Abstract
Reproductive genetic carrier screening (RGCS) for hundreds of different genetic conditions is technically available for prospective parents, but these tests have not been integrated in a public health policy except for specific sub-groups. We aimed to provide an overview of the perspectives of multiple professional stakeholder groups in order to enhance a responsible implementation of population-based reproductive genetic carrier screening. We conducted a systematic literature search using eight online databases focussing on studies that were published from January 2009 to January 2021. We selected articles dealing with attitudes and opinions from different professional stakeholders, in particular healthcare professionals and policymakers, on how to implement a policy about carrier screening for a reproductive purpose. We identified 18 studies that met our inclusion criteria. Based on our inductive analysis, we identified ten themes categorized in both clinical and program management challenges: ensuring availability of RGCS to all couples who request the test, embedding RGCS as a test offer before pregnancy, providing clear and reliable information, ensuring voluntary participation, developing genetic counselling pre- and post-testing (after positive or negative result), avoiding psychological harm, ensuring equal access, avoiding social pressure, educating and involving a broad spectrum of non-genetic health care professionals, and promoting an independent non-commercial organisational structure. We highlight one major stumbling block on how to responsibly inform couples about hundreds different genetic conditions within constraints regarding time and ability of non-genetic professionals. We promote further research to tackle the issues brought up by this systematic review through pilot studies. Trial Registration: PROSPERO International Prospective Register of Systematic Reviews PROSPERO 2021 # CRD42021233762; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=233762.
Similar content being viewed by others
Introduction
Through reproductive genetic carrier screening (RGCS), prospective parents can acquire information about whether they have an increased risk of conceiving a child affected with a recessive genetic condition [1]. Due to recessive inheritance patterns, most at-risk couples are unaware of their carrier status as they do not have a known family history of the genetic condition [2]. Therefore, most carrier couples only learn about their reproductive risks following the birth of a child with a specific genetic condition. Carrier screening in the preconception period provides benefits to couples that turn out to be at high risk, by allowing them to make informed reproductive decisions [3]. At-risk couples have the option to undergo prenatal diagnosis, preimplantation genetic testing for monogenic conditions (PGT-M), gamete donation, adoption or refraining from having children altogether. In some cases, RGCS could also contribute to the improvement of the quality of care for affected offspring by allowing early diagnosis and therapeutic procedures.
Several traditional carrier screenings programs have been implemented for a small number of conditions like Thalassemia, Tay-Sachs disease or Cystic Fibrosis, mostly in countries where the consanguinity rate or the heterozygote prevalence for these diseases are very high [4]. Beyond these frequent recessive disorders, most autosomal recessive and X-linked recessive disorders are individually rare. The total number of autosomal recessive genes for recognizable phenotypes may be between 9000 and 10,100, which suggests that the currently known autosomal recessive genes represent only ~20% of the total [2]. Collectively, recessive genetic conditions affect as many as 2% of live births [5], accounting for approximately 20% of infant mortality and 10% of all paediatric hospitalizations [6]. At last, based on 6447 exome sequences of healthy, genetically unrelated Europeans of two distinct ancestries, it has been calculated that every individual is a carrier of at least 2 pathogenic variants in currently known autosomal-recessive (AR) genes and that 0.8%–1% of European couples are at risk of having a child affected with a severe AR genetic disorder [7].
Following the adoption of next-generation sequencing in the mid-2000s, screening for multiple recessive disorders in a single test became feasible, leading to the development of expanded screening test panels. These technological developments have triggered a two-fold transition: it not only allows the simultaneous screening of several hundreds of diseases, but also refers to a screening offer that is universal regardless of ethnicity, geographic origin or family history [8].
Although several professional health societies recommend to offer RGCS, many technical challenges still remain with regard to which disorders should be included, how to interpret variants and how to incorporate newly discovered genetic diseases into existing screening programmes [2]. There are also many issues with regard to the moral acceptability, the economical affordability and policy questions regarding its implementation. RGCS is allowed and offered in many countries [9], sometimes promoted by private companies but not usually funded by states. Within Europe, RGCS has not been integrated as a public health approach or policy and is not reimbursed except for only one or very few conditions as thalassemia in Cyprus or for consanguineous couples/founder populations (The Netherlands) or in case of family history (United Kingdom) [9].
To explain the low implementation rates of such a screening test, various issues could be raised. The complexities of genetic sequencing raise substantial technical challenges, such as the ways DNA variations are interpreted and reported. Some authors have described a number of complex cases of misclassified variants that could have resulted in significant patient harm [10]. Furthermore, following others that developed a framework highlighting the multiple components involved in genetic screening program, it is recognized that numerous stakeholders (including patient group representatives, genetics experts, policymakers etc.), each with their own values, preferences, expectations and concerns, influence various decisions to implement such a policy [11]. Therefore, an important point is to understand what are the professional stakeholders’ attitudes and opinions about RGCS. We hypothesized the discrepancies between them might hinder on how to fairly implement a RGCS program as a public health policy. In order to comprehend where the stumbling blocks remain, from a clinical and management point of view, we conducted a systematic review to collect professional stakeholders’ attitudes and opinions towards RGCS.
In 2021, the American College of Medical Genetics and Genomics (ACMG) recommends that the phrase “expanded carrier screening” be replaced by “carrier screening” [12]. Therefore, we decided not use any longer the adjective “expanded” in this manuscript. However, the search string included this term, as it was regularly used beforehand.
Methods
Design and search strategy
We conducted a systematic search in order to identify empirical studies that focus on perspectives and attitudes of healthcare professionals, policy makers and patients’ advocacy organizations towards reproductive genetic carrier screening. Because pan-ethnic or expanded carrier screening was introduced to the market in 2009, studies published prior to 2009 were not included. We developed a search string for the concept of “carrier screening”, for an example, hereby the terms used in PubMed: (expanded[tiab] OR universal[tiab] OR pan-ethnic[tiab] OR preconception[tiab]) AND ((“Genetic Carrier Screening”[MeSH] OR “Genetic Carrier*“[tiab]) OR ((Carrier[tiab] OR preconception*[tiab] OR “heterozygote detection”[tiab]) AND (screen*[tiab] OR test*[tiab] OR detect*[tiab]))). We did not create search strings for the concepts of “views” or “stakeholders” that encompass a wide range of meanings in different databases, but we included these concepts in the inclusion and exclusion criteria.
We searched for relevant publications in eight online databases (Pubmed, Web of Science core collection, CINAHL (EBSCO), Embase, Cochrane Library (central), Scopus, Proquest Central, international HTA). The publication range was from January 2009 to January 2021. The reference lists of the included publications were hand searched in order to find any additional publications warranting inclusion in the review (i.e. snowballing method). Finally, as a ‘related search’ strategy, we searched the first 200 hits for each of the included articles on Google Scholar to identify potentially relevant studies.
We were guided by the PRISMA guidelines for systematic reviews [13] to set up this review. The study was registered in the international prospective register of systematic reviews (PROSPERO 2021 # CRD42021233762; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=233762).
Inclusion and exclusion criteria
Studies were included in the review if they met all of the following criteria: empirical studies on how to implement a policy about carrier screening; the stakeholders belonging to one of the following population: healthcare professionals (e.g. geneticists, gynaecologists, obstetricians, reproductive / infertility physicians, midwives, general practitioners, psychologists), public health specialists (e.g. legal experts, health economists, sociologists, policymakers) or patient’s advocacy organisations; and studies published between January 2009 and January 2021.
Studies/articles were excluded if they met any of the following criteria: studies assessing the interest of patients or couples in or uptake of genetic tests aimed at obtaining non-reproductive medical information; studies focused on genetic tests targeting dominant genetic disorders; studies assessing the interest in or uptake of a carrier screening test targeting only one gene (e.g. CFTR) or very few genes or within specific communities (e.g. Ashkenazi Jewish Community); publications other than original research articles (e.g. guidelines, commentaries, opinion pieces and studies using secondary data, reviews); publications in a language other than English.
Search outcomes
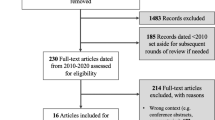
Our initial search identified 9479 articles. After removing duplications using EndNote, 5388 articles remained. First, article titles, then full abstracts were independently screened against the inclusion and exclusion criteria by two researchers (LP, MR). Any disagreement between the reviewers was resolved by discussion until consensus was reached. After excluding articles based on titles and abstracts, 20 articled remained. The full texts articles were read and screened by the same reviewers and 17 articles met the inclusion criteria. Snowball sampling led to one additional relevant article to include. The additional search in Google Scholar did not lead to additional articles. A total of 18 articles were included in this review. Fig. 1 graphically summarizes the literature search process.
Data analysis
Three researchers (LP, LB, PB) independently read and assessed every paper to identify recurrent themes in the data. The researchers defined themes and merged various thematic categories together.
Quality appraisal
Three researchers (LP, MR, MS) performed independently an indicative quality appraisal of each of the included articles using the tool developed by Hawker et al. [14]. By using this system, we were able to evaluate the given methodological rigour of the included study. In case of disagreement, the specific item was discussed until mutual agreement. No articles were excluded from our systematic review based on their methodological quality.
Results
Quality appraisal
The results of the quality appraisal are summarized in Table 1. All studies included in this review had well-structured abstracts except one [15]. In addition, the full-text articles included in this review provided a concise literature review and a clear statement aim. The methodology of the included studies was clearly explained and appropriate to the study aim.
All studies provided a fairly detailed description of the data analysis performed. However, the sample size was not always justified and specific groups were often targeted using convenience sampling. Some articles did not address ethical or potential bias issues. For example in Arjunan et al. (2020), the relationship between researchers and participants had not been adequately considered. Most studies gained ethical approval and only a few studies addressed ethical issues in more detail. The results section of the included articles reported results directly related to the aims and were logical and easy to understand. The transferability and generalizability of some of the reported results are questionable mainly because of different national contexts.
Study characteristics
A detailed overview of the underlying study methods of the empirical studies included in this systematic review is presented in Table 2. Experts in academia, public health systems, and commercial companies and healthcare professionals representing a range of disciplines relevant to RGCS are scientists, molecular geneticists, genetic counselors, clinical geneticists, paediatricians, midwives, bioethicists, legal experts, theologians, political party representatives, general practitioners, obstetricians and gynaecologists.
Among the 18 articles included, seven articles used a quantitative methodology (online surveys) and nine articles used a qualitative approach (eight with semi-structured interviews and one focus-group methodology). Although the results from Molster et al. (2017) were based on a workshop during a geneticists’ conference, we decided to include this article in the systematic review as the framework and methodology are very close to a social science approach (methods, results and discussion). Lastly, one article described an alternative methodology applying a validated severity algorithm to assign objective severity classifications to 176 genetic diseases commonly found on RGCS panels [16].
The Arjunan et al. (2020) article deals with geneticists’ views about the genes to be included in the genes panel. The Lazarin et al. (2014) article asked healthcare professionals to rate the severity of selected inherited diseases. Although the technical issues are indeed one of the stakes to be considered in order to design RGCS, they were scarcely quoted and were not considered as relevant as others categories dealing with the implementation issue. Therefore, the Arjunan et al. (2020) and the Lazarin et al. (2014) articles were removed from the thematic analysis and were not further discussed.
Main findings
The main study findings of this systematic review are summarised in Table 3. After an inductive analysis and ongoing discussion within the team, we classified the different themes into two categories. The theme clinical challenges holds subthemes focusing on the management of genetic tests offered in the clinic. The theme program management challenges holds subthemes focusing on future policy challenges. Some subthemes could have been classified under both main themes.
Clinical challenges
At the clinical level, various challenges were identified concerning the offer of RGCS:
-
(1)
Ensuring availability of RGCS to all couples who request the test
Some clinical geneticists believed that ideally, RGCS should be implemented systematically for all reproductive-aged couples to ensure equal access [17]. While acknowledging that this offer to the general population is premature at present, there was consensus among these participants that RGCS should be made available to couples willing to take the test to enhance their reproductive autonomy. In an American study, more than half of the obstetricians surveyed indicated that they already provided RGCS upon patient request [18]. Clinical geneticists also believed that RGCS could be offered to all people who use assisted reproduction [17].
-
(2)
Embedding RGCS as a test offer before pregnancy
In earlier studies, clinical geneticists [19] as well as obstetricians [18] indicated that the optimal time for RGCS would be prior to conception. This viewpoint was commonly shared by many interviewees with different background in eight studies [16, 18,19,20,21,22,23,24]. One of the most frequently quoted criteria to offer RGCS before pregnancy was to maximise reproductive choices for couples. Then reproductive partners and/or prospective parents can make informed reproductive choices either to avoid or to accept the genetic risk within their lineage.
-
(3)
Providing clear and reliable information
There was a strong agreement among several healthcare professionals that RGCS should be based on a highly individualised decision and that couples should receive information explaining limitations and reduce misperceptions [17,18,19,20,21,22,23, 25,26,27,28]. Various stakeholders have already experienced a dilemma acknowledging that it is essential to communicate the clinical significance of the diseases being tested and that it is impossible to provide detailed information about all of the included conditions [18, 21]. Indeed, some clinical and molecular geneticists voiced concerns that prospective parents may become overwhelmed when facing the amount of information, which may confuse rather than enlighten them to make the best reproductive choices [19, 27].
To address this issue, genetic counsellors focused on simplicity rather than the inclusion of all information. A generic consent model was most commonly proposed by participants to convey pre-test information [26]. In addition, to recognise differences among various disorders, a tiered approach to consent was proposed, where diseases could be grouped into categories based on common characteristics [19]. Healthcare professionals could provide general information during counselling and refer to information leaflets or the internet for detailed information [21]. Pre-test counselling for RGCS can take the form of an informational brochure or audio-visual [26] or a website [21]. According to some clinical geneticists, a public offer may best include educational tools that convey objective information, including the limitations of RGCS [19]. This was recommended to avoid overloading prospective parents with too much information during a consultation and reduce the workload for healthcare providers [19].
Different healthcare professionals also indicated that the general public’s knowledge is currently insufficient [21] and expressed doubt regarding the general public’s ability to comprehend issues surrounding RGCS [17]. A basic understanding of carrier screening is needed to educate patients about their testing options and obtain informed consent [18, 28]. According to various genetic professionals, the genetic literacy required to understand the information provided, including the implications of results and the complex choices the couples may face in a positive test result, should be enhanced, as the stakes could be poorly understood by themselves and/or health professionals [25].
-
(4)
Ensuring voluntary participation
Geneticists in Molster et al. (2017) worried that a routine or state-funded offer of RGCS may create social pressure, coercion or obligation to participate for a given couple. Some healthcare professionals voiced concerns about the notion of a social responsibility to avoid giving birth to a child with a genetic condition [22]. In various studies, due to the sensitive nature of reproductive decisions, clinical and molecular geneticists stressed the fact that RGCS should be a voluntary offer for couples that corresponds with the couple’s personal values and is based on informed consent [19, 25].
However, RGCS was also viewed by healthcare policymakers as an opportunity to make informed reproductive decisions [29]. To maximise voluntary participation, a repeat-visit before the test has been suggested by geneticists and general practitioners in order to ensure that only motivated prospective parents take the test [19, 23]. In another study, one respondent argued that it would be good to have people pay a small amount out-of-pocket to ensure a more considered decision [21].
-
(5)
Developing genetic counselling pre- and post-testing (after positive or negative results)
There was consensus among clinical geneticists that patients should receive genetic counselling before and after RGCS, and that pre and post-test counselling should be provided by a clinician with expertise in communicating genetic information [20]. The time needed for counselling and coordinating follow-up studies as well as comfort with counselling after a positive result lead a majority of obstetricians gynaecologists and other professionals involved in reproductive medicine to state that a post-test consultation with a genetic counsellor would be helpful, if not essential [28]. Clinical geneticists emphasised that face-to-face genetic counselling should be provided for couples where screening identifies that both members carry a pathogenic mutation associated with the same autosomal recessive disorder [19, 25, 26]. Additionally, half of obstetricians and gynaecologists were comfortable discussing negative test results (i.e. no pathogenic variations identified) [30].
Over half of genetic counsellors had concerns about the amount of time spent counselling patients regarding RGCS results and time needed to coordinate follow-up testing [26], although not all healthcare professionals in this study practised reproductive genetic counselling and offered RGCS. Alternatively, general practitioners claimed that the allocated time of 20 min was sufficient for the majority of sessions to efficiently inform the couples in their day-to-day practices [23]. General practitioners expected pre-test counselling sessions to last longer for couples with low educational backgrounds and poor genetic literacy [23]. The appropriate time to get the genetic results of RGCS was only discussed once. Eight-week turnaround time was considered acceptable by the general practitioners for non-pregnant couples [23].
-
(6)
Avoiding psychological harm
Different psychological impacts have been reported or expected for prospective parents when having RGCS, namely: (i) increased anxiety or false expectations that they have been “promised” a healthy baby;[25] (ii) anxiety among parents because they might discover some aspects of themselves they did not know, such as being a carrier of a genetic disorder; [22, 24] (iii) concerns that the residual risk of having an affected child after a negative test result (i.e. no pathogenic variations identified for the couple) would lead to undue anxiety in couples where only one partner is found to carry a disease-associated variant in one gene;[17] (iv) worry, anxiety, guilt and even fright if parents realized their genome was imperfect and might pass on their affected genes to their children [27]. Clinical geneticists agreed that identifying a couple at risk could have negative impact on the emotional well-being of the couple, or create tension in their relationship [31].
Program Management Level
At the organizational level various challenges were also identified with regard to the offer of RGCS:
-
(7)
Ensuring Equal Access
According to most participants in the included interview studies, attention should be given to ensuring equity of access for all prospective parents [17, 24]. According to healthcare professionals, scientists, patient organizations and policymakers in the Netherlands, excessively high costs of screening might exclude people with a lower socio-economic status [21]. In Sweden, some informants were worried that RGCS would precipitate a health equity problem since only those who can afford it will buy preconception RGCS from private companies [22]. Furthermore, it was underlined that follow-up interventions for individuals identified as carriers, such as PGT-M, are provided in the private sector within Australia, meaning there can be significant costs to individuals, thereby worsening equal access [25].
Some healthcare professionals, scientists, patients’ organisations and policymakers expressed concerns about the increasing inequality due to high costs when offered by private companies [21].
-
(8)
Avoiding social pressure
Although most respondents agreed to assign no moral responsibility to parents to undergo screening, some obstetricians or clinical geneticists brought up the notion of moral responsibility [22]. It was mentioned that implementing RGCS could lead to a feeling of responsibility and could encourage prospective parents to accept RGCS because of their perception that the state or the society are entrusting them with such a responsibility [29]. When asking healthcare professionals from reproductive medicine whether “participation in RGCS before pregnancy is socially responsible behaviour”, they mostly agreed, although a significant proportion disagreed or were neutral [28]. Assigning responsibility upon prospective parents to undergo preconception RGCS may be viewed as a form of compulsion [22], in conflict with the principle of voluntary participation.
This higher responsibility has been linked to the potential for pressure to test, either by the society’s expectations or the view that preconception RGCS is a recommendation of healthcare professionals [22, 27]. If an RGCS program is organized and reimbursed by a government, some respondents have argued that this might foster the perception of genetic testing being “routine” and that screening is mandated by the government, and thus not voluntary. People may feel social pressure, coercion, or obligation to participate [25]. Some participants stated that blame could be placed on parents who decide to opt out of RGCS screening, particularly if it results in children with a monogenic condition. This may be coupled with increased stigmatization of those born with disabilities [29]. Therefore, an RGCS program, either direct-to-consumer or publicly funded, should consider this issue and guarantee that prospective parents will not be blamed for whatever choice they make [19].
-
(9)
Educating and involving a broad spectrum of non-genetic health care professionals
According to molecular and clinical geneticists, a successful population-wide carrier screening program would require active involvement of non-genetic health care professionals from the primary care level, such as general practitioners, midwives, obstetricians-gynaecologists to inform and educate prospective parents about RGCS [17]. There is a general agreement by the obstetricians-gynaecologists for further medical education and training programs when RGCS is offered within their clinical practice [30]. Most stakeholders (including healthcare professionals, patients’ organizations or policymakers) agreed that screening should be offered by a medically trained professional to assure sufficient counselling [21].
Based on some surveys including mainly non-geneticist professionals, the need for information to educate healthcare professionals on RGCS was almost a unanimous request [22]. Only a third (33%) of obstetricians and gynaecologists were comfortable with being responsible for counselling patients prior to the test, and even less (24.9%) were comfortable explaining the results of RGCS [18]. According to another survey, continuing education regarding autosomal recessive inheritance and transmission risks was needed, as demonstrated by the lower scores on knowledge questions related to these topics [28]. Geneticists thought a program is not just about the screening test itself but should include education sessions towards healthcare professionals and the general public [25]. The program should encompass the benefits, risks, harms, consequences, uncertainties around genetics and RGCS, and impact on individuals and society. Further, there was a view that program information should also outline the different conditions screened, testing procedure, interpretation of results, and implications. In general, according to different stakeholders more attention should be paid to genetics education, and carrier screening more specifically, by assigning a central role to this topic initially or during continuing medical education [21]. Geneticists or obstetricians emphasized the importance of educated counsellors and stressed that RGCS should not be offered to the general population without taking preparatory measures, not only for healthcare professionals but also for the general public [17, 24].
-
(10)
Promoting an independent non-commercial organisation structure
Several healthcare professionals indicated the need for a rigorous regulatory framework [22]. When discussing the implementation of RGCS in a fair and ethical manner, one could expect the government to step in and take the responsibility to organise the debate about screening and to develop this policy as a public health program. However, placing the responsibility of execution with the government may also be misinterpreted and could be seen as a kind of societal pressure to take the test [22]. Given the European Society of Human Genetics’ recommendation that RGCS programs should not aim for a high uptake, various stakeholders thought that the responsibility could also be assigned to an independent non-commercial organisation instead of the government [21]. It is noteworthy that these two articles were based only on European healthcare professionals and policymakers’ opinions.
Discussion
We conducted a systematic review about policymakers, patient’s advocacy groups and healthcare professionals’ attitudes, all main stakeholders for a potential public healthcare policy, to comprehend where some of the stumbling blocks remain when considering how to fairly offer RGCS in the general population.
Our systematic review identified different principles related to the ethically responsible implementation of RGCS. However, more often than not, principles of voluntary participation and equal access were not discussed in the different articles. Voluntary participation, linked to the autonomy of each human being, was mentioned only in four out of eighteen articles included in this systematic review [19, 22, 25, 27]. Although the fourteen remaining articles did not claim the contrary, we had expected this criterion to be present in most articles. If RGCS would be considered by healthcare professionals as systematically carried out, it may raise ethical concerns as voluntary participation stands at the core of such a new health public policy. Furthermore, the equity of access principle was almost only mentioned by European professionals. These European countries have national health care insurance systems in common based on solidarity. Therefore, to consider a carrier screening program as truly accessible that should allow every couple to access RGCS regardless of one’s income, it would require a public reimbursement approach [25]. As an example, the Swedish healthcare values refer to a non-discriminatory principle where everyone is viewed as equal, included within a collective solidarity and where the most in need have the highest priority for healthcare attention [29]. The lack of focus on these ethical principles could mean that participants took them for granted or they did not view them as critical.
Stipulating RGCS should be offered before pregnancy appears to stand at the core of such an offer. As quoted many times by different professional stakeholders [18,19,20,21,22,23,24, 26], this time slot opens the widest range of reproductive choices and allows the greatest autonomy for couples. Different professional stakeholders also felt that an expertise in communicating genetic information is required and therefore a specific training is needed beforehand [17, 18, 20,21,22,23,24,25,26, 28, 30]. Various primary care providers (general practitioners, gynaeco-obstetricians, midwives) are likely able to administer screening programs without the benefit of detailed genetics knowledge, as long as they have a basic understanding of the concepts related to screening and are provided with appropriate resources, such as expert assistance from laboratories, medical geneticists, and genetic counsellors (Ready, 2012). This criterion of specific training was commonly shared and appears pivotal to the implementation an RGCS program.
In this systematic review, we highlight the issue of the pre-test information. An RGCS program has intrinsically to deal with numerous different diseases. One recent article published by Best et al. in 2021, based upon a systematic review methodology, has gathered health practitioners’ perceptions of the barriers and enablers to the implementation of RGCS [32]. Some of the themes identified in both systematic reviews overlap and confirm professionals’ need for appropriate resources to provide fair information to prospective parents when implementing RGCS. Of note, Best et al. (2021) combined the findings from articles focussing on single gene carrier screening (mainly cystic fibrosis) and only 10 articles out of 26 dealing with RGCS. As our systematic review about RGCS demonstrates, there is a specific issue about the composition of screening test-panels. Indeed, the numerous different conditions already included in the current panels (up to more than one thousand) need to be handled. This broad amount of different genetic diseases has to deal with a wide range in severity, age of onset, organ involvements and potential treatments. Therefore, such a program should not be only the extrapolation of what is already implemented for only several or even dozens of genetic conditions. This complexity in terms of information and comprehension for prospective parents would make it impossible to provide detailed pre-test counselling about each disorder. Furthermore, some professionals predict that there will be additional technical limitations with the inability to fully rule out the possibility of severe recessive diseases due to rare mutations especially as the genetic background could vary between different ethnic origins [20]. Therefore, the numerous numbers of different genetic conditions and the residual risk of a severe genetic disease that may vary according to the ethnic origins trigger different issues on how to provide fair information to prospective couples. An RGCS program requires new ways to fairly inform the future parents before they consent and specific training management for professionals. At last, the large number of different genetic conditions screened would also raise the price of each RGCS test that need to be taken into account, but this issue is beyond the scope of this study.
Therefore, in an attempt to help healthcare professionals to deal with RGCS, guidelines have been endorsed by different professional societies. In United States, the American College of Obstetricians and Gynaecologists (ACOG) considers RGCS to be an acceptable screening strategy and recommended above all that conditions selected for screening panels should meet several of seven criteria for disease inclusion on RGCS panels [33]. Interestingly, the guideline suggests also pre-test education should be detailed to couples. Indeed, the healthcare professionals should focus on the limitations of screening, verbally or by using videos, or online resources especially about the residual risk because not every possible disease-producing mutation or allele would be screened and because de novo mutations may arise. However, this guideline did not mention any details about healthcare professionals’ need to be trained or what an informed consent is. In 2021, the American College of Medical Genetics and Genomics (ACMG) endorsed a statement to help geneticists and other clinicians to provide quality medical services in RGCS [12]. Interestingly, information that should be given to the patient during pre-test and post-test consultations is listed. Although this statement underlined that RGCS can be both time and labour intensive and should be provided by trained healthcare professionals, it is not detailed on how to either educate them appropriately or include this offer in their practices. In Europe, the European Society of Human Genetics also endorsed a guideline on RGCS that deals with a responsible implementation [34]. The word “responsible” refers to how this implementation should enhance the best autonomy, assess the impact on psychological well-being, avoid stigmatisation and discrimination and look for equity and fairness. Acknowledging that informed consent for each genetic condition would remain an ethical challenge, the authors suggested the use of generic consent as a way to safeguard informed decision-making. In this approach, prospective couples would be told that the procedure is carried out to identify individuals/couples at risk to give birth to a child with a severe disability. Some of these diseases, as well as associated clinical symptoms, could be briefly mentioned as examples and illustrated by educational tools. Although this European guideline details many items brought up in this systematic review, it did not mention how to implement these in day-to-day practises. For example, providing adequate counselling is time-consuming and may be challenging for healthcare professionals without a background in genetics. As the clinical geneticists could not provide RGCS to every prospective parent on their own, the kind of non-geneticist healthcare professionals to be involved and how to train them still need to be identified. Due to the risks for false positive and negative results, some authors state that carrier screening should not be held to the same standards as diagnostic testing [10]. Therefore, in order to investigate the items listed throughout this study where the discrepancies remain between the professional stakeholders and the ways and means to responsibly implement this new kind of genetic tests for a whole population, pilot studies at a state level are required.
As far as we know, only one publication described a protocol study investigating limitations in infrastructure and bottlenecks through the day-to-day professional’s practises and couples’ lives in Australia [35]. Given that lack of embedded evaluation plans might still hinder the implementation process, we promote further research and pilot studies to tackle the issues brought up by this systematic review. Along with implementation science that deals within methods translating research findings into practical and useful outcomes in order to launch a new health policy, many challenges remain to be overcome [36]. In particular, a common language, agenda and convergent attitudes should be shared, in an RGCS program, between the healthcare professionals and the policymakers.
Conclusion
We highlight the need for couples’ voluntary participation and fair access to RGCS. Furthermore, we highlight the need for clear and reliable information to timely inform couples, non-genetic professionals and the general public to comprehend the complexities of carrier screening for numerous different genetic conditions. Resource constraints should also be addressed when genetic education programs are implemented. Facing these issues appears to be pivotal before fairly implementing an RGCS program. These research studies should investigate at a state level how to best regulate the implementation of such a health policy, including social and human sciences for legal, health economic, psychological or sociological considerations beforehand.
Limitations
First, due to the scope of this systematic review, some topics were not addressed. Although program costs are salient in a topic dealing with public health policies, economic related issues were not considered. These issues require health economic knowledge that could not be addressed here and depend on healthcare systems specific from each country. Although the authors assume that technical issues (for example the number and the nature of genes to be included in the panels or the variants of unknown significance management) themselves raise ethical questions, we did not include them because these are not directly linked to a fair RGCS implementation.
Furthermore, due to including only publications written in English, some relevant publications about healthcare or policymakers’ attitudes might have been missed. Finally, although primary care physicians are key actors in this genetic offer that should reach out each couple, their opinions are lacking through this systematic review.
References
Dive L, Newson AJ. Ethical issues in reproductive genetic carrier screening. Med J Aust. 2021;214:165–7.
Antonarakis SE. Carrier screening for recessive disorders. Nat Rev Genet. 2019;20:549–61.
Langlois S, Benn P, Wilkins-Haug L. Current controversies in prenatal diagnosis 4: pre-conception expanded carrier screening should replace all current prenatal screening for specific single gene disorders. Prenat Diagn. 2015;35:23–8.
Kraft SA, Duenas D, Wilfond BS, Goddard KAB. The evolving landscape of expanded carrier screening: challenges and opportunities. Genet Med J Am Coll Med Genet. 2019;21:790–7.
Schneider JL, Goddard KAB, Davis J, Wilfond B, Kauffman TL, Reiss JA, et al. Is it worth knowing? focus group participants’ perceived utility of genomic preconception carrier screening. J Genet Couns. 2016;25:135–45.
Kumar P, Radhakrishnan J, Chowdhary MA, Giampietro PF. Prevalence and patterns of presentation of genetic disorders in a pediatric emergency department. Mayo Clin Proc. 2001;76:777–83.
Fridman H, Yntema HG, Mägi R, Andreson R, Metspalu A, Mezzavila M, et al. The landscape of autosomal-recessive pathogenic variants in European populations reveals phenotype-specific effects. Am J Hum Genet. 2021;108:608–19.
van der Hout S, Dondorp W, de Wert G. The aims of expanded universal carrier screening: autonomy, prevention, and responsible parenthood. Bioethics 2019;33:568–76.
Delatycki MB, Alkuraya F, Archibald A, Castellani C, Cornel M, Grody WW, et al. International perspectives on the implementation of reproductive carrier screening. Prenat Diagn. 2020;40:301–10.
Silver J, Norton ME. Expanded carrier screening and the complexity of implementation. Obstet Gynecol. 2021;137:345–50.
Andermann A, Blancquaert I, Déry V. Genetic screening: a conceptual framework for programmes and policy-making. J Health Serv Res Policy. 2010;15:90–7.
Gregg AR, Aarabi M, Klugman S, Leach NT, Bashford MT, Goldwaser T, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med J Am Coll Med Genet 2021;23:1793–806.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700.
Hawker S, Payne S, Kerr C, Hardey M, Powell J. Appraising the evidence: reviewing disparate data systematically. Qual Health Res. 2002;12:1284–99.
Thompson J, Vogel Postula K, Wong K, Spencer S. Prenatal genetic counselors’ practices and confidence level when counseling on cancer risk identified on expanded carrier screening. J Genet Couns. 2019;28:908–14.
Arjunan A, Bellerose H, Torres R, Ben-Shachar R, Hoffman JD, Angle B, et al. Evaluation and classification of severity for 176 genes on an expanded carrier screening panel. Prenat Diagn. 2020;40:1246–57.
Janssens S, Chokoshvili D, Vears D, De Paepe A, Borry P. Attitudes of European geneticists regarding expanded carrier screening. J Obstet Gynecol Neonatal Nurs Jognn. 2017;46:63–71.
Benn P, Chapman AR, Erickson K, Defrancesco MS, Wilkins-Haug L, Egan JFX, et al. Obstetricians and gynecologists’ practice and opinions of expanded carrier testing and noninvasive prenatal testing. Prenat Diagn. 2014;34:145–52.
Janssens S, Chokoshvili D, Vears DF, De Paepe A, Borry P. Pre- and post-testing counseling considerations for the provision of expanded carrier screening: exploration of European geneticists’ views. BMC Med Ethics. 2017;18:46.
Cho D, McGowan ML, Metcalfe J, Sharp RR. Expanded carrier screening in reproductive healthcare: perspectives from genetics professionals. Hum Reprod Oxf Engl 2013;28:1725–30.
Holtkamp KCA, Vos EM, Rigter T, Lakeman P, Henneman L, Cornel MC. Stakeholder perspectives on the implementation of genetic carrier screening in a changing landscape. BMC Health Serv Res. 2017;17:146. 16.
Matar A, Kihlbom U, Höglund AT. Swedish healthcare providers’ perceptions of preconception expanded carrier screening (ECS)-a qualitative study. J Community Genet. 2016;7:203–14.
Schuurmans J, Birnie E, van den Heuvel LM, Plantinga M, Lucassen A, van der Kolk DM, et al. Feasibility of couple-based expanded carrier screening offered by general practitioners. Eur J Hum Genet Ejhg. 2019;27:691–700.
Stark Z, Massie J, McClaren B, Ioannou L, Cousens N, Lewis S, et al. Current practice and attitudes of Australian obstetricians toward population-based carrier screening for inherited conditions. Twin Res Hum Genet J Int Soc Twin Stud. 2013;16:601–7.
Molster CM, Lister K, Metternick-Jones S, Baynam G, Clarke AJ, Straub V, et al. Outcomes of an international workshop on preconception expanded carrier screening: some considerations for governments. Front Public Health. 2017;5:25.
Lazarin GA, Detweiler S, Nazareth SB, Ashkinadze E. Genetic counselors’ perspectives and practices regarding expanded carrier screening after initial clinical availability. J Genet Couns. 2016;25:395–404.
Matar A, Hansson MG, Höglund AT. Values and value conflicts in implementation and use of preconception expanded carrier screening - an expert interview study. BMC Med Ethics. 2019;20:25.
Ready K, Haque IS, Srinivasan BS, Marshall JR. Knowledge and attitudes regarding expanded genetic carrier screening among women’s healthcare providers. Fertil Steril. 2012;97:407–13.
Matar A, Hansson MG, Höglund AT. « A perfect society »- Swedish policymakers’ ethical and social views on preconception expanded carrier screening. J Community Genet. 2019;10:267–80.
Briggs A, Nouri PK, Galloway M, O’Leary K, Pereira N, Lindheim SR. Expanded carrier screening: a current survey of physician utilization and attitudes. J Assist Reprod Genet. 2018;35:1631–40.
Chokoshvili D, Janssens S, Vears D, Borry P. Designing expanded carrier screening panels: results of a qualitative study with European geneticists. Pers Med. 2016;13:553–62.
Best S, Long J, Theodorou T, Hatem S, Lake R, Archibald A, et al. Health practitioners’ perceptions of the barriers and enablers to the implementation of reproductive genetic carrier screening: A systematic review. Prenat Diagn. 2021;41:708–19.
Committee Opinion No. 690. Carrier Screening in the Age of Genomic Medicine. Obstet Gynecol. 2017;129:e35–40.
Henneman L, Borry P, Chokoshvili D, Cornel MC, van El CG, Forzano F, et al. Responsible implementation of expanded carrier screening. Eur J Hum Genet EJHG. 2016;24:e1–12.
Ong R, Edwards S, Howting D, Kamien B, Harrop K, Ravenscroft G, et al. Study protocol of a multicentre cohort pilot study implementing an expanded preconception carrier-screening programme in metropolitan and regional Western Australia. BMJ Open. 2019;9:e028209.
Rapport F, Clay-Williams R, Churruca K, Shih P, Hogden A, Braithwaite J. The struggle of translating science into action: Foundational concepts of implementation science. J Eval Clin Pr. 2018;24:117–26.
Acknowledgements
This work has been carried out within the framework of the FHU GenOMedS thanks to the support of the Health cooperation group of University Hospitals of the Great West (GCS HUGO) and the National Alliance for Life Sciences and Health (Aviesan). The authors wish to thank Thomas Vandendriessche, Kristel Paque and Krizia Tuand, the biomedical reference librarians of the KU Leuven Libraries – 2Bergen – learning Centre Désiré Collen (Leuven, Belgium), for their help in conducting the systematic literature search. We also thank Zoë Claesen who participated in the study review process.
Funding
Rennes University Hospital (France).
Author information
Authors and Affiliations
Contributions
LP, EVS and PB designed the study. The comprehensive search approach, selection and screening of articles were carried out by LP and MR. The quality appraisal was performed by LP, LB, MR and MS. A first draft of the article was written by LP and critically discussed and revised by EVS, MS, MR and PB. PB coordinated the study. All the authors have approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pasquier, L., Reyneke, M., Beeckman, L. et al. Attitudes of professional stakeholders towards implementation of reproductive genetic carrier screening: a systematic review. Eur J Hum Genet 31, 395–408 (2023). https://doi.org/10.1038/s41431-022-01274-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01274-9
This article is cited by
-
April, again
European Journal of Human Genetics (2023)