Abstract
UDP-3-O-acyl-N-acetylglucosamine deacetylase (LpxC) is an essential enzyme in the biosynthesis of Lipid A, an active component of lipopolysaccharide (LPS), from UDP-3-O-acyl-N-acetylglicosamine. LPS is a major component of the cell surface of Gram-negative bacteria. LPS is known to be one of causative factors of sepsis and has been associated with high mortality in septic shock. TP0586532 is a novel non-hydroxamate LpxC enzyme inhibitor. In this study, we examined the inhibitory effect of TP0586532 on the LPS release from Klebsiella pneumoniae both in vitro and in vivo. Our results confirmed the inhibitory effect of TP0586532 on LPS release from the pathogenic bacterial species. On the other hand, meropenem and ciprofloxacin increase the level of LPS release. Furthermore, the effects of TP0586532 on LPS release and interleukin (IL)-6 production in the lung were determined using a murine model of pneumonia caused by K. pneumoniae. As observed in the in vitro study, TP0586532 showed the marked inhibitory effect on LPS release in the lungs, whereas meropenem- and ciprofloxacin-treated mice showed higher levels of LPS release and IL-6 production in the lungs as compared to those in the lungs of vehicle-treated mice. Moreover, TP0586532 used in combination with meropenem and ciprofloxacin attenuated the LPS release and IL-6 production induced by meropenem and ciprofloxacin in the lung. These results indicate that the inhibitory effect of TP0586532 on LPS release from pathogenic bacteria might be of benefit in patients with sepsis.
Similar content being viewed by others
Introduction
Lipopolysaccharide (LPS) is considered as one of the major factors involved in the pathophysiology of sepsis. Septic shock caused by LPS is known to be associated with high mortality rates [1]. LPS induces pro-inflammatory cytokine responses, including of interleukin (IL)-6. IL-6 is one of the most well-recognized causes of the excessive inflammatory response in cases of sepsis [2]. High levels of IL-6 production are known to be associated with a high risk of death [3]. In addition, the serum levels of IL-6 were higher in patients with septic shock than in those with sepsis [4]. Some antibiotics have been reported to induce an increase of LPS release [5, 6], which might induce further IL-6 production and cause clinical deterioration in patients with sepsis. In fact, some previous study referred the association between administration of antibiotics and an increase in the risk of mortality in patients with septic shock [7,8,9]. However, there are inadequate data to reach any definitive conclusion concerning this matter.
Some drugs of the class of β-lactam antibiotics which interfere with cell wall synthesis have been shown to induce LPS release both in vitro and in vivo [5, 6, 10]. These antibiotics have been shown to induce morphological changes in pathogens [11]. Antibiotic-induced filamentation of bacterial cells is known to be associated with higher levels of LPS release [12]. These β-lactam antibiotics, which are first-line therapeutic agents for severe infections, including sepsis, are known to induce antibiotics-induced LPS release and might potentially have adverse effects in patients with sepsis [13].
UDP-3-O-acyl-N-acetylglucosamine deacetylase (LpxC) is a rate-limiting step in the biosynthetic pathway of lipid A, a component of LPS. LpxC inhibitors have been shown to reduce the levels of LPS in the outer membranes of Gram-negative bacteria. According to Fujiwara’s study, RC-01, which is a hydroxamate LpxC inhibitor, has been shown to reduce the LPS release and to attenuate antibiotic-induced LPS release from Pseudomonas aeruginosa in vitro [14]. Furthermore, Lin et al. showed in their in vivo study, that an LpxC inhibitor, which did not have the bacterial killing effect against Acinetobacter baumannii in vitro, reduced the LPS release and LPS-induced immune responses in the serum in a murine model of infection caused by A. baumannii [15]. They showed in their study that A. baumannii exposed to LpxC inhibitors increase their susceptibility to phagocytosis by macrophages. Bacteria are digested by lysosomal enzymes during the process of phagocytosis [16]. Therefore, the levels of LPS released might be lower during bacterial killing by phagocytosis than during bacterial killing by antibacterial drugs. Klebsiella pneumoniae is well known as a causative pathogen of pneumonia and urinary tract infections. K. pneumoniae has been reported to be the third most common causative microorganism of sepsis in patients admitted to intensive care units in Japan, following methicillin-resistant Staphylococcus aureus and Escherichia coli [17]. In addition, K. pneumoniae is the most common carbapenem-resistant Enterobacteriaceae isolated in the United States, accounting for half of the isolates, and is a world threat [18]. TP0586532 (Fig. 1) is a novel non-hydroxamate LpxC inhibitor which has been shown to have a broad spectrum of antibacterial activity against carbapenem-resistant Enterobacteriaceae and exert potent efficacy in murine models of systemic infection, and pneumonia caused by Gram-negative pathogens [19, 20]. To investigate the LPS release associated with the bacterial killing activity of antimicrobial agents, the inhibitory effects of TP0586532 on LPS release from K. pneumoniae, which is killed by TP0586532, were examined in vitro and in vivo. Furthermore, the degree of reduction of antibiotic-induced LPS release and IL-6 production in the lungs treated with TP0586532 in combination with antibiotics were determined using a murine model of pneumonia caused by K. pneumoniae.
Materials and methods
Bacterial strains
K. pneumoniae 4124, a clinically isolated strain from sputum in a Japanese hospital, was used in both the in vitro and in vivo studies. Heart Infusion Agar (Nippon Becton Dickinson Co., Ltd., Tokyo, Japan) and Mueller Hinton II Broth (Nippon Becton Dickinson Co., Ltd.) were used for the bacterial cultures.
Compounds
TP0586532 was synthesized by Taisho Pharmaceutical Co, Ltd., (Saitama, Japan). The drug was dissolved in dimethyl sulfoxide (for the in vitro studies) or 11 w/v% sulfobutylether-β-cyclodextrin sodium salt (11 v/w% SBE-β-CD, for the in vivo studies). Ceftazidime was purchased from Sigma-Aldrich (St. Louis, MO). Ciprofloxacin was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Meropenem was purchased from Sumitomo Dainippon Pharma Co., Ltd. (Osaka, Japan) and U.S. Pharmacopeial Convention (Rockville, MD). Cilastatin was purchased from Sigma-Aldrich and AvaChem Scientific (San Antonio, TX). Meropenem was combined with cilastatin at ratio of 1:1 for the in vivo study. Ceftazidime was dissolved in distilled water. Ciprofloxacin and meropenem were dissolved in distilled water (for in vitro studies) or saline (for the in vivo studies). Cilastatin was dissolved in saline.
Determination of the minimum inhibitory concentrations
The MICs were determined by two-fold microdilution in broth according to the Clinical & Laboratory Standards Institute (CLSI) standard [21].
In vitro LPS release study
K. pneumoniae 4124 was cultured to log phase in MHIIB and suspended in MHIIB, so that the suspension contained approximately 106 CFU ml−1. The cultures were exposed to 0.5-, 1-, and 2-fold of the MICs (0.5 MIC, 1 MIC, and 2 MIC) of the antibacterial agents. For the combined antibiotic testing, 1 MIC of meropenem, ciprofloxacin or ceftazidime was combined with 0.25 MIC, 0.5 MIC, and 1 MIC of TP0586532 or ciprofloxacin. The cultures were incubated at 35 °C for 4 h. At 1, 2, and 4 h (for the single antibiotic testing) or at 2 and 4 h (for the combined antibiotic testing) after the addition of antibacterial agents, aliquots of cultures were inoculated on HIA for colony counting. The amounts of LPS released from the bacteria were measured using a LPS-specific Limulus amebocyte lysate chromogenic endpoint assay (Endospecy, Seikagaku Co., Tokyo, Japan). In order to remove the LPS associated with the bacteria from the cultures, the cultures were filtered through a 0.45-μm filter, and the supernatants were used for the assay. The amounts of LPS were determined by comparison with a standard curve run in the same experiment. All experiments were performed in duplicate or more.
Animals
All experimental procedures, including the animal handling, were conducted with the approval of the Institutional Animal Care and Use Committee of Taisho Pharmaceutical Co., Ltd., and were in accordance with the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, 2006). Four-week-old female ICR mice (SLC Japan, Shizuoka, Japan) were used for all the in vivo experiments. The mice were maintained in controlled temperature (23 °C ± 3 °C) and humidity (50% ± 20%) conditions under a 12-h light/dark cycle (lights on at 07:00 h). Food and water were provided ad libitum to the animals.
Murine model of pneumonia
Mice anesthetized with ketamine-xylazine were injected intranasally with 0.05 ml of the K. pneumoniae 4124 suspension (approximately 7 × 107 CFU/mouse). At 1.5, 3, and 6 h after the bacterial inoculation, the mice (n = 6 in each treatment group) were given a subcutaneous injection of a single dose of TP0586532, meropenem/cilastatin, or ciprofloxacin at 100, 25, or 3 mg kg−1, respectively. These doses were designed to show a similar bacterial killing capacity in the lung after treatment. Meropenem/cilastatin and ciprofloxacin were combined with 1, 10, or 100 mg kg−1 dose−1 of TP0586532. Control mice were treated with placebo (11 v/w% SBE-β-CD) alone. At 3, 6, and 9 h after inoculation, lung samples were removed and homogenized with 1 ml of saline using a Multi-beads Shocker (Yasui Kikai, Osaka, Japan). For determination of the bacterial concentrations, the homogenates were diluted with saline and plated on HIA. Viable cell counts of K. pneumoniae in the lung were estimated by counting the bacterial colonies on the agar medium. For the analyses of LPS and IL-6, the lung homogenates were centrifuged for 10 min at 10,000 × g and filtered through a 0.45-μm filter. The supernatants were frozen until the analyses. The amounts of LPS in the lung supernatant samples were measured as described above [15, 22]. The IL-6 levels in the lung supernatant samples were measured by IL-6 mouse Quantikine ELISA (R&D systems. Inc., Minneapolis, MN). All sample concentrations were determined by comparison with a standard curve run in the same experiment.
Statistical analysis
In vivo LPS and IL-6 levels were compared using Wilcoxon test or Steel’s non-parametric multiple comparison test. *: p < 0.05 and **: p < 0.01 values were considered as denoting significance.
Results
TP0586532 reduced LPS release from K. pneumoniae in vitro
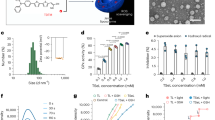
The MICs of TP0586532, meropenem, ciprofloxacin, and ceftazidime against K. pneumoniae 4124 used in this study were 2, 0.03, 0.03, and 0.25 µg ml−1, respectively. The efficacy of bacterial killing and inhibitory effect on LPS release by TP0586532 for K. pneumoniae 4124 were examined. The viable cell counts in the cultures and the levels of LPS released into the culture supernatants at 1, 2, and 4 h post-treatment are shown in Fig. 2. The viable cell counts of K. pneumoniae 4124 increased logarithmically until 4 h in the no agent group, while the counts in the TP0586532-, meropenem-, and ciprofloxacin-treated groups decreased in a concentration-dependent manner (Fig. 2a–c). The level of LPS released into the culture supernatants in the no agent group at 4 h was 4811 EU ml−1. On the other hand, the levels of LPS released into the culture supernatants in the TP0586532-, meropenem-, ciprofloxacin-, and ceftazidime-treated groups at 4 h were 58–225, 2837–10937, 1770–13106, and 4809–5321 EU ml−1, respectively (Fig. 2e–h). Thus, the level of LPS released into the culture supernatant in the TP0586532-treated group was lower than that in the no agent group (Fig. 2e), whereas the levels of LPS released into the culture supernatants in the meropenem-, ciprofloxacin-, and ceftazidime-treated groups were comparable or higher than those in the no agent group (Fig. 2f–h).
Efficacy of bacterial killing and inhibitory effects on LPS release in the cultures of K. pneumoniae 4124. Viable cell counts in the cultures treated with TP0586532 (a), meropenem (b), ciprofloxacin (c), and ceftazidime (d). LPS levels in the cultures treated with TP0586532 (e), meropenem (f), ciprofloxacin (g), and ceftazidime (h). Each symbol represents the mean and SEM (n = 3–4). 0.5 MIC (□), 1 MIC (♦), 2 MIC (○), and no agent (–)
TP0586532 reduced LPS release in the lungs of a murine model of pneumonia caused by K. pneumoniae
Viable cell counts and the levels of LPS and IL-6 production in the lungs of mice treated with antibacterial agents were measured. The changes in the viable cell counts in the lungs are shown in Fig. 3a. The viable cell counts in the lungs of mice treated with TP0586532, meropenem/cilastatin, or ciprofloxacin decreased by approximately 2 log in comparison with those in the lungs of the vehicle-treated mice at 6 and 9 h after the inoculation. Therefore, TP0586532, meropenem/cilastatin and ciprofloxacin showed equivalent efficacy of bacterial killing at each of the tested doses in the murine model of pneumonia. The levels of LPS released into the lungs are shown in Fig. 3b. The levels of LPS released into the lungs of the vehicle-treated mice increased over time until 9 h after the inoculation. On the other hand, the levels of LPS released into the lungs of the mice treated with TP0586532 were significantly reduced in comparison with those of the vehicle-treated mice at 6 and 9 h after the inoculation. Notably, the levels of LPS released into the lungs of the mice treated with meropenem/cilastatin were significantly higher than those in the lungs of the vehicle-treated mice at 3, 6, and 9 h after the inoculation. Similarly, the levels of LPS released into the lungs of the mice treated with ciprofloxacin were also significantly higher than those of the vehicle-treated mice at 3 and 9 h after the inoculation. In addition, the levels of IL-6 in the lungs of the mice treated with meropenem/cilastatin or ciprofloxacin were increased as compared to those in the lungs of the vehicle-treated mice at 6 h after the inoculation (Fig. 3c), while there was no difference in the IL-6 levels in the lungs between the TP0586532-treated and vehicle-treated mice.
Efficacy of bacterial killing and inhibitory effects on LPS release and IL-6 production in the lungs in a murine model of pneumonia caused by K. pneumoniae 4124. Viable cell counts (a), LPS levels (b), and IL-6 levels (c) are shown. Each symbol represents the mean and SEM (n = 6). TP0586532 (100 mg kg−1 dose−1); MEM, meropenem/cilastatin (25 mg kg−1 dose−1); CIP, ciprofloxacin (3 mg kg−1 dose−1). Statistical comparisons of LPS release and IL-6 production were performed by the Wilcoxon test (vs. vehicle in the same time point, *: p < 0.05, **: p < 0.01)
TP0586532 attenuated antibiotic-induced LPS release from K. pneumoniae in vitro
Our study showed that monotherapy of meropenem or ciprofloxacin increased the levels of LPS released into the lungs of the treated mice, whereas monotherapy of TP0586532 reduced the levels of LPS in the lungs of the treated mice. Therefore, we evaluated whether combined use of TP0586532 could reduce the induction of LPS release by other antibiotics such as meropenem and ciprofloxacin. First, the in vitro inhibitory effect of TP0586532 on antibiotic-induced LPS release was examined (Fig. 4). Addition of TP0586532 along with meropenem, ciprofloxacin or ceftazidime reduced the viable cell counts of K. pneumoniae 4124 to the same or greater degree as compared to that observed with the addition of meropenem, ciprofloxacin, or ceftazidime alone (Fig. 4a–c). The antibiotic-induced LPS release into the culture supernatants was attenuated by combined treatment with TP0586532 (Fig. 4e–g). The efficacy of addition of TP0586532 was observed even at 0.25 MIC. On the other hand, the amount of LPS released into the culture supernatant following treatment with the combination of meropenem and ciprofloxacin was comparable to that following treatment with meropenem alone (Fig. 4h).
Efficacy of bacterial killing and inhibitory effects of TP0586532 in combination on LPS release induced by other antibiotics in the cultures of K. pneumoniae 4124. Viable cell counts in the cultures treated with meropenem plus TP0586532 (a), ciprofloxacin plus TP0586532 (b), ceftazidime plus TP0586532 (c), and meropenem plus ciprofloxacin (d). LPS levels in the cultures treated with meropenem plus TP0586532 (e), ciprofloxacin plus TP0586532 (f), ceftazidime plus TP0586532 (g), and meropenem plus ciprofloxacin (h). Each symbol represents the mean and SEM (n = 3). Meropenem, ciprofloxacin, or ceftazidime alone (□), combined with 0.25 MIC TP0586532 or ciprofloxacin (♦), combined with 0.5 MIC TP0586532 or ciprofloxacin (○), combined with 1 MIC TP0586532 or ciprofloxacin (▲), and no agent (–)
TP0586532 attenuated antibiotic-induced LPS release and IL-6 production in the lungs in a murine model of pneumonia caused by K. pneumoniae
Viable cell counts and levels of LPS release and IL-6 production in the lungs of mice treated with TP0586532 in combination with meropenem/cilastatin or ciprofloxacin were determined. The changes in the viable cell counts in the lungs are shown in Fig. 5a. The viable cell counts in the lungs of mice treated with meropenem/cilastatin or ciprofloxacin alone or meropenem/cilastatin or ciprofloxacin in combination with TP0586532 decreased by approximately 2 log in comparison with those in the vehicle-treated mice. Therefore, the bacterial killing activities of all the treatments were equivalent in the murine model of pneumonia. The levels of LPS released into the lungs of the antibiotic-treated mice are shown in Fig. 5b. Combined administration of TP0586532 with meropenem/cilastatin or ciprofloxacin markedly attenuated the LPS release induced by meropenem/cilastatin or ciprofloxacin alone. The attenuated effect was observed at the doses of 10 and 100 mg kg−1dose−1 of TP0586532 administered in combination with meropenem/cilastatin or ciprofloxacin. Similar to the effect on the antibiotic-induced LPS release, combined administration of TP0586532 with meropenem/cilastatin or ciprofloxacin also attenuated the amount of IL-6 produced into the lungs (Fig. 5c). The effect on IL-6 production of combined administration of TP0586532 with meropenem/cilastatin was observed even at the dose of 1 mg kg−1 dose−1 of TP0586532. These effects in the ciprofloxacin-treated mice were observed in a dose-dependent manner from 1 mg kg−1 dose−1 of TP0586532.
Efficacy of bacterial killing and inhibitory effects of TP0586532 in combination on LPS release and IL-6 production induced by other antibiotics in the lungs of a murine model of pneumonia caused by K. pneumoniae 4124 at 6 h after inoculation. Viable cell counts (a), LPS levels (b), and IL-6 levels (c) are shown. Each symbol represents the mean and SEM (n = 6). MEM, meropenem/cilastatin; CIP, ciprofloxacin. Statistical comparisons of LPS release and IL-6 production were performed by the Steel test (*: p < 0.05, TP0586532 vs. vehicle, meropenem/cilastatin plus TP0586532 vs. meropenem/cilastatin alone, ciprofloxacin plus TP0586532 vs. ciprofloxacin alone)
Discussion
According to the WHO 2020 report [23], there were estimated to be 49 million cases of sepsis in 2017, leading to the sepsis-related death of 11 million people worldwide. This was estimated approximately 20% of the world’s annual mortality rate. The mortality rate for sepsis is very high, with an estimated mortality rate of 26.7% for hospitalized patients treated for sepsis and 42.6% for patients in intensive care units treated for sepsis [24]. In addition, the WHO estimated that one in four sepsis cases in 2017 occurred in a hospital. In order to properly treat sepsis, it is important to identify symptoms early and treat the underlying infection with empiric antibiotic therapy [25]. However, some antimicrobial agents have been reported to increase the LPS release during bacterial killing activity [8]. In addition, it has also been reported that excessive immune response caused of IL-6 induced by LPS might deteriorate the pathogenesis of sepsis [9]. TP0586532, a non-hydroxamate LpxC inhibitor, reduced the total LPS level of E. coli in a concentration-dependent manner [20] and thus are expected to inhibit LPS release during bacterial killing activity. Therefore, we investigated the effects of TP0586532 on LPS release during bacterial killing activity and on IL-6 production in a murine model of pneumonia caused by K. pneumoniae. Furthermore, we investigated the effects of TP0586532 in combination with meropenem or ciprofloxacin on LPS release and IL-6 production induced by these antibiotics.
This study revealed that TP0586532 reduced the levels of LPS released from K. pneumoniae both in vitro (Fig. 2) and in vivo (Fig. 3). Furthermore, use of TP0586532 in combination with meropenem or ciprofloxacin also attenuated the antibiotic-induced LPS release both in vitro (Fig. 4) and in vivo (Fig. 5). These effects were observed even at low doses of TP0586532 where TP0586532 exhibited no antimicrobial activity. Meropenem and ceftazidime induced high levels of LPS release both in vitro and in vivo, consistent with previous reports [6, 11]. In addition, the present study also revealed that administration of ciprofloxacin increased the levels of LPS released into the lungs in the murine model of pneumonia. Ciprofloxacin has been reported to induce bacterial filamentation [26] and LPS release in vitro [27]. However, Kawai et al. reported that ciprofloxacin reduced the levels of LPS and cytokines released by the bacteria in a murine model of pneumonia caused by K. pneumoniae [28]. The discrepant results between their study and our study could due to differences in the bacterial burden and/or timing of samplings. In the study by Kawai et al., the lung levels of LPS in the ciprofloxacin-treated mice were measured at 24 to 96 h after the inoculation. At these time points, the viable cell counts in the bronchoalveolar lavage fluid were 1 to 3 log10 CFU ml−1. On the other hand, in our study, the measurements were performed at 3, 6, and 9 h after the inoculation, and the viable cell counts in the lungs were over almost 6 log10 CFU ml−1. LPS release might be induced by ciprofloxacin when the bacterial burden at the infected site is high. Therefore, our model might represent a more severe model of the acute phase with a high bacterial burden. There is the report that LPS-hypersensitive C3H/HeN mice treated with ceftazidime showed higher lethality than untreated mice [5]. In addition, in a mouse model of septic shock, LPS binding and neutralizing peptide in combination with ceftazidime improved the survival rate [29]. These reports suggest that antibiotic-induced LPS release exerts an influence on the therapeutic effect of the antibiotic. LpxC inhibitors might prevent the reduction in the therapeutic effect of antibiotics associated with antibiotic-induced LPS release. Further investigation is needed to the relationship of the therapeutic effect and the antibiotic-induced LPS release.
IL-6 is one of the important cytokines with both pro- and anti-inflammatory effects, and acts as a major regulator of host defenses against bacteria [30]. However, in sepsis, IL-6 is known to induce excessive inflammatory responses and cytokine storm. Some studies were reported that mice or rats treated with anti-IL-6 antibody showed prolonged survival in the cecum ligation and puncture-induced sepsis model [31, 32]. These reports suggest that overexpression of IL-6 can induce aggravation of symptoms in sepsis. The FDA has approved the emergency use of tocilizumab, an IL-6 receptor antibody, for the treatment of COVID-19 infection, which is currently raging worldwide. Although there is not enough evidence to support the use of tocilizumab for sepsis, it is clear that IL-6 plays an important role in cytokine syndromes [33], and further research for sepsis is expected. Our results showed that the LpxC inhibitor TP0586532 might suppress excessive inflammatory responses by inhibiting LPS release and IL-6 production. However, several cytokines other than IL-6 are known to be related to the excessive inflammatory responses in sepsis [34]. The limitations of our study are that no cytokines other than IL-6 were evaluated. Further studies are need on the inhibition of LPS release and the excessive inflammatory responses.
It has been reported that excessive inflammation and immunosuppression occur simultaneously in the early phase of sepsis [35]. Production of inflammatory cytokines in excess both activated regulatory T cells and induced anti-inflammatory cytokines, including IL-10 [36]. This status is called compensatory anti-inflammatory response syndrome (CARS). Both systemic inflammatory response syndrome (SIRS) and CARS are induced more strongly in more critically ill patients [37]. Exhaustion of T cells as a result of prolonged inflammatory events and anti-inflammatory responses are known to be associated with worsening of the disease state and a poor prognosis in patients with sepsis [38]. Expressions of programmed death (PD)-1 and cytotoxic T lymphocyte antigen (CTLA)-4 on T cells have been reported to be upregulated in patients with sepsis [39, 40]. Therefore, these molecules are expected as promising therapeutic targets [41]. In a murine model of sepsis, while anti-PD-1 and anti-CTLA-4 antibody improved the survival rates, high doses of anti-CTLA-4 worsened the survival rates [42, 43]. Thus, a proper balance between inflammatory events and anti-inflammatory responses may be important for the success of therapy in cases of sepsis. In addition, in the cecum ligation and puncture rat model, it was reported that the survival rate using low dose tocilizumab was higher than that using high dose [32]. It has been suggested that minimal levels of IL-6 signaling are required to maintain immune system balance, especially in the acute phase of early sepsis. Combined use of TP0586532 with meropenem or ciprofloxacin attenuated the release of LPS and IL-6 production induced by meropenem and ciprofloxacin. Furthermore, it is important to note that TP0586532 did not completely suppress IL-6 production. These results indicate the possibility that TP0586532 attenuates the IL-6 which are excessively produced. Therefore, LpxC inhibitors might be expected to reduce the adverse effects caused by overproduction of IL-6. In the present study, we conducted no histopathological analysis and clinical conditions analysis such as survival rate and body weight. These are limitations of this study and further studies are needed.
For the management of sepsis, identification of the source of infection and prompt administration of antibiotics are still essential. Delays in the administration of antibiotics have been reported to be associated with increased odds of hospital mortality in cases of septic shock [44]. Selection of an antibiotic(s) that does not induce excessive inflammatory responses might be important for mitigating septic shock.
In conclusion, the inhibitory effect exerted by TP0586532 on LPS release by other antibacterial drugs may be of benefit in patients with sepsis. Future studies are needed for a clearer elucidation of the effect of LpxC inhibitors on disordered immune and excessive inflammatory responses.
References
Morrison DC, Ryan JL. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–32.
Bosmann M, Ward PA. The inflammatory response in sepsis. Trends Immunol. 2013;34:129–36.
Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–63.
Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine 2005;29:169–75.
Kirikae T, Kirikae F, Saito S, Tominaga K, Tamura H, Uemura Y, et al. Biological characterization of endotoxins released from antibiotic-treated Pseudomonas aeruginosa and Escherichia coli. Antimicrob Agents Chemother. 1998;42:1015–21.
Hilliard JJ, Melton JL, Hall L, Abbanat D, Fernandez J, Ward CK, et al. Comparative effects of carbapenems on bacterial load and host immune response in a Klebsiella pneumoniae murine pneumonia model. Antimicrob Agents Chemother. 2011;55:836–44.
Puskarich MA, Trzeciak S, Shapiro NI, Arnold RC, Horton JM, Studnek JR, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39:2066–71.
Evans ME, Pollack M. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J Infect Dis. 1993;167:1336–43.
Brandenburg K, Heinbochel L, Correa W, Lohner K. Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis. Biochim Biophys Acta. 2016;1858:971–9.
Goscinski G, Tano E, Löwdin E, Sjölin J. Propensity to release endotoxin after two repeated doses of cefuroxime in an in vitro kinetic model: higher release after the second dose. J Antimicrob Chemother. 2007;60:328–33.
Jackson JJ, Kropp H. beta-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J Infect Dis. 1992;165:1033–41.
Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev. 2002;15:95–110.
Lepper PM, Held TK, Schneider EM, Bölke E, Gerlach H, Trautmann M. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med. 2002;28:824–33.
Fujiwara M, Nakagawa S, Eto M, Mizunaga S Inhibition of LpxC Activity and Elimination of Lipopolysaccharide (LPS) Release by T-1228 (RC-01). [Abstract] American Society for Microbiology Microbe 2019, abstr AAR-741.
Lin L, Tan B, Pantapalangkoor P, Ho T, Baquir B, Tomaras A, et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. MBio 2012;3:e00312–12.
Uribe-Querol E, Rosales C. Control of Phagocytosis by Microbial Pathogens. Front Immunol. 2017;8:1368.
Japanese Association for Infectious Diseases/Japanese Society of Chemotherapy, The JAID/JSC Guide/Guidelines to Clinical Management of Infectious Disease Preparing Committee, Sepsis working group, Arakawa S, Kasai M, Kawai S, Sakata H, Mayumi T. The JAID/JSC guidelines for management of infectious diseases 2017 – Sepsis and catheter-related bloodstream infection. J Infect Chemother. 2021;27:657–77.
Castanheira M, Doyle TB, Kantro V, Mendes RE, Shortridge D. Meropenem-Vaborbactam activity against carbapenem-resistant enterobacterales isolates Collected in U.S. hospitals during 2016 to 2018. Antimicrob Agents Chemother. 2020;64:e01951–19.
Ushiyama F, Takashima H, Matsuda Y, Ogata Y, Sasamoto N, Kurimoto-Tsuruta R, et al. Lead optimization of 2-hydroxymethyl imidazoles as non-hydroxamate LpxC Inhibitors: discovery of TP0586532. Bioorg Med Chem. 2020;30:115964 https://doi.org/10.1016/j.bmc.2020.115964.
Fujita K, Takata I, Yoshida I, Okumura H, Otake K, Takashima H, et al. TP0586532, a Non-Hydroxamate LpxC Inhibitor, has in vitro and in vivo antibacterial activities against Enterobacteriaceae. J Antibiot. (Tokyo). 2021. https://www.nature.com/articles/s41429-021-00486-3. Online ahead of print.
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard—Tenth Edition. M07–A11. Clinical and Laboratory Standards Institute, Wayne, PA. 2018.
Norimatsu M, Morrison DC. Correlation of antibiotic-induced endotoxin release and cytokine production in Escherichia coli-inoculated mouse whole blood ex vivo. J Infect Dis. 1998;177:1302–7.
GLobal Report On The Epidemiology And Burden Of Sepsis. World Health Organization. 2020. https://apps.who.int/iris/bitstream/handle/10665/334216/9789240010789-eng.pdf.
Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46:1552–62.
Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–55.
Mason DJ, Power EG, Talsania H, Phillips I, Gant VA. Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother. 1995;39:2752–8.
Crosby HA, Bion JF, Penn CW, Elliott TS. Antibiotic-induced release of endotoxin from bacteria in vitro. J Med Microbiol. 1994;40:23–30.
Kawai S, Nakagawa T, Sakayori S, Kobayashi O, Kamiya S. Effect of ciprofloxacin on levels of lipopolysaccharide and cytokines in experimentally induced Gram-negative bacterial pneumonia in mice. J Infect Chemother. 2006;12:119–23.
Wu G, Fan X, Li L, Wang H, Ding J, Hongbin W, et al. Interaction of antimicrobial peptide s-thanatin with lipopolysaccharide in vitro and in an experimental mouse model of septic shock caused by a multidrug-resistant clinical isolate of Escherichia coli. Int J Antimicrob Agents. 2010;35:250–4.
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88.
Honda S, Sato K, Totsuka N, Fujiyama S, Fujimoto M, Miyake K, et al. Marginal zone B cells exacerbate endotoxic shock via interleukin-6 secretion induced by Fcα/μR-coupled TLR4 signalling. Nat Commun. 2016;7:11498.
Ibrahim YF, Moussa RA, Bayoumi AMA, Ahmed AI-SF. Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-κB/JNK: a possible role of P-glycoprotein. Inflammopharmacology 2020;28:215–30.
Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020;52:731–3.
Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators Inflamm. 2013;2013:165974.
Ono S, Tsujimoto H, Hiraki S, Aosasa S. Mechanisms of sepsis-induced immunosuppression and immunological modification therapies for sepsis. Ann Gastroenterol Surg. 2018;2:351–8.
Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27:669–84.
Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–90.
Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019;10:782.
Chen CW, Mittal R, Klingensmith NJ, Burd EM, Terhorst C, Martin GS, et al. Cutting edge: 2B4-mediated coinhibition of CD4+ T cells underlies mortality in experimental sepsis. J Immunol. 2017;199:1961–6.
Shao R, Fang Y, Yu H, Zhao L, Jiang Z, Li CS. Monocyte programmed death ligand-1 expression after 3–4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit Care. 2016;20:124.
Patil NK, Guo Y, Luan L, Sherwood ER. Targeting immune cell checkpoints during sepsis. Int J Mol Sci. 2017;18:2413.
Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–40.
Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose dependent effect of anti-CTLA-4 on survival in sepsis. Shock 2011;36:38–44.
Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196:856–63.
Acknowledgements
We thank Dr. Yusuke Honma for his helpful discussions and technical support for the experiments, Ms. Ai Shoji, Mr. Tomonori Aida and Ms. Satoko Murakami for their technical support for the experiments, and Mr. Fumihito Ushiyama for the synthesis of TP0586532.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujita, K., Takata, I., Yoshida, I. et al. TP0586532, a non-hydroxamate LpxC inhibitor, reduces LPS release and IL-6 production both in vitro and in vivo. J Antibiot 75, 136–145 (2022). https://doi.org/10.1038/s41429-021-00498-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-021-00498-z