2299–306.
Smith JA, Mackensen F, Sen HN, Leigh JF, Watkins AS, Pyatetsky D, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116:1544–51.
Cann M, Ramanan AV, Crawford A, Dick AD, Clarke SLN, Rashed F, et al. Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol Online J. 2018;16:51.
Gregory AC, Kempen JH, Daniel E, Kacmaz RO, Foster CS, Jabs DA, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology. 2013;120:186–92.
Tappeiner C, Klotsche J, Schenck S, Niewerth M, Minden K, Heiligenhaus AL. Temporal change in prevalence and complications of uveitis associated with juvenile idiopathic arthritis: data from a cross-sectional analysis of a prospective nationwide study. Clin Exp Rheumatol. 2015;33:936–44.
Simonini G, Paudyal P, Jones G, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology. 2013;2013:825–31.
Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, et al. (SYCAMORE Study Group). Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N Engl J Med. 2017;376:1637–46.
Interim Clincical Comissioning Policy: Adalimumab for Children with Severe Refractory Uveitis. D12X02. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/11/d12x02-paediatric-uveitis-anti-tnf.pdf.
Jaffe GJ, Dick AD, Brezin AP, Ngyuen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375:932–43.
Foot B, Stanford M, Rahi J, Thompson J. The British Ophthalmological Surveillance Unit: an evaluation of the first 3 years. Eye. 2002;16:1–7.
Acknowledgements
We would like to thank all reporting ophthalmologists who reported cases and returned questionnaires: J Adepegba, J Ashworth, A Bates, H Bunting, MP Clarke, E Damato, J Escardo-Paon, C Edelsten, J Ferris, C Funnell, A Gaur, V Geh, C Gibbon, C Guly, E Hughes, M Hopes, RC Humphrey, S Jain, NP Jones, A Joseph, C Kanawati, A Kulkarni, U Mahmood, RHC Markham, C Moraitis, CE Morton, A Patwardhan, J Pauw, A Quinn, MR Stanford, J Self, J Thompson, M Tsimpida, J Vodden, S West.
Funding
The British Ophthalmological Surveillance Unit (BOSU) arm of the study was awarded funding by Moorfields Eye Charity (grant ST1407K). The Scottish Ophthalmological Surveillance Unit (SOSU) arm of the study was awarded a grant from the Ross Foundation/Royal College of Ophthalmologists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koay, Sy., Johnson, M., Xing, W. et al. Childhood uveitis not associated with juvenile idiopathic arthritis: a national survey of incidence, management and visual outcomes. Eye 35, 2573–2578 (2021). https://doi.org/10.1038/s41433-020-01267-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01267-2